Abstract
Background
Hypersensitivity pneumonitis (HP), an immune-mediated inflammatory interstitial lung disease (ILD), can result from exposure to several well-recognized antigens. Despite antigen avoidance, progressive pulmonary fibrosis and death can occur, suggesting that additional factors may contribute to disease activity. We hypothesized that the presence of autoimmunity might impact clinical course in patients with HP. In this study, we examined an HP cohort to identify those with HP and autoimmune features (HPAF), and determine its prevalence and outcomes.
Methods
The University of Chicago ILD registry was screened to identify patients with HP. Patients were characterized as HPAF if they had an autoimmune disease or features of autoimmunity, defined as the presence of specific connective tissue disease symptoms and serologies. Demographics, clinical characteristics, and outcomes were compared between groups. Survival analysis was performed using Cox regression to identify predictors of transplant-free survival in this cohort.
Results
One hundred twenty patients with chronic, fibrotic HP were identified. Of these, 18/120 (15%) were characterized as HPAF. Compared to those without evidence of autoimmunity, patients with HPAF had a higher proportion of females (54% vs. 83%, respectively; p=0.02) but were otherwise similar with regard to clinical characteristics. The presence of autoimmunity was an independent predictor of increased mortality (HR 4.45; 95% CI 1.43 – 13.88; p=0.01) after multivariable adjustment.
Conclusions
Fifteen percent of patients with chronic, fibrotic HP displayed evidence of a concurrent defined autoimmune disease or autoimmune features suggestive of CTD. The presence of autoimmunity in patients with chronic, fibrotic HP may portend a poorer prognosis. Future studies are needed to validate these findings and determine the impact of immunosuppressive treatment.
Keywords: autoimmunity, connective tissue disease, hypersensitivity pneumonitis, interstitial lung disease, pulmonary fibrosis, immunosuppressive therapy
Interstitial lung disease (ILD) encompasses a heterogeneous group of diffuse parenchymal lung diseases often characterized by inflammation and scarring of the pulmonary parenchyma, resulting in significant morbidity and mortality(1). While it is well-recognized that ILD can develop secondary to connective tissue disease (CTD), there is increasing awareness that features of autoimmunity are common among patients characterized as having idiopathic interstitial pneumonia (IIP),(2) and that systematic evaluation of patients with IIP can reveal a previously unrecognized autoimmune process(3-6) The clinical implications of autoimmune features in those with IIP who fail to meet established rheumatologic criteria remain unclear, but some studies suggest an improved prognosis (7, 8).
Hypersensitivity pneumonitis (HP) is an ILD caused by a wide variety of small organic particles. These antigens include fungi, proteins from animals and insects and some chemical compounds.(9) As these antigens are ubiquitous, it remains unclear why only a small fraction of exposed individuals develop HP. One explanation may lie with abnormal T cell function, as individuals with HP do not suppress T-cell proliferation after exposure to known antigen when compared to healthy controls. (10) T cell dysregulation is also common among individuals with several CTDs (11-13), and raises the question of whether autoimmune disease is more likely to be present among patients with HP.
In this study, we systematically assessed an HP cohort to identify patients with autoimmune features (HPAF), defined as the presence of documented autoimmune disease or contemporaneous autoimmune serologies and clinical features suggestive of an undifferentiated connective tissue disease (UCTD)(7). We then characterized clinical features and outcomes among patients with HPAF and compared them to those HP patients without evidence of autoimmunity.
Materials and Methods
Study Population
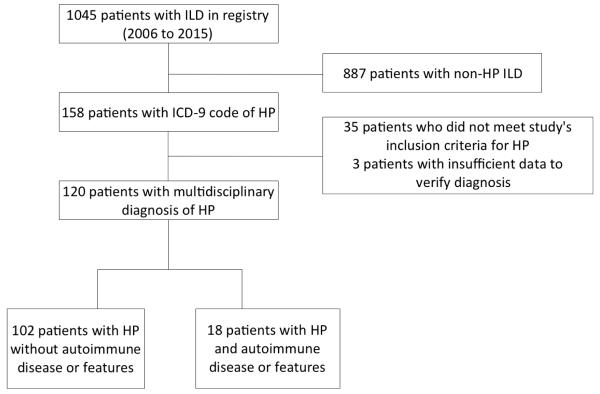
This retrospective analysis was conducted at the University of Chicago with approval of our Institutional Review Board (IRB #14163A) and all patients provided informed consent. We identified consecutive patients aged ≥18 years who enrolled in the University of Chicago ILD registry, were diagnosed with HP based on multidisciplinary evaluation, and were seen in the ILD clinic between January 1, 2006 and February 28, 2015. (Fig 1). Data was extracted using the electronic medical record. Variables collected included demographic data (age, race/ethnicity, gender), symptoms, co-morbid conditions (autoimmune disease, coronary artery disease, gastroesophageal reflux), history of tobacco use, use of chronic corticosteroid and immunosuppressive therapy, physical examination findings such as body mass index (BMI), crackles and clubbing, assessment of environmental antigen exposures (avian, mold, hot tub, unknown), laboratory data including serologies and C-reactive protein (CRP), serial PFTs including percent predicted total lung capacity (TLC), forced vital capacity (FVC) and diffusion capacity of the lung for carbon monoxide (DLCO), six minute walk test and histopathologic findings. An experienced pulmonary pathologist with expertise in ILD previously reviewed all surgical lung biopsies. Outcomes assessed included all-cause mortality, lung transplantation and ≥ 10% decline in FVC. Outcomes were ascertained by review of medical records, telephone interviews and the Social Security Death Index.
Figure 1.
Consort diagram
Enrollment criteria
All patients in the University of Chicago ILD registry are assessed for exposure to antigens commonly associated with HP, regardless of referring diagnosis. A diagnosis of HP was based on multidisciplinary evaluation of patients’ clinical features, HRCT findings and surgical lung biopsy results by physicians with expertise in ILD. Patients were diagnosed with HP and were included in the current study if they met the following criteria:
(1) HRCT features compatible with HP: mosaic attenuation/air trapping, centrilobular nodules, reticulation, traction bronchiectasis, and honeycombing. (2) Surgical lung biopsy specimens, when obtained, demonstrated presence of a histologic pattern consistent with HP. This included lymphocytic predominant interstitial infiltrates, with/without poorly formed granulomas, distributed in a bronchiolocentric pattern. Histologic fibrosis was also noted when present. (3) Exclusion of an alternative etiology for these findings. The presence of antibodies to serum precipitins supported the diagnosis, but was not a requirement.
HRCT Review and Scoring
Features suggesting HP and pulmonary fibrosis, as defined by reticulation, traction bronchiectasis or honeycombing pattern, were recorded. All HRCTs were systematically reviewed and scored by a senior chest radiologist (S.M.) who was blinded to clinical phenotype. The mean extent of these morphological features was scored to the nearest 5% in all three zones of each lung as previously described (14, 15) resulting in a semi-quantitative HRCT fibrosis score. These findings were graded on a scale of 1-4: 1 - normal attenuation; 2 - reticular abnormality; 3 - traction bronchiectasis; and 4 - honeycombing. Independent assessment was made for each of these four HRCT findings in three (upper, middle and lower) zones of each lung. The score for each zone was determined by multiplying the grading scale score by the percentage involvement for each zone. The average score of all six zones was calculated as the total score for each patient. Using this method, the highest score was 203 points and the lowest score was 100 points.
Autoimmune Features
Patients were classified as HPAF if they had a documented diagnosis of autoimmune disease(16-19) including scleroderma, Sjogren’s disease, idiopathic inflammatory myopathy (IIM), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and ulcerative colitis. Patients were also classified as HPAF if at least one specific CTD symptom and one serologic test suggestive of autoimmune disease were present, as previously proposed by Corte et al(7). Specific CTD symptoms included Raynaud’s phenomenon, arthralgias/joint swelling, morning stiffness, dry mouth/dry eyes and proximal muscle weakness. Positive autoimmune serologies were an anti-nuclear antibody (ANA) ≥1:320, a rheumatoid factor (RF) ≥ 3x upper limit of normal, or a positive anti-neutrophil cytoplasmic antibody (ANCA), cyclic citrullinated peptide (aCCP); Jo1; dsDNA; ribonucleoprotein (anti-RNP); Scl-70; SS-A/Ro or SS-B/La. Patients were stratified based on HPAF status and further sub-stratified based on the presence of defined autoimmune disease.
Statistical Analysis
Continuous variables were reported as means with standard deviation (SD) and compared using a two-tailed student’s t-test. Categorical variables were reported as counts and percentages and compared using the Chi-square test or Fisher’s exact test, as appropriate. A p-value of <0.05 was considered to be significant. Survival analysis was performed using univariate and multivariable Cox proportional hazards analysis along with the unadjusted log rank test and plotted using the Kaplan-Meier survival estimator. Transplant-free survival was defined as time from diagnosis to death or lung transplantation. Patients who were lost to follow-up were censored at that time. All data analysis was performed in Stata version 13.1 (Stata Corp; College Station, Texas).
Results
Of 1045 patients enrolled in our ILD registry, 158 had an ICD-9 diagnosis code of HP (Fig 1). Of these 158, 38 were excluded due to an alternate ILD diagnosis or insufficient data to verify their diagnosis, leaving 120 patients with HP based on a multidisciplinary review of clinical data, radiographic and/or pathologic findings and a compatible exposure history. All 120 patients had chronic, fibrotic HP based on HRCT and/or SLB. Eighteen patients (15%) met criteria for HPAF. Of these patients, nine had a documented autoimmune disease (scleroderma, Sjogren’s disease, IIM, SLE, RA, ulcerative colitis) and nine displayed features of autoimmunity (Table 1 and Supplemental Table 1).
Table 1.
Autoimmune disease or features in Hypersensitivity Pneumonitis
| Autoimmune disease or features | n (%) |
|---|---|
| Autoimmune disease* | 9 (50.0) |
| Scleroderma | 1 (5.6) |
| Sjogren’s disease | 2 (11.1) |
| Idiopathic inflammatory myopathy | 1 (5.6) |
| Systemic lupus erythematosus | 2 (11.1) |
| Rheumatoid arthritis | 4 (22.2) |
| Ulcerative colitis | 1 (5.6) |
| Autoimmune features | 9 (50.0) |
Some patients had more than one autoimmune disease
Baseline demographics and clinical characteristics of the entire cohort revealed that mean age at diagnosis of HP was 63 years (±10 years); with a mean BMI of 32 (±8) and a Caucasian (83%) and female predominance (58%). Comorbid clinical conditions included gastroesophageal reflux (59%) and coronary artery disease (21%). Fifty-eight percent had a prior smoking history and 72% required chronic systemic corticosteroid use for their ILD. On examination, most had crackles (85%) while a minority of patients had digital clubbing (24%). Exposure to avian antigens (47%) was the most common environmental exposure identified.
When comparing baseline characteristics between HPAF and HP patients without evidence of autoimmunity, HPAF had a higher proportion of females (83% vs. 54%, respectively; p=0.02) and demonstrated less digital clubbing (6% vs. 28% p=0.045) (Table 2). There were no differences between groups with regard to other demographic characteristics, antigen exposure, radiographic or histopathologic features, or pulmonary function testing (Tables 2 and 3). When comparing autoimmune serologies and clinical features between groups (Table 4), those meeting predefined criteria for HPAF had a higher prevalence of positive ANA, SS-A, anti-dsDNA, digital arthralgias, morning stiffness and dry eyes and dry mouth than patients with HP who did not meet these criteria.
Table 2.
Baseline Characteristics in HP patients with and without Autoimmune Features
| Characteristics | HPAF (n=18) |
HP without AF (n=102) |
p-value |
|---|---|---|---|
| Age, mean (±SD) | 62.2 (9.5) | 62.7 (10.5) | 0.858 |
| Female gender, n (%) | 15 (83.3) | 55 (53.9) | 0.020 |
| Race/Ethnicity | |||
| Caucasian, n (%) | 13 (72.2) | 87 (85.3) | 0.170 |
| Hispanic, n (%) | 1 (5.6) | 9 (8.8) | 1.000 |
| African American, n (%) | 2 (11.1) | 5 (4.9) | 0.282 |
| Asian, n (%) | 2 (11.1) | 1 (0.98) | 0.059 |
| BMI, mean (±SD) | 31.1 (8.2) | 32.6 (8.0) | 0.470 |
| Ever smoker, n (%) | 8 (44.4) | 62 (60.8) | 0.195 |
| Chronic systemic corticosteroid use, n (%) | 16 (61.5) | 70 (74.5) | 0.195 |
| Other immunosuppressive therapy, n (%) | 5 (27.8) | 31 (30.4) | 0.823 |
| Antigen exposure | |||
| Avian, n (%) | 8 (44.4) | 48 (47.1) | 0.838 |
| Mold, n (%) | 6 (33.3) | 29 (28.4) | 0.673 |
| Hot tub, n (%) | 0 (0) | 3 (2.9) | 0.611 |
| Unknown, n (%) | 4 (22.2) | 34 (33.3) | 0.350 |
| Gastroesophageal reflux, n (%) | 13 (72.2) | 58 (56.9) | 0.222 |
| Coronary artery disease, n (%) | 4 (22.2) | 21 (20.6) | 0.875 |
| Crackles, n (%) | 15 (83.3) | 87 (85.3) | 0.830 |
| Clubbing, n (%) | 1 (5.6) | 28 (27.5) | 0.045 |
HP = Hypersensitivity Pneumonitis; HPAF= HP with Autoimmune features as defined in the text; AF = Autoimmune features
Table 3.
Assessment of Lung Function, HRCT and Pathologic Characteristics in HP
| Characteristics | HPAF (n=18) |
HP without AF (n=102) |
p-value |
|---|---|---|---|
| Pulmonary Function Tests | |||
| TLC‘ (% predicted) (±SD) | 72.9 (16.0) | 72.3 (18.4) | 0.889 |
| FVC@ (% predicted) (±SD) | 63.7 (20.3) | 65.1 (18.7) | 0.773 |
| DLCO (% predicted) (±SD) | 55.1 (26.7) | 54.3 (24.2) | 0.906 |
| Oxygen therapy, n (%) | 7 (38.9) | 61 (59.8) | 0.099 |
| 6MWT distance (feet) (±SD) | 1004.3 (424) | 1120.0 (451) | 0.328 |
| HRCT features | |||
| HRCT fibrosis score, mean (±SD) | 123.3(14.0) | 124.0(17.4) | 0.862 |
| Mosaic attenuation, n (%) | 15 (83.3) | 88 (86.3) | 0.741 |
| Centrilobular nodules, n (%) | 11 (61.1) | 40 (39.2) | 0.083 |
| Ground glass opacities, n (%) | 17 (94.4) | 97 (95.1) | 0.631 |
| Reticulation, n (%) | 14 (77.8) | 78 (76.5) | 1.000 |
| Traction bronchiectasis, n (%) | 14 (77.8) | 84 (82.4) | 0.644 |
| Honeycombing, n (%) | 7 (38.9) | 43 (42.2) | 0.795 |
| Absence of lower zone predominant fibrosis, n (%) | 10 (55.6) | 59 (57.8) | 0.856 |
| Histopathologic features | |||
| Surgical lung biopsy obtained, n (%) | 14 (77.8) | 60 (58.8) | 0.127 |
| Poorly formed granulomas, n (%) | 11 (78.6) | 37 (61.7) | 0.233 |
| Lymphoplasmacytic infiltration/GC, n (%) | 6 (42.9) | 13 (21.7) | 0.170 |
| Honeycombing with UIP pattern, n (%) | 7 (50.0) | 28 (46.7) | 0.822 |
HP = Hypersensitivity Pneumonitis; HPAF= Hypersensitivity Pneumonitis with Autoimmune features as defined in the text; AF = Autoimmune features;
n=112;
n=114; 6MWT = 6 minute walk test, n=109; HRCT= High-resolution computerized tomography scan; GC = Germinal centers
Table 4.
Autoimmune Serologic Tests and Clinical characteristics
| Characteristics | HPAF (n=18) |
HP without AF (n=102) |
p-value |
|---|---|---|---|
| Autoimmune serologic tests | |||
| ANA ≥ 1:320, n (%) | 11 (61.1) | 23 (22.5) | 0.001 |
| RF ≥ 3x upper limit normal, n (%) | 0 (0) | 1 (1.0) | 1.000 |
| Anti-ENA RNP, n (%) | 1 (5.5) | 0 (0) | 0.154 |
| Anti-topoisomerase (Scl-70), n (%) | 1 (5.5) | 1 (1.0) | 0.285 |
| Anti-Ro (SS-A), n (%) | 2 (11.1) | 0 (0) | 0.023 |
| Anti-La (SS-B), n (%) | 0 (0) | 1 (1.0) | 1.000 |
| Anti-dsDNA, n (%) | 3 (16.7) | 1 (1.0) | 0.011 |
| Anti-CCP, n (%) | 2 (11.1) | 1 (1.0) | 0.061 |
| C-reactive protein‘, mean (±SD) | 13.6 (26.2) | 9.9 (25.6) | 0.575 |
| Autoimmune clinical features | |||
| Raynaud’s phenomenon, n (%) | 3 (16.7) | 6 (5.9) | 0.109 |
| Arthralgias/multiple joint swelling, n (%) | 6 (33.3) | 3 (2.9) | <0.001 |
| Morning stiffness, n (%) | 6 (33.3) | 3 (2.9) | <0.001 |
| Dry mouth/dry eyes, n (%) | 12 (66.7) | 23 (22.5) | <0.001 |
| Proximal muscle weakness, n (%) | 2 (11.1) | 2 (2.0) | 0.106 |
HP = Hypersensitivity Pneumonitis; HPAF= Hypersensitivity Pneumonitis with Autoimmune features as defined in the text; AF = Autoimmune features
During the study period, 28% of the entire HP cohort either died or underwent lung transplantation (Table 5 and Supplemental Fig 1). Mean survival was 16.5 months (±13.0 months) in HPAF compared to 29.7 months (±21.5 months) (p=0.103) in HP without autoimmune features. There were no statistically significant differences between groups with regard to number of deaths (28% vs 24%, respectively; p=0.698) or lung transplantation (6% vs 4%, respectively; p=0.75). The number of patients with a 10% or more decline in percentage predicted FVC did not differ (50% in HPAF vs 30% in HP without autoimmune features; p=0.178) and the time to FVC decline was similar in both groups (26±16 months vs 18±18 months; p=0.337).
Table 5.
Outcomes in patients with HP
| Characteristics | HPAF (n=18) |
HP without AF (n=102) |
p-value |
|---|---|---|---|
| Deceased, n (%) | 5 (27.8) | 24 (23.5) | 0.698 |
| Mean survival period, months (±SD) | 16.5 (13.0) | 29.7 (21.5) | 0.103 |
| Transplanted, n (%) | 1 (5.6) | 4 (3.9) | 0.749 |
| Mean Time to transplant, months (±SD) | 23.6 (0) | 33.2 (28.4) | 0.528 |
| 10% FVC decline*, n (%) | 6 (50) | 24 (30.4) | 0.178 |
| Mean Time to 10% FVC decline, months (±SD) | 26.2 (16) | 18.2 (18.2) | 0.337 |
HP = Hypersensitivity Pneumonitis; HPAF= Hypersensitivity Pneumonitis with Autoimmune features as defined in the text; AF = Autoimmune features
Follow up FVC available for 91 HP patients; 12 and 79, with and without autoimmune disease or features respectively.
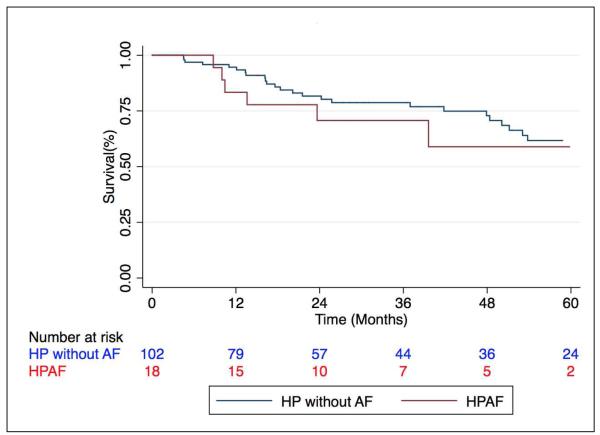
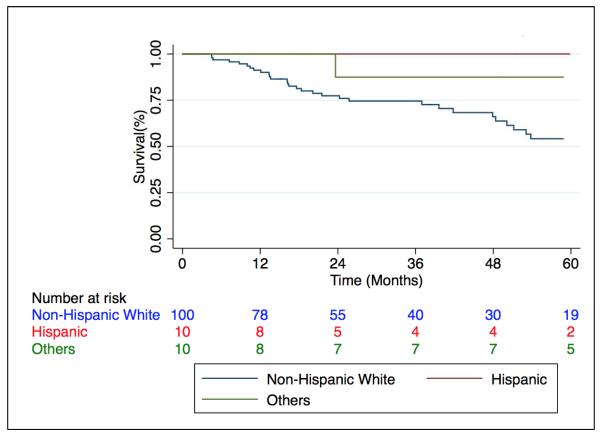
The unadjusted comparison of HPAF to HP subjects without autoimmune features demonstrated no difference in survival between groups (p=0.59) (Fig 2). HPAF was sub-stratified based on the presence of defined autoimmune disease and no survival difference was detected (p=0.47). However, when stratification of the entire HP cohort was performed by race/ethnicity, survival was greatest among non-Caucasian subjects (p=0.036) (Fig 3). Stratification of the entire HP cohort based on identification of an inciting antigen showed that antigen detection had no effect on prognosis (data not shown).
Figure 2. Survival among patients with HP stratified by HPAF status.
Mean survival time in those with coexistent HP and autoimmune disease or features (HPAF) was 17.7 ± 12 months compared to 30.2 ± 22 months (p=0.59) in those with HP alone. HP = Hypersensitivity pneumonitis. AF = autoimmune features.
Figure 3. Survival among patients with HP stratified by Race/Ethnicity.
Caucasians with HP demonstrate a reduced survival time compared with non-Caucasians (p=0.036). HP = Hypersensitivity Pneumonitis.
Unadjusted predictors of transplant-free survival included Caucasian race (HR 8.42; 95% CI 1.15–61.68; p=0.036), BMI (HR 0.95; 95% CI 0.90–0.99; p=0.034), presence of digital clubbing (HR 3.47; 95% CI 1.77–6.81; p<0.001), HRCT fibrosis score (HR 1.04; 95% CI 1.02–1.05; p<0.001) (Supplemental Fig 2), and oxygen therapy (HR 4.38; 95% CI 1.81-10.59; p=0.001) (Table 6). After adjustment for age, gender, race, BMI, digital clubbing, oxygen therapy and disease severity (HRCT fibrosis score and percentage predicted FVC and DLCO), multivariable Cox regression analysis demonstrated HPAF to be an independent factor predicting mortality or lung transplantation (HR 4.45; 95% CI 1.43-13.88; p=0.01). Predictors of transplant-free survival in multivariable Cox regression analysis also included non-Caucasian race (HR 9.33; 95% CI 1.14–76.30; p=0.037) and HRCT fibrosis score (HR 1.02; 95% CI 1.00–1.04; p=0.045).
Table 6.
Variables Predicting Survival in Patients with HP
| Unadjusted (n=120) |
Adjusted* (n=118) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HR | p-value | 95% CI | HR | p-value | 95% CI |
| Autoimmune disease or features (HPAF) | 1.28 | 0.591 | 0.53 – 3.10 | 4.45 | 0.010 | 1.43 – 13.88 |
| Age | 1.02 | 0.184 | 0.99 – 1.06 | 1.00 | 0.893 | 0.95 – 1.05 |
| Gender | 1.09 | 0.798 | 0.55 – 2.17 | 1.73 | 0.151 | 0.82 – 3.65 |
| Caucasian race | 8.42 | 0.036 | 1.15 – 61.68 | 9.33 | 0.037 | 1.14 – 76.30 |
| BMI | 0.95 | 0.034 | 0.90 – 0.99 | 0.95 | 0.076 | 0.89 – 1.01 |
| Digital clubbing | 3.47 | <0.001 | 1.77 – 6.81 | 1.34 | 0.469 | 0.60 – 2.99 |
| HRCT fibrosis score | 1.04 | <0.001 | 1.02 – 1.05 | 1.02 | 0.045 | 1.00 – 1.04 |
| FVC@ (% predicted) | 0.94 | <0.001 | 0.92 – 0.97 | 0.97 | 0.059 | 0.94 – 1.00 |
| DLCO (% predicted) | 0.96 | <0.001 | 0.95 – 0.98 | 0.99 | 0.489 | 0.97 – 1.01 |
| Oxygen therapy | 4.38 | 0.001 | 1.81 – 10.59 | 2.51 | 0.094 | 0.85 – 7.38 |
Adjusted for all variables listed above;
n=119;
HP = Hypersensitivity Pneumonitis; HPAF= Hypersensitivity Pneumonitis with Autoimmune features as defined in the text;
Discussion
In this study, we showed that a significant minority of patients with HP displayed evidence of a concurrent defined autoimmune disease or autoimmune features suggestive of CTD. Patients with HPAF were predominantly female. We found that the presence of autoimmune disease or autoimmune features independently predicted worse outcomes for patients with HP after controlling for disease severity and radiographic extent of fibrosis. To our knowledge, this is the first study of autoimmunity in HP and identifies clinical factors associated with worse outcomes.
Predictors of survival in HP have previously been reported in the literature. Similar to Sansores et al, we found that digital clubbing was prevalent and predicted worse outcomes on univariate analysis(20). Mooney et al. studied predictors of transplant-free survival in HP, and found that crackles, pulmonary function tests, and oxygen therapy were associated with outcome on univariate analysis. (15) While we confirmed a similar association between oxygen therapy and transplant-free survival in our entire cohort, we additionally found that a lower BMI was a poor prognostic marker. We also found that FVC and DLCO, key physiologic parameters of the ILD-GAP risk prediction model, were predictors of poor outcome. Lastly, like Mooney et al, we found that the HRCT fibrosis score independently predicted worse prognosis in our entire HP cohort. (15)
To our knowledge, the role of race/ethnicity in predicting outcomes has not been previously studied in HP. In multivariable analysis, we found that Caucasian subjects were 9.33 times more likely to die or undergo a lung transplant during follow-up than non-Caucasian patients. These findings may support a genetic role as has been suggested in prior studies by others, in the pathogenesis or disease course of HP but require validation(21, 22).
This is the first systematic study to examine the presence of autoimmune diseases or specific CTD features in HP and their impact on patient outcomes. As might be expected given the higher prevalence of autoimmune diseases in women(23), our HPAF cohort was predominantly female. Although digital clubbing is commonly seen in HP,(20) this was an uncommon finding in our HPAF patients. After controlling for disease severity as measured by pulmonary function tests and HRCT fibrosis score, we found that the presence of autoimmune disease or specific predefined autoimmune features remained a significant risk factor for poor outcomes.
The reasons underlying an association between autoimmunity and HP are unclear. Alteration of the pulmonary epithelium by inhaled environmental agents has been shown to promote post-translational modifications, which eventually alter respiratory mucosal proteins and results in the production of several citrullinated self-proteins(24). This pathophysiologic process may explain the autoimmune phenomenon observed in our study in which almost half of those subjects with HP who did not meet our HPAF criteria had either a positive ANA or CTD symptoms. Perhaps like cigarette smokers(25, 26) and coal workers who develop RA(27), silica-exposed individuals who develop RA and SLE(28-31), farmers at increased risk of primary systemic vasculitis(32) and the World Trade Center firefighters/rescue workers who developed diverse CTDs(33), inhaled environmental exposures may trigger autoimmune processes in genetically susceptible individuals.
Our study has several limitations. First, this is a retrospective study, although all data were prospectively acquired. Second, our cohort is drawn from a single tertiary referral center with expertise in ILD and these findings require external validation. Third, we recognize that beyond those patients with autoimmune disease, definitions of autoimmune features have varied in the literature.(2, 7, 8) We chose previously published and frequently cited criteria for autoimmune features that utilize more specific symptoms and serologies.
Conclusion
Our findings show that HP may coexist with autoimmune disease or features in the same patient and manifest as advanced ILD with increased mortality. Careful consideration should be given to the coexistence of both processes especially in female patients. Whether pre-existing autoimmunity delays recognition of HP and/or contributes to a more exuberant inflammatory response deserves further study. It is currently unknown if chronic environmental antigen exposure precipitates and/or exacerbates autoimmune disease. Recognition of autoimmunity in patients with HP may have an impact on subsequent ILD management and disease course.
Supplementary Material
Highlights.
Features of autoimmunity are prevalent in hypersensitivity pneumonitis (HP)
Presence of autoimmune disease or features predicts worse outcomes for HP patients
Recognition of autoimmunity in HP may impact management and disease course
Acknowledgments
This investigation was supported by an NIH T32 training grant (T32-HL007605).
Dr. Vij has received funding from Genentech for the conduct of research in interstitial lung disease. Dr. Strek has institutional grants from the NIH, Bristol-Myers Sqibb ,Gilead, InterMune, and MedImmune for the conduct of clinical trials in IPF. She has received honoraria for serving on a Data Monitoring Committee for Boehringer Ingelheim and an advisory board for InterMune.
Abbreviation List
- aCCP
anti-citrullinated protein antibody
- ANA
antinuclear antibody
- ANCA
antineutrophil cytoplasmic antibody
- BMI
body mass index
- CAD
coronary artery disease
- CI
Confidence Interval
- CRP
C-reactive protein
- CTD
connective tissue disease
- DLCO
diffusion capacity of the lung for carbon monoxide
- FVC
forced vital capacity
- GER
gastroesophageal reflux
- HLA
human leukocyte antigen
- HP
Hypersensitivity pneumonitis
- HR
hazard ratio
- HRCT
high-resolution computed tomagraphy
- IIP
idiopathic interstitial pneumonia
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- IRB
institutional review board
- OR
odds ratio
- PFT
pulmonary function testing
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- SD
standard deviation
- SLB
surgical lung biopsy
- SLE
systemic lupus erythematosus
- TLC
total lung capacity
- UCTD
undifferentiated connective tissue disease
- UIP
usual interstitial pneumonia
Footnotes
Conflict of Interest Disclosures: Drs. Adegunsoye, Oldham, and Montner, along with Ms. Demchuk, have nothing to disclose.
Author contribution: Conception and design: AA, JO, SM, RV, MS. Data collection and interpretation: AA, JO, CD, SM, RV, MS. Data analysis and interpretation: AA, JO, RV, MS. Drafting the manuscript for important intellectual content: AA, JO, RV, MS. Dr. Adegunsoye is the guarantor of this paper and takes responsibility for the integrity of the work as a whole, from inception to published article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. American journal of respiratory and critical care medicine. 2005;172:268–279. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 2.Kinder BW, Shariat C, Collard HR, Koth LL, Wolters PJ, Golden JA, Panos RJ, King TE., Jr Undifferentiated connective tissue disease-associated interstitial lung disease: changes in lung function. Lung. 2010;188:143–149. doi: 10.1007/s00408-009-9226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 4.Alhamad EH. Interstitial lung diseases in Saudi Arabia: A single-center study. Annals of thoracic medicine. 2013;8:33–37. doi: 10.4103/1817-1737.105717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009;136:23–30. doi: 10.1378/chest.08-2572. [DOI] [PubMed] [Google Scholar]
- 6.Fischer A, Pfalzgraf FJ, Feghali-Bostwick CA, Wright TM, Curran-Everett D, West SG, Brown KK. Anti-th/to-positivity in a cohort of patients with idiopathic pulmonary fibrosis. The Journal of rheumatology. 2006;33:1600–1605. [PubMed] [Google Scholar]
- 7.Corte TJ, Copley SJ, Desai SR, Zappala CJ, Hansell DM, Nicholson AG, Colby TV, Renzoni E, Maher TM, Wells AU. Significance of connective tissue disease features in idiopathic interstitial pneumonia. The European respiratory journal. 2012;39:661–668. doi: 10.1183/09031936.00174910. [DOI] [PubMed] [Google Scholar]
- 8.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest. 2011;140:1292–1299. doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. American journal of respiratory and critical care medicine. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 10.Girard M, Israel-Assayag E, Cormier Y. Impaired function of regulatory T-cells in hypersensitivity pneumonitis. The European respiratory journal. 2011;37:632–639. doi: 10.1183/09031936.00055210. [DOI] [PubMed] [Google Scholar]
- 11.Beavis PA, Gregory B, Green P, Cribbs AP, Kennedy A, Amjadi P, Palfreeman AC, Feldmann M, Brennan FM. Resistance to regulatory T cell-mediated suppression in rheumatoid arthritis can be bypassed by ectopic foxp3 expression in pathogenic synovial T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16717–16722. doi: 10.1073/pnas.1112722108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander T, Sattler A, Templin L, Kohler S, Gross C, Meisel A, Sawitzki B, Burmester GR, Arnold R, Radbruch A, Thiel A, Hiepe F. Foxp3+ Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Annals of the rheumatic diseases. 2013;72:1549–1558. doi: 10.1136/annrheumdis-2012-202216. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology. 2012;51:1540–1549. doi: 10.1093/rheumatology/kes090. [DOI] [PubMed] [Google Scholar]
- 14.Oda K, Ishimoto H, Yatera K, Naito K, Ogoshi T, Yamasaki K, Imanaga T, Tsuda T, Nakao H, Kawanami T, Mukae H. High-resolution CT scoring system-based grading scale predicts the clinical outcomes in patients with idiopathic pulmonary fibrosis. Respiratory research. 2014;15:10. doi: 10.1186/1465-9921-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooney JJ, Elicker BM, Urbania TH, Agarwal MR, Ryerson CJ, Nguyen ML, Woodruff PG, Jones KD, Collard HR, King TE, Koth LL. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144:586–592. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 16.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiodt M, Umehara H, Vivino F, Zhao Y, Dong Y, Greenspan D, Heidenreich AM, Helin P, Kirkham B, Kitagawa K, Larkin G, Li M, Lietman T, Lindegaard J, McNamara N, Sack K, Shirlaw P, Sugai S, Vollenweider C, Whitcher J, Wu A, Zhang S, Zhang W, Greenspan J, Daniels T, Sjogren's International Collaborative Clinical Alliance Research G American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis care & research. 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennish L, Labitigan M, Budoff S, Filopoulos MT, McCracken WA, Swearingen CJ, Yazici Y. Utility of the new rheumatoid arthritis 2010 ACR/EULAR classification criteria in routine clinical care. BMJ open. 2012;2 doi: 10.1136/bmjopen-2012-001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA, Jr., Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Muller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Ellen Csuka M, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Annals of the rheumatic diseases. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 19.Urowitz MB, Gladman DD, Ibanez D, Sanchez-Guerrero J, Romero-Diaz J, Gordon C, Bae SC, Clarke AE, Bernatsky S, Fortin PR, Hanly JG, Isenberg D, Rahman A, Wallace DJ, Ginzler E, Petri M, Bruce IN, Merrill JT, Nived O, Sturfelt G, Dooley MA, Alarcon GS, Fessler B, Steinsson K, Ramsey-Goldman R, Zoma A, Khamashta M, Manzi S, van Vollenhoven R, Ramos-Casals M, Aranow C, Stoll T. American College of Rheumatology criteria at inception, and accrual over 5 years in the SLICC inception cohort. The Journal of rheumatology. 2014;41:875–880. doi: 10.3899/jrheum.130704. [DOI] [PubMed] [Google Scholar]
- 20.Sansores R, Salas J, Chapela R, Barquin N, Selman M. Clubbing in hypersensitivity pneumonitis. Its prevalence and possible prognostic role. Archives of internal medicine. 1990;150:1849–1851. [PubMed] [Google Scholar]
- 21.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. American journal of respiratory and critical care medicine. 2006;173:188–198. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falfan-Valencia R, Camarena A, Pineda CL, Montano M, Juarez A, Buendia-Roldan I, Perez-Rubio G, Resendiz-Hernandez JM, Paramo I, Vega A, Granados J, Zuniga J, Selman M. Genetic susceptibility to multicase hypersensitivity pneumonitis is associated with the TNF-238 GG genotype of the promoter region and HLA-DRB1*04 bearing HLA haplotypes. Respiratory medicine. 2014;108:211–217. doi: 10.1016/j.rmed.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. The American journal of pathology. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 25.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, Young A, British Rheumatoid Interstitial Lung N Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology. 2014;53:1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 26.Doyle TJ, Dellaripa PF, Batra K, Frits ML, Iannaccone CK, Hatabu H, Nishino M, Weinblatt ME, Ascherman DP, Washko GR, Hunninghake GM, Choi AM, Shadick NA, Rosas IO. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest. 2014;146:41–50. doi: 10.1378/chest.13-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kart L, Sarikaya S, Gurel A, Altin R, Armutcu F, Tor M, Ozdolap S. Rheumatoid factor seropositivity and rheumatoid symptoms in coal worker's pneumoconiosis. Clinical rheumatology. 2003;22:365–366. doi: 10.1007/s10067-003-0727-0. [DOI] [PubMed] [Google Scholar]
- 28.Rocha MC, Santos LM, Bagatin E, Cohen Tervaert JW, Damoiseaux JG, Lido AV, Longhini AL, Torello CO, Queiroz ML. Genetic polymorphisms and surface expression of CTLA-4 and PD-1 on T cells of silica-exposed workers. International journal of hygiene and environmental health. 2012;215:562–569. doi: 10.1016/j.ijheh.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Speck-Hernandez CA, Montoya-Ortiz G. Silicon, a Possible Link between Environmental Exposure and Autoimmune Diseases: The Case of Rheumatoid Arthritis. Arthritis. 2012;2012:604187. doi: 10.1155/2012/604187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas CD, Amft N, Reid PT. Systemic lupus erythematosus complicating simple silicosis. Occupational medicine. 2014;64:387–390. doi: 10.1093/occmed/kqu060. [DOI] [PubMed] [Google Scholar]
- 31.Stolt P, Kallberg H, Lundberg I, Sjogren B, Klareskog L, Alfredsson L, group Es Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Annals of the rheumatic diseases. 2005;64:582–586. doi: 10.1136/ard.2004.022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane SE, Watts RA, Bentham G, Innes NJ, Scott DG. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis and rheumatism. 2003;48:814–823. doi: 10.1002/art.10830. [DOI] [PubMed] [Google Scholar]
- 33.Webber MP, Moir W, Zeig-Owens R, Glaser MS, Jaber N, Hall C, Berman J, Qayyum B, Loupasakis K, Kelly K, Prezant DJ. Nested case-control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis & rheumatology. 2015;67:1369–1376. doi: 10.1002/art.39059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.