Supplemental Digital Content is available in the text.
Keywords: diabetes mellitus, type 2; extracellular fluid; high-density lipoprotein cholesterol; low-density lipoprotein cholesterol; macrophages
Abstract
Objective—
Patients with type 2 diabetes mellitus (T2D) have an increased risk of cardiovascular disease, the mechanism of which is incompletely understood. Their high-density lipoprotein (HDL) particles in plasma have been reported to have impaired cholesterol efflux capacity. However, the efflux capacity of HDL from interstitial fluid (IF), the starting point for reverse cholesterol transport, has not been studied. We here investigated the cholesterol efflux capacity of HDL from IF and plasma from T2D patients and healthy controls.
Approach and Results—
HDL was isolated from IF and peripheral plasma from 35 T2D patients and 35 age- and sex-matched healthy controls. Cholesterol efflux to HDL was determined in vitro, normalized for HDL cholesterol, using cholesterol-loaded macrophages. Efflux capacity of plasma HDL was 10% lower in T2D patients than in healthy controls, in line with previous observations. This difference was much more pronounced for HDL from IF, where efflux capacity was reduced by 28% in T2D. Somewhat surprisingly, the efflux capacity of HDL from IF was lower than that of plasma HDL, by 15% and 32% in controls and T2D patients, respectively.
Conclusion—
These data demonstrate that (1) HDL from IF has a lower cholesterol efflux capacity than plasma HDL and (2) the efflux capacity of HDL from IF is severely impaired in T2D when compared with controls. Because IF comprises the compartment where reverse cholesterol transport is initiated, the marked reduction in cholesterol efflux capacity of IF-HDL from T2D patients may play an important role for their increased risk to develop atherosclerosis.
Patients with type 2 diabetes (T2D) display an increased risk for premature cardiovascular disease and death.1 Several factors may contribute to this, including hyperglycemia, dyslipidemia, and inflammation.1 Levels of plasma low-density lipoprotein (LDL) cholesterol are usually not markedly elevated in T2D patients; instead they often present with reduced high-density lipoprotein (HDL) cholesterol levels. Importantly, lipid-lowering therapy is of clear benefit for T2D patients.2
We recently investigated the hypothesis that T2D patients might have increased levels of LDL in interstitial fluid (IF) because of an enhanced leakage over the vascular wall.3 However, we unexpectedly observed the opposite, ie, that T2D patients have less apolipoprotein B (apoB)–containing lipoproteins (very low-density lipoprotein and LDL) in IF relative to serum compared with healthy controls.3 These results indicated an increased accumulation or catabolism of apoB-containing particles in the interstitial compartment in T2D. In contrast, the level of HDL cholesterol in IF did not differ between T2D patients and healthy controls.3
HDL metabolism plays an important role for the development of atherosclerosis, and the inverse relationship between HDL cholesterol levels and cardiovascular disease is well established.4 However, attempts to pharmacologically increase plasma HDL-cholesterol failed to show clinical benefit.5–7 It has recently become evident that the functional properties of HDL particles are important for their atheroprotective function, more than the circulating levels per se.8 One major function of HDL is its key role in reverse cholesterol transport, ie, transport of cholesterol from peripheral tissues to the liver for subsequent biliary and fecal excretion. In this process, the capacity of the HDL particle to promote cholesterol efflux from peripheral cells is considered a crucial step.9 Thus, measurements of this efflux capacity have shown to be independently related to the risk for cardiovascular events.10 Accordingly, T2D patients generally have been demonstrated to display reduced cholesterol efflux capacity.11–14
As reverse cholesterol transport is initiated in the extravascular compartment in peripheral tissues,9 evaluating efflux capacity of HDL from IF might be more relevant than studying plasma HDL. Various aspects of HDL metabolism have previously been studied in IF (ie, peripheral lymph).15–17 However, to the best of our knowledge, there are no reports on HDL-mediated cholesterol efflux using HDL from IF, neither in healthy subjects nor in T2D patients. Therefore, we measured in vitro cholesterol efflux capacity of HDL isolated from both IF and plasma obtained from a previous study of T2D patients and healthy controls.3
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Lipoprotein Sizes in Serum and IF From T2D Patients and Healthy Controls
LDL and HDL particle diameters, calculated from the fast performance liquid chromatography (FPLC) retention times, are shown in the Table (absolute retention times are given in Table I in the online-only Data Supplement and the FPLC profiles are given in Figure IA and IB in the online-only Data Supplement). Compared with controls, the calculated HDL particle diameters in T2D patients were reduced by 6.3% and 4.6% in serum and IF, respectively. When comparing sizes of HDL particles in IF with those in serum in the same study groups, we found that the HDL particle size in IF was increased by 4.4% in controls and by 6.4% in T2D patients (Table).
Table.
LDL and HDL Sizes and Lipid Composition of HDL in Serum and IF From T2D Patients and Controls
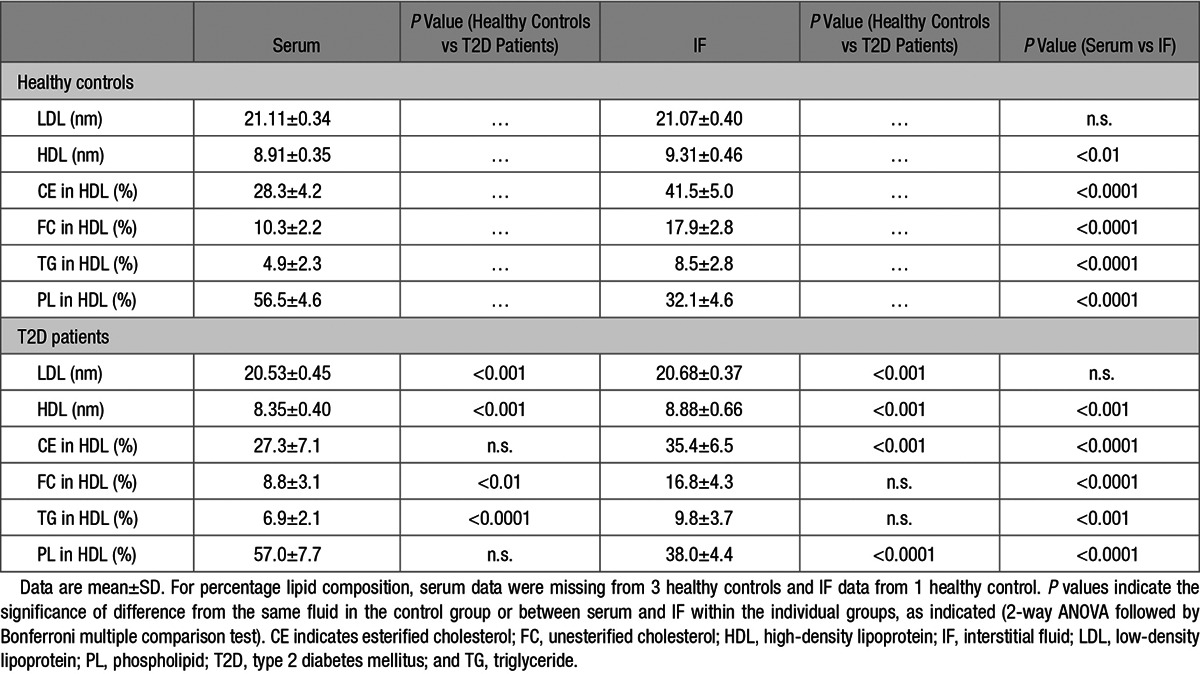
The LDL particle size in T2D patients was smaller than in controls, by 2.7% and 1.9% in serum and IF, respectively. LDL size did not differ between serum and IF, neither in T2D patients nor in controls (Table).
Lipid Composition of HDL in Serum and IF From T2D Patients and Healthy Controls
Phospholipid content of HDL from serum and IF was analyzed by FPLC (the FPLC profiles are shown in Figure IIC and IID in the online-only Data Supplement). T2D patients were found to have reduced levels of phospholipids in HDL from both serum and IF when compared with controls (Table II in the online-only Data Supplement). As described previously,3 triglyceride and unesterified cholesterol contents of HDL, together with esterified cholesterol content of HDL (calculated from total cholesterol and unesterified cholesterol) were determined and are shown in Table II in the online-only Data Supplement; the FPLC profiles for unesterified cholesterol and triglycerides are given in Figures IC and ID and IIA and IIB. The percentage composition of HDL lipids in relation to total (Table) was calculated from the lipid data in Table II in the online-only Data Supplement. From these calculations, it was found that serum HDL from T2D patients had decreased content of unesterified cholesterol and increased triglyceride content compared with healthy controls, whereas for esterified cholesterol and phospholipids, there were no differences (Table). HDL from IF from T2D patients had a reduced content of esterified cholesterol and an increased content of phospholipids. For unesterified cholesterol and triglycerides, there were no differences (Table). When comparing lipid composition in HDL from serum and IF, respectively, it was found that HDL from IF contained more esterified cholesterol, unesterified cholesterol, and triglycerides, but substantially less phospholipids than did HDL from serum, in both T2D patients and healthy controls (Table).
HDL-Mediated Cholesterol Efflux Capacity in Plasma and IF in T2D Patients and Healthy Controls
Cholesterol efflux to HDL, normalized for HDL cholesterol, was assayed using a standardized in vitro system based on THP-1–derived macrophage foam cells loaded with 3H-cholesterol.18 As expected, plasma HDL from T2D patients had a 10% lower efflux capacity when compared with healthy controls (Figure). The efflux capacity of IF-HDL was reduced by 28% in samples from T2D patients compared with those from controls (Figure). Comparing the efflux capacities of HDL from IF with those from plasma in controls and in T2D, respectively, it was found that efflux to HDL-IF was 15% lower in healthy controls whereas the reduction in T2D was even more pronounced (32%; Figure).
Figure.
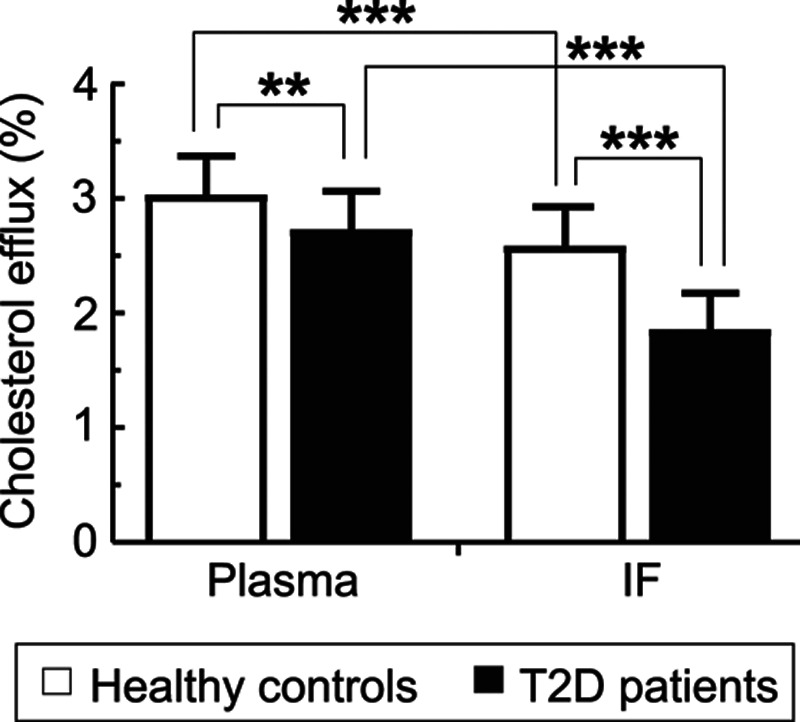
Cholesterol efflux capacity of high-density lipoprotein from plasma and interstitial fluid (IF) from type 2 diabetes mellitus (T2D) patients and healthy controls. Data are presented as mean±SD. Plasma was available from all subjects, whereas IF was available from 21 healthy controls and 24 T2D patients. **P<0.01, ***P<0.001 (2-way ANOVA followed by Bonferroni multiple comparison test).
Discussion
To the best of our knowledge, cholesterol efflux measurements using HDL isolated from IF have not been reported previously. In our study, we could demonstrate that cholesterol efflux to HDL of interstitial origin was clearly of lower magnitude than that to plasma HDL, in both healthy controls and T2D patients. Because the process of cholesterol loading of HDL particles is thought to mainly take place in peripheral tissues,16,17 the evaluation of cholesterol efflux capacity of HDL from IF may, therefore, be of higher physiological relevance than measurements of the efflux capacity of HDL from plasma.
We could confirm the results of previous reports by showing a 10% reduction in plasma HDL cholesterol efflux in T2D patients in comparison with healthy subjects.11–14 However, not all studies have been able to detect differences between T2D patients and healthy controls,19,20 and 1 study has reported an increased efflux capacity in T2D patients.21 These incongruent results might be related to not only the varying degree of severity of disease of the patients studied but also differences in the cell systems used in the efflux assays. It is important to note that the reduced capacity for cholesterol efflux that we observed for HDL from plasma of T2D patients was much more pronounced for HDL from IF.
A potential limitation of our study is the fact that some T2D patients were on statin and fibrate treatments, alone or in combination, a situation that might have improved cholesterol efflux.18,22,23 However, also in this case, the published literature is not consistent because unaltered21,23,24 or even decreased24 efflux capacity has been observed. We did not see any significant difference comparing T2D patients with or without lipid-lowering therapy in this respect (Table III in the online-only Data Supplement). The fact that many patients had documented cardiovascular disease should also be noted.
Small HDL particles have been reported to be more efficient to promote cholesterol efflux from cells.25,26 Our finding of reduced HDL particle size in IF and serum from T2D patients may at first appear somewhat puzzling although the observation of a reduced size of serum HDL in T2D is in agreement with previous work.27 It should be pointed out that the analysis of different subclasses of HDL is not possible using our technique. Increased glycation of HDL in T2D may contribute to the reduced cholesterol efflux28 although such a phenomenon has also not been found universally.21 From our calculated data on percentage composition of the different lipids in HDL, we show that the percentage of esterified cholesterol in serum HDL, the main component of the core of HDL, did not differ between healthy controls and T2D patients. For HDL from IF, the percentage of esterified cholesterol was reduced in T2D patients, which could contribute to the reduced HDL size.
Previous work has concluded that HDL from peripheral lymph carries more cholesterol than can be explained by transendothelial transfer of HDL from plasma,16 and that the infusion of apoAI/phosphatidylcholine discs increases the cholesterol content of HDL in peripheral lymph.17 Those results support the hypothesis that HDL acquires cholesterol within peripheral tissues. From this reasoning, we expected that the cholesterol efflux capacity should be higher for HDL isolated from IF than from plasma. Instead, we found the opposite. The reduced cholesterol efflux capacity of HDL from IF compared with those from plasma was accompanied by increased HDL size, in both patients and controls. This relationship fits well with the previously reported association between small HDL and increased efflux capacity.25,26
The phospholipid content of HDL is shown to be one of the major drivers of cholesterol efflux.29 The reduced cholesterol efflux to IF-HDL may, therefore, at least in part, be explained by the substantially reduced phospholipid content in those particles compared with serum HDL. These results further support the importance of HDL-phospholipid for cholesterol efflux capacity.
Interestingly, it has been shown in vitro that phospholipid hydrolysis by endothelial lipase contributes to transendothelial transport of HDL.30 This may very well explain the reduced level of HDL-phospholipid in IF compared with serum. This transendothelial movement of HDL also reduces HDL size.30 In this study, we found increased HDL size in IF, which may be explained by increased percentage of esterified cholesterol. Although not completely comparable, our results showing larger HDL size in suction blister fluid are also in agreement with the reported increased abundance of large HDL particles in peripheral prenodal lymph.16 As HDL particles are proposed to acquire cholesterol in IF before returning to plasma via the lymph, it is reasonable that HDL size is increased in lymph. HDL isolated from suction blister fluid may already have acquired saturating levels of cholesterol from peripheral cells, resulting in larger size and decreased detected efflux capacity.
Analyzing samples from the same patients and controls, we recently showed that T2D patients have unexpectedly reduced levels of apoB-containing lipoproteins in IF, possibly mirroring an increased uptake by, or adhesion to, interstitial constituents. In contrast, the IF:serum ratio for HDL was not influenced by T2D.3 The present demonstration of a significantly lower cholesterol efflux to HDL from IF suggests that, in addition to an increased peripheral disposal of apoB-containing lipoproteins, the capacity to remove deposited cholesterol is reduced in T2D. Altogether, our results indicate that the interstitial compartment, which should reflect the metabolic conditions in the arterial wall, may be an important location for several disturbances of cholesterol metabolism that might explain the increased propensity for atherosclerosis in T2D.
In conclusion, the capacity to promote cholesterol efflux is considerably lower for HDL from IF than HDL from plasma, both in healthy controls and T2D patients. Moreover, in IF from T2D patients, the efflux capacity of HDL is strongly impaired when compared with that in healthy controls. Interventions directed at promoting HDL-mediated cholesterol efflux in the IF compartment may be a successful new way to target the increased risk of atherosclerosis and cardiovascular disease in T2D.
Acknowledgments
We thank Catharina Sjöberg, research nurse, for assisting in sample collection and Lilian Larsson, laboratory technician, for assistance with fast performance liquid chromatography analyses.
Sources of Funding
This study was supported by the Swedish Research Council (K2013-55X-15075-10-3 and K2014-55X-07137-30-5), the Stockholm City Council (ALF; 20130327, 20120278), the Swedish Heart-Lung Foundation (20130518 and 20120539), the NovoNordisk Foundation, the Knut and Alice Wallenberg Foundation, the Cardiovascular Program at the Karolinska Institute/Stockholm City Council, and the Netherlands Organization for Scientific Research (VIDI Grant 917-56-358 to U.J.F. Tietge).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- FPLC
- fast performance liquid chromatography
- IF
- interstitial fluid
- T2D
- type 2 diabetes mellitus
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.116.307385/-/DC1.
Significance
This study shows that cholesterol efflux to high-density lipoprotein from interstitial fluid is strongly suppressed in type 2 diabetes mellitus patients. This study analyzes cholesterol efflux in interstitial fluid, an important measurement because the first step in reverse cholesterol transport, namely loading of high-density lipoprotein with cholesterol, occurs in the interstitial compartment. The results contribute important knowledge to understand why type 2 diabetes mellitus patients are at increased risk of developing atherosclerosis.
References
- 1.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins R, Armitage J, Parish S, Sleigh P, Peto R Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 3.Apro J, Parini P, Broijersén A, Angelin B, Rudling M. Levels of atherogenic lipoproteins are unexpectedly reduced in interstitial fluid from type 2 diabetes patients. J Lipid Res. 2015;56:1633–1639. doi: 10.1194/jlr.P058842. doi: 10.1194/jlr.P058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W Atherosclerosis Risk in Communities Study Group. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 5.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 6.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GG, Olsson AG, Abt M, et al. dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 8.Triolo M, Annema W, Dullaart RP, Tietge UJ. Assessing the functional properties of high-density lipoproteins: an emerging concept in cardiovascular research. Biomark Med. 2013;7:457–472. doi: 10.2217/bmm.13.35. doi: 10.2217/bmm.13.35. [DOI] [PubMed] [Google Scholar]
- 9.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syvänne M, Castro G, Dengremont C, De Geitere C, Jauhiainen M, Ehnholm C, Michelagnoli S, Franceschini G, Kahri J, Taskinen MR. Cholesterol efflux from Fu5AH hepatoma cells induced by plasma of subjects with or without coronary artery disease and non-insulin-dependent diabetes: importance of LpA-I:A-II particles and phospholipid transfer protein. Atherosclerosis. 1996;127:245–253. doi: 10.1016/s0021-9150(96)05962-x. [DOI] [PubMed] [Google Scholar]
- 12.Autran D, Attia N, Dedecjus M, Durlach V, Girard-Globa A. Postprandial reverse cholesterol transport in type 2 diabetic patients: effect of a lipid lowering treatment. Atherosclerosis. 2000;153:453–460. doi: 10.1016/s0021-9150(00)00428-7. [DOI] [PubMed] [Google Scholar]
- 13.Attia N, Nakbi A, Smaoui M, Chaaba R, Moulin P, Hammami S, Hamda KB, Chanussot F, Hammami M. Increased phospholipid transfer protein activity associated with the impaired cellular cholesterol efflux in type 2 diabetic subjects with coronary artery disease. Tohoku J Exp Med. 2007;213:129–137. doi: 10.1620/tjem.213.129. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Tan KC, Shiu SW, Wong Y. Determinants of leukocyte adenosine triphosphate-binding cassette transporter G1 gene expression in type 2 diabetes mellitus. Metabolism. 2008;57:1135–1140. doi: 10.1016/j.metabol.2008.03.020. doi: 10.1016/j.metabol.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Miller NE, Olszewski WL, Hattori H, Miller IP, Kujiraoka T, Oka T, Iwasaki T, Nanjee MN. Lipoprotein remodeling generates lipid-poor apolipoprotein A-I particles in human interstitial fluid. Am J Physiol Endocrinol Metab. 2013;304:E321–E328. doi: 10.1152/ajpendo.00324.2012. doi: 10.1152/ajpendo.00324.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanjee MN, Cooke CJ, Wong JS, Hamilton RL, Olszewski WL, Miller NE. Composition and ultrastructure of size subclasses of normal human peripheral lymph lipoproteins: quantification of cholesterol uptake by HDL in tissue fluids. J Lipid Res. 2001;42:639–648. [PubMed] [Google Scholar]
- 17.Nanjee MN, Cooke CJ, Garvin R, Semeria F, Lewis G, Olszewski WL, Miller NE. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J Lipid Res. 2001;42:1586–1593. [PubMed] [Google Scholar]
- 18.Triolo M, Annema W, de Boer JF, Tietge UJ, Dullaart RP. Simvastatin and bezafibrate increase cholesterol efflux in men with type 2 diabetes. Eur J Clin Invest. 2014;44:240–248. doi: 10.1111/eci.12226. doi: 10.1111/eci.12226. [DOI] [PubMed] [Google Scholar]
- 19.Dullaart RP, Annema W, de Boer JF, Tietge UJ. Pancreatic β-cell function relates positively to HDL functionality in well-controlled type 2 diabetes mellitus. Atherosclerosis. 2012;222:567–573. doi: 10.1016/j.atherosclerosis.2012.03.037. doi: 10.1016/j.atherosclerosis.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Dullaart RP, van Tol A. Twenty four hour insulin infusion impairs the ability of plasma from healthy subjects and Type 2 diabetic patients to promote cellular cholesterol efflux. Atherosclerosis. 2001;157:49–56. doi: 10.1016/s0021-9150(00)00691-2. [DOI] [PubMed] [Google Scholar]
- 21.Low H, Hoang A, Forbes J, Thomas M, Lyons JG, Nestel P, Bach LA, Sviridov D. Advanced glycation end-products (AGEs) and functionality of reverse cholesterol transport in patients with type 2 diabetes and in mouse models. Diabetologia. 2012;55:2513–2521. doi: 10.1007/s00125-012-2570-9. doi: 10.1007/s00125-012-2570-9. [DOI] [PubMed] [Google Scholar]
- 22.Guerin M, Egger P, Soudant C, Le Goff W, van Tol A, Dupuis R, Chapman MJ. Dose-dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apoB-containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis. 2002;163:287–296. doi: 10.1016/s0021-9150(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 23.Franceschini G, Calabresi L, Colombo C, Favari E, Bernini F, Sirtori CR. Effects of fenofibrate and simvastatin on HDL-related biomarkers in low-HDL patients. Atherosclerosis. 2007;195:385–391. doi: 10.1016/j.atherosclerosis.2006.10.017. doi: 10.1016/j.atherosclerosis.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Sviridov D, Hoang A, Ooi E, Watts G, Barrett PH, Nestel P. Indices of reverse cholesterol transport in subjects with metabolic syndrome after treatment with rosuvastatin. Atherosclerosis. 2008;197:732–739. doi: 10.1016/j.atherosclerosis.2007.07.007. doi: 10.1016/j.atherosclerosis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Camont L, Lhomme M, Rached F, Le Goff W, Nègre-Salvayre A, Salvayre R, Calzada C, Lagarde M, Chapman MJ, Kontush A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33:2715–2723. doi: 10.1161/ATVBAHA.113.301468. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 26.Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 27.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 28.Duell PB, Oram JF, Bierman EL. Nonenzymatic glycosylation of HDL and impaired HDL-receptor-mediated cholesterol efflux. Diabetes. 1991;40:377–384. doi: 10.2337/diab.40.3.377. [DOI] [PubMed] [Google Scholar]
- 29.Fournier N, Paul JL, Atger V, Cogny A, Soni T, de la Llera-Moya M, Rothblat G, Moatti N. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler Thromb Vasc Biol. 1997;17:2685–2691. doi: 10.1161/01.atv.17.11.2685. [DOI] [PubMed] [Google Scholar]
- 30.Robert J, Lehner M, Frank S, Perisa D, von Eckardstein A, Rohrer L. Interleukin 6 stimulates endothelial binding and transport of high-density lipoprotein through induction of endothelial lipase. Arterioscler Thromb Vasc Biol. 2013;33:2699–2706. doi: 10.1161/ATVBAHA.113.301363. doi: 10.1161/ATVBAHA.113.301363. [DOI] [PubMed] [Google Scholar]


