ABSTRACT
OBJECTIVES:
To discuss how patient considerations and the initial wound environment can affect wound treatment and summarize the way in which the initial US Wound Registry measures capture aspects of the DIME (Debridement/devitalized tissue, Infection or inflammation, Moisture balance, and wound Edge preparation/wound depth) principles.
DISCUSSION:
The treatment of chronic wounds often involves extended hospital stays and long-term outpatient follow-up visits with costly advanced therapeutic interventions. As complex care is required for chronic wounds, treatment guidelines such as DIME have evolved to include consideration of patient-centered concerns and etiology, as well as features of wound bed preparation. The US healthcare system is in the midst of transitioning to a quality-based system. However, as wound care is not yet a recognized specialty, it is poorly represented in the current approved quality-based measures.
CONCLUSION:
This article helps to identify the practice guidelines that are not currently represented by quality metrics.
KEYWORDS: wound care, chronic wounds, comorbidities, quality measures, DIME
INTRODUCTION
Patients with chronic wounds often bear significant financial burdens associated with their treatment, in addition to loss of productivity, anxiety, and decreased quality of life.1–3 The treatment of chronic wounds often involves extended hospital stays and long-term outpatient follow-up visits with costly advanced therapeutic interventions.3,4 The current US payment system rewards healthcare providers according to the number of interventions performed without a feedback mechanism for quality of care, outcome, or patient-centered concerns.5 In the hope of controlling costs while improving the quality of care, efforts are underway to transition the US healthcare payment system from the current “volume-driven” model to one that is determined by value and that links cost to improved patient outcomes and satisfaction. This is being accomplished by linking reimbursement to the reporting of “quality measures,” usually designed to assess the clinician’s adherence to specialty-specific clinical practice guidelines.
Wound care guidelines have taken on new importance now that they serve as the principal ingredients for quality measures and, through quality measures, clinician reimbursement. Many types of guidelines exist, particularly in wound care where technological advances have allowed a variety of wound care strategies to be developed. Although many different types of guidelines exist, the elements of wound bed preparation, controlling infection/inflammation, and maintaining moisture balance are common to all guidelines.1,6–8 Unfortunately, few clinical practice guidelines address patient-centered concerns, despite the fact that outcomes are improved when patient-centered issues are addressed prior to initiating wound care treatments.9 One wound care guideline, DIME (Debridement/devitalized tissue, Infection or inflammation, Moisture balance, wound Edge preparation and wound depth), has evolved to include a holistic patient-centered approach to wound care. The DIME approach first assesses and addresses patient concerns and the underlying comorbidities before treatment of the wound begins (Figure 1, Table 1).9–11
Figure 1.
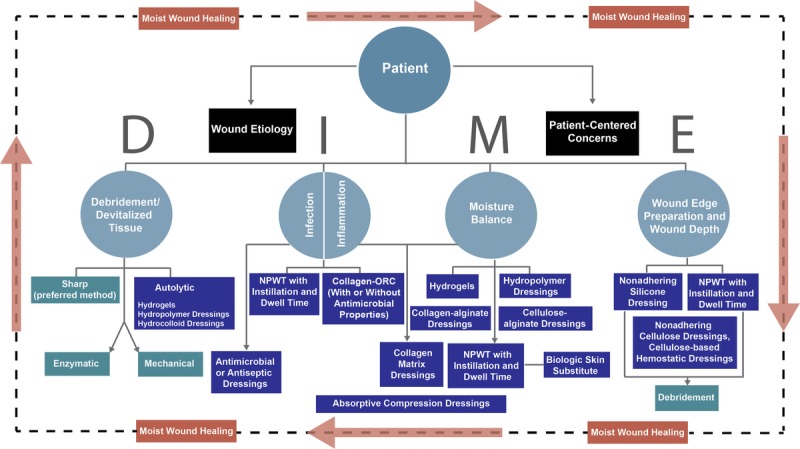
SCHEMATIC OF DIME WOUND TREATMENT STRATEGY
Table 1.
INTRODUCTION TO DIME

Abbreviations: NPWT, negative-pressure wound therapy; ORC, oxidized regenerated cellulose.
In 2006, the US Centers for Medicare & Medicaid Services (CMS) established the Physician Quality Reporting Initiative, now called the Physician Quality Reporting System (PQRS), which initially provided incentive payments to qualified healthcare professionals reporting quality measure data on Medicare patients. Now moving into the penalty phase, US healthcare providers who fail to successfully report PQRS measures in 2016 will lose 4% of their Medicare revenue in 2018. The CMS also has many quality programs targeting hospitals.12 The Affordable Care Act includes a new value-based payment modifier that will be used to provide differential payments to healthcare providers, inpatient facilities, outpatient departments, and accountable care organizations based on quality and cost of care.13 When these changes are combined, substantial reductions in reimbursement are on the horizon for both wound care clinicians and hospital-based outpatient wound centers unless a suite of quality measures is developed that can convey the evidence-based practice of wound care.
Most quality measures were created by specialty societies; however, because wound care is not yet a specialty recognized by the American Board of Medical Specialties, wound care quality measure development has been poorly focused. In 2007, the American Medical Association convened a Physician Consortium for Performance Improvement initiative in collaboration with the American Society of Plastic Surgeons to develop wound care quality measures,14 2 of which were incorporated into PQRS by CMS. These 2 “overuse” measures were (1) performing a swab culture of any wound and (2) not using a saline wet-to-dry dressing.15 Despite being the only 2 wound care measures in PQRS, these measures were limited in their ability to reflect wound care principles like DIME and have since been retired from PQRS. For example, the swab culture measure was intended to encourage better methods of wound culture (such as biopsy for quantitative culture), thereby limiting the overuse of antibiotics in chronic wounds; however, as written, practitioners passed the measure by never performing a swab culture on any wound. Although the wound dressing quality measure was intended to promote the selection of dressings that improve wound moisture balance, the measure was not specifically written to accomplish moisture balance and thus did a poor job of measuring what it is designed to assess. Both of these PQRS measures were retired after the 2014 reporting period, leaving no wound care–specific quality measures within the PQRS system.
In 2009 and 2011, the US Wound Registry (USWR) submitted several evidence-based wound-care quality measures to CMS during annual open “calls for measures.” None were accepted, presumably because the suggested measures had not been endorsed by the National Quality Forum, a lengthy and expensive process to which the American Medical Association Physician Consortium for Performance Improvement measures had also not been subjected. The USWR is a nonprofit organization that has been a CMS-approved quality registry since 2008 when PQRS was initiated. In 2014, the USWR was accepted by CMS as a Qualified Clinical Data Registry under a new program that allows experienced registries to develop their own quality measures. The CMS has accepted 21 new quality measures specific to wound care developed by the USWR in conjunction with the member organizations of the Alliance of Wound Care Stakeholders, which healthcare providers can report to satisfy their PQRS requirements.16 With the retirement of the only wound care–specific quality measures from traditional PQRS, the qualified clinical data registry process is currently the only mechanism by which wound care clinicians can report relevant quality measures as part of PQRS participation.
In this article, the authors discuss how patient considerations and the initial wound environment can affect wound treatment and summarize the way in which the initial USWR measures capture aspects of the DIME principles. The authors also highlight those practice guidelines that are not currently represented by any quality metrics, in order to provide suggested areas for future measure development.
SEARCH STRATEGY AND SELECTION CRITERIA
Data for this review were identified by search of MEDLINE and PubMed. References were chosen from relevant articles using the search terms “DIME,” “wound care,” “chronic wounds,” “wound healing”, and “quality measures.” Only articles published in English between 1990 and 2014 were included.
PATIENT HEALTH HISTORY AND CONDITION OF THE WOUND CAN AFFECT IMPLEMENTATION AND SUCCESS OF TREATMENT
Patient considerations and etiology are major factors in wound treatment and wound healing. Patient demographics, comorbidities, psychosocial stress and anxiety, and medication use often alter the wound healing process.1–3,17–21 Comorbidities, such as patient age, diabetes, arterial disease, obesity, smoking, and malnutrition, can alter the progression of wound healing and the treatment tolerated by the patient.3,17–20 In addition, medication use (such as steroids) can affect and/or alter healing processes in patients.17,21 Studies have indicated that patients with chronic nonhealing wounds additionally suffer from stress and anxiety related to the wound and wound treatment.1,2 Little emphasis has been placed on the importance of evaluating wound-related quality of life or the way in which various treatments may affect wound quality of life with or without associated healing. Stalled, complex, or chronic wounds are long-lasting wounds that are unable to complete the healing process because of a number of underlying comorbidities or impairments, including the presence of dead tissue, ongoing inflammation, infection, and deficiencies in epithelialization.22,23
Stalled/chronic wounds have unbalanced wound biochemistries,24,25 including increased proteases and inflammatory mediators, decreased protease inhibitors,23,25,26 high bacterial loads,27–29 and hyperproliferative wound edges.30 This imbalance favors the stalling of wound healing in the inflammatory stage. Devitalized tissue also impedes the wound healing process and should be assessed (Figure 2). This tissue, with extremely reduced blood and oxygen flow, needs to be removed from the wound in order to “jumpstart” the wound healing process.31 For effective wound healing to take place, infection and inflammation need to be mitigated (Figure 3).32 To that end, Robson et al27 discussed different factors involved in altered wound healing following infection. Here, they reported that the presence of chronic granulation tissue severely decreased the amount of antibiotics that reached the wound infection, thus prolonging the healing process.27 In addition, Gardner et al29 evaluated the reliability of clinical tools to assess the signs and symptoms of localized infections in chronic wounds. They found the “Clinical Signs and Symptoms Checklist,” which focuses on primary and secondary signs of infection, to be reliable and complemented clinician wound infection assessments.29
Figure 2.
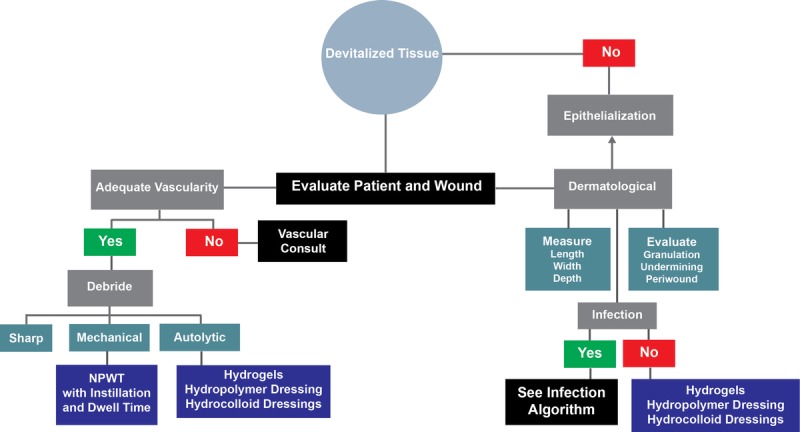
DEVITALIZED TISSUE TREATMENT PATHWAYS
Figure 3.
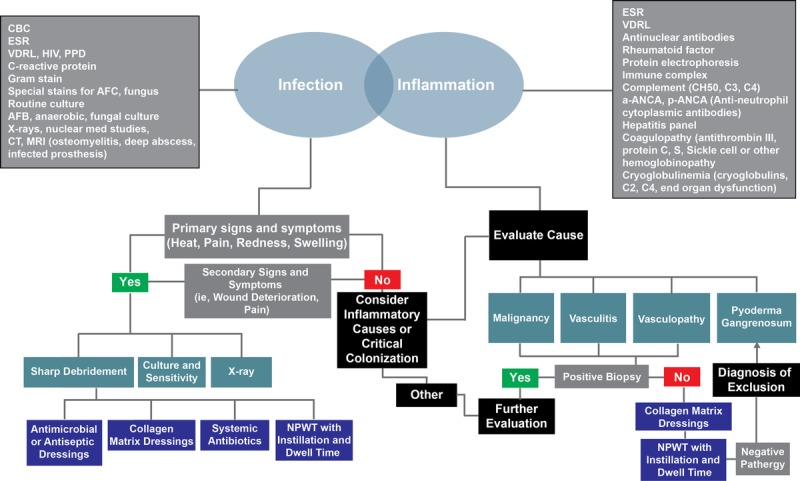
WOUND INFECTION AND INFLAMMATION TREATMENT PATHWAYS
DIME AND WOUND CARE QUALITY MEASURES
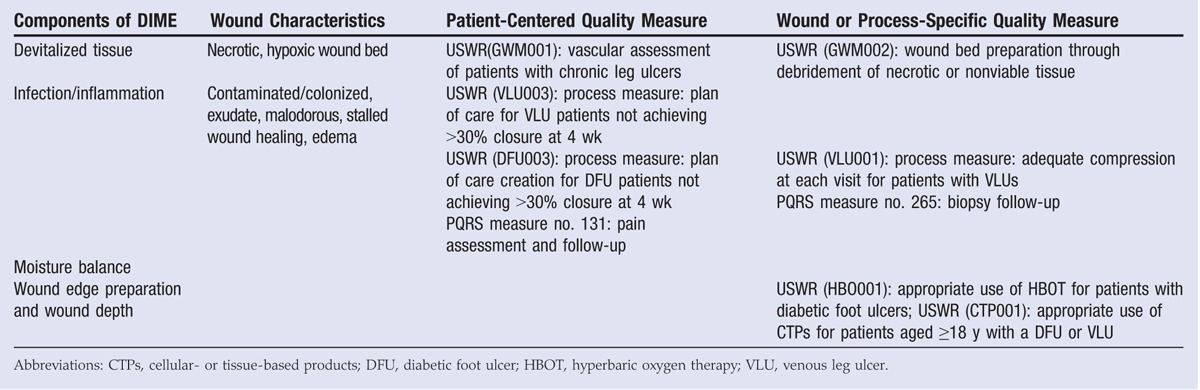
The DIME strategy provides overarching principles for the assessment and treatment of a wound. For instance, wound etiology and biochemistry affect the ability of a wound to heal; as such, the DIME strategy calls for extensive wound bed preparation to accelerate endogenous healing and remove factors that prevent wound repair.33,34 Active treatments utilize external mechanisms of action to remove necrotic tissue and/or exudate from the wound, including sharp/surgical, enzymatic, or mechanical debridement; negative-pressure wound therapy (NPWT); and NPWT with instillation and a dwell time (Table 2). Passive treatments allow the patient’s endogenous healing mechanisms to remove barriers to healing and include autolytic debridement, hydrogels (hydropolymer dressings), hydrocolloid dressings, antimicrobial or antiseptic dressings, collagen-oxidized regenerated cellulose dressings, collagen-alginate dressings, cellulose-alginate dressings, and nonadherent silicone dressings (Table 2). An additional wound care treatment includes cellular- and tissue-based products (allografts, xenographs, dermal matrices, and composite skin) that aid in wound closure and can function as replacement skin (Table 2).35
Table 2.
ACTIVE, PASSIVE, AND BIOLOGIC SKIN SUBSTITUTE WOUND TREATMENT
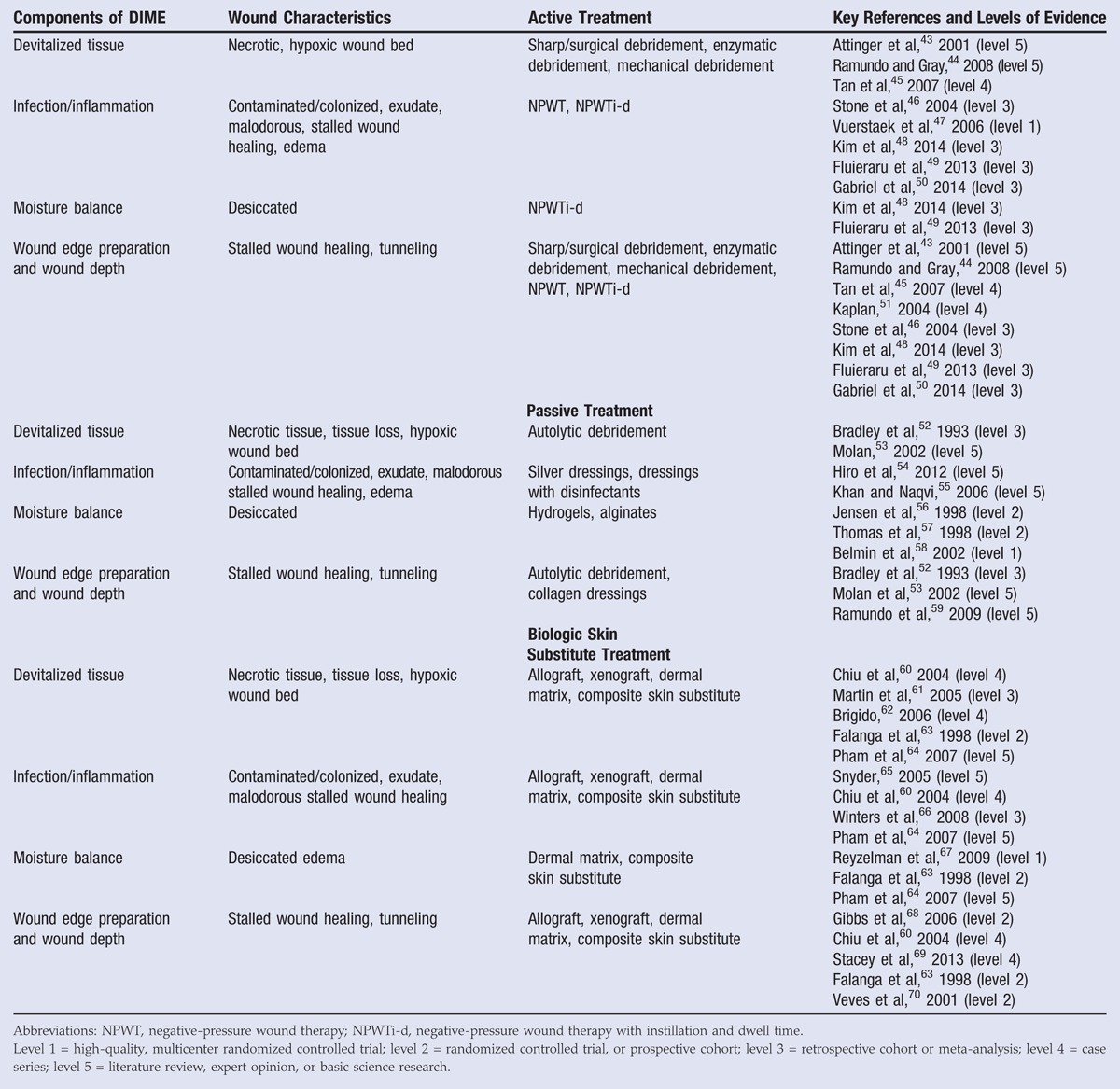
Putting these DIME concepts into clinical practice and then measuring whether care was actually accomplished are the fundamental challenges of the entire quality measure movement. For example, debridement of necrotic or nonviable tissue, a major tenet of the DIME principle, has been captured in “Process Measure: Wound Bed Preparation through Debridement of Necrotic or Nonviable Tissue.” This quality measure assesses the percentage of patients with nonviable tissue who underwent any form of debridement. Although the DIME principle mentions the issue of tissue hypoxia, it does not discuss the framework within which ischemia is assessed prior to debridement or as part of the evaluation for active or passive wound treatments. The “Process Measure: Vascular Assessment of Patients with Chronic Leg Ulcers” accounts for the percentage of patients with lower-extremity leg ulcers undergoing any type of vascular assessment (eg, handheld Doppler, ankle-brachial index, transcutaneous oximetry, skin perfusion pressure, and so on). In addressing the issue of stalled wounds, 2 process measures require the creation of a plan of care for diabetic foot ulcers and venous ulcers that have failed to achieve 30% reduction in surface area within 4 weeks of care. Such a plan of care could include NPWT or the use of cellular and/or tissue-derived products. Another process measure focusing on the appropriate use of cellular and/or tissue-derived products measures the percentage of patients undergoing this treatment who have first undergone vascular assessment and measurement of hemoglobin A1c (Figure 4, Table 3).
Figure 4.
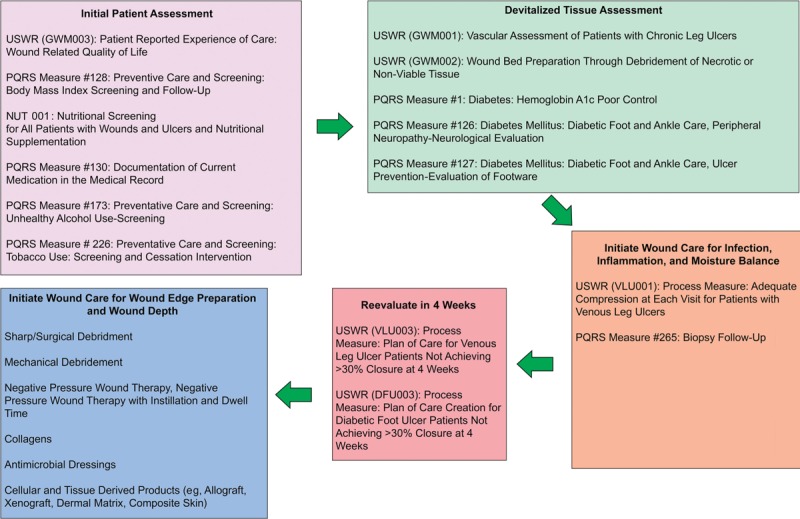
FLOWCHART OF QUALITY MEASURES UTILIZED IN THE DIME WOUND TREATMENT STRATEGY
Table 3.
REPRESENTATIVE QUALITY MEASURES RELATED TO DIME
Infection can become a significant hurdle in chronic wound healing. Quality measures should include both the resolution of infection through approved treatment methods (eg, topical antimicrobial treatment and systemic antibiotics), as well as process measures for identifying infection through tissue biopsy or validated quantitative swab technique. It is important to identify and properly treat wound infection; however, the use of wound surface culture technique was a PQRS overuse measure that failed when a swab culture was performed. Although this measure was intended to promote the use of better culture techniques (eg, Levine swab or biopsy for quantitative culture), its design likely prevented it from being used as a way to improve quality wound care (Figure 4, Table 3).
Maintaining wound moisture balance allows for progression of wound healing, which can be achieved through various pathways (Figure 5). Quality measures detailing the types of dressings that provide continued moisture, manage wound exudate, and protect periwound skin are required. Currently, no quality measures exist that focus on wound moisture. As such, additional measures for wound moisture assessment may be warranted, especially in the case of stalled wound healing following intervention (Figure 4, Table 3).
Figure 5.
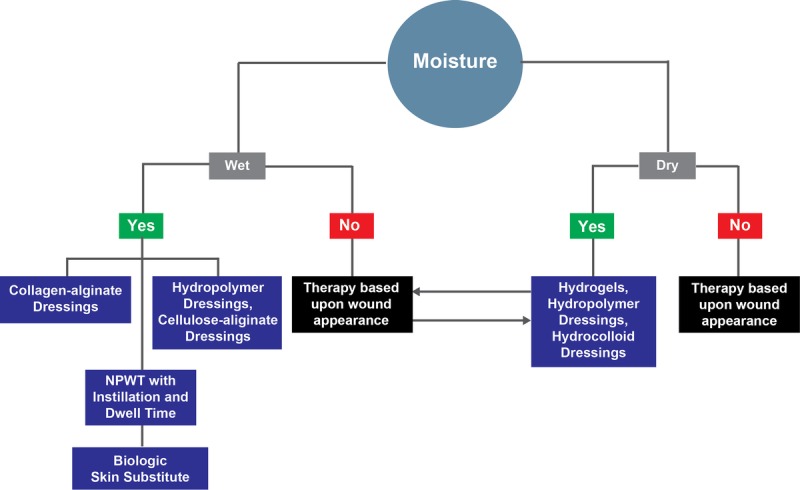
WOUND MOISTURE TREATMENT PATHWAYS
It is important to note that some aspects of DIME are difficult to encapsulate within the quality measure framework. For example, those components that focus on wound tunneling and undermining or rolled wound edges provide awareness of certain aspects of the wound that require intervention; however, this aspect of DIME is difficult to protocolize because several possible actions might be acceptable (Figure 6). An additional challenge is that wound treatment should be sequential as wound environment and healing processes change throughout the treatment plan.36 As such, identifying wound healing milestones that indicate a need to switch to a different treatment plan is necessary to provide the best quality of care.36–39
Figure 6.
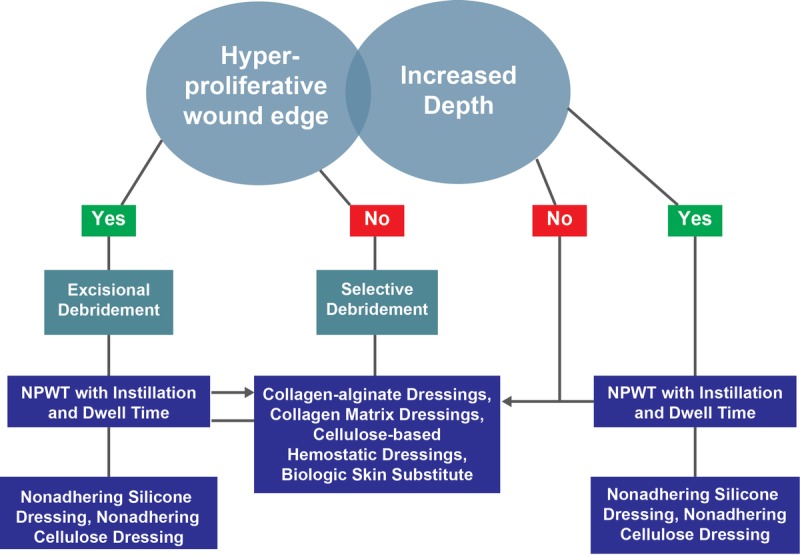
WOUND EDGE AND DEPTH TREATMENT PATHWAYS
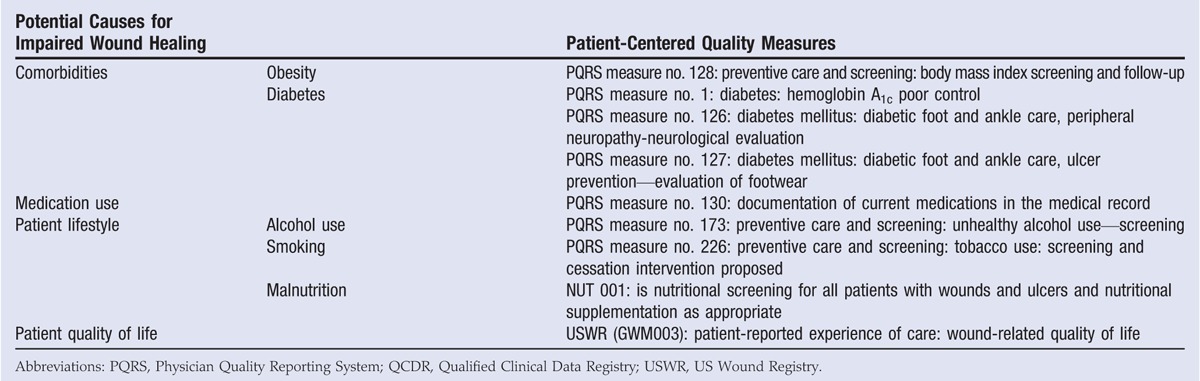
The DIME approach to wound healing has a patient-centered component; however, patient-centered quality measures are few and far between. Previous studies have shown that patient comorbidities (eg, obesity, diabetes), medication use, and lifestyle factors (eg, smoking, malnutrition) can alter wound healing.3,17–21 At point of care, healthcare providers should evaluate patient comorbidities and lifestyle factors and recommend treatment to improve any potential deficiencies (such as high blood glucose, hypertension, or malnutrition) before focusing on wound treatment. Currently, only a handful of quality measures regarding patient-centered components exist (Table 4). More patient-related wound outcomes should be developed. Similarly, patient-reported experience of care can be assessed by measuring the wound-related quality of life at the initial consultation and the final visit, which may provide valuable data to assess the effect of wound treatment on quality of life whether healing is achieved. These quality-of-life measures should be utilized to gauge the effect of wound treatment on the patient. As clinical practice guidelines continue to be refined, the focus should be toward the challenge of putting them into use within patient care.
Table 4.
QUALITY MEASURES FOR ASSESSING PATIENT FACTORS AFFECTING WOUND HEALING
DISCUSSION
Over the years, patient-centered wound treatment has become a prominent portion of wound care. This holistic approach builds a comprehensive treatment plan that takes the patient’s concerns, support system, and environment into consideration during the development and implementation of the treatment plan, which may lead to improved patient compliance and wound healing.2
Advances in wound care have created a variety of wound care strategies; even though quality measures have become an important tool in evaluating patient care and treatment outcomes, quality measures for wound care are limited. Currently, “plan of care” quality measures are used in the treatment of stalled, complex, or chronic wounds. However, quality measures are lacking that endorse sequential treatment and continued wound assessment in wounds stalled following intervention. The wound care community needs to collaborate on developing specific quality measures to address this area of need.
Although the CMS prefers to measure outcome of care, it is possible to measure the appropriateness of clinical interventions through “process measures” as long as these processes can be shown to contribute to the desired outcome. Thus, in the field of wound care, improved patient care may need to begin with the reporting of process measures.
Wound care provided through quality improvement programs that offer feedback to clinicians on performance across various quality measures allows practices to compare themselves with others across the country through benchmarking. Maximizing adherence to quality-of-care guidelines should be a high priority for the optimal management of wounds.
CONCLUSIONS
The US healthcare system is transitioning to a quality-based system. Wound care, still an emerging specialty, is poorly represented in the current approved quality-based measures. It is clear that the lack of suitable wound care quality measures threatens the survival of wound care provider practitioners and services services and therefore, must be urgently addressed. Quality measures are based upon current evidence, validated guidelines, and best practices; however, patient-centered concerns must be considered when advising diagnostic and treatment protocols. In addition, wound healing should be regularly reevaluated to ensure current treatment plans are promoting healing.
KEY REVIEW POINTS.
Patient demographics, comorbidities, psychosocial stress and anxiety, and medication use alter the wound healing process.
Quality measures are based on current evidence, validated guidelines, and “best practices”; however, patient-centered concerns must be considered when advising diagnostic and treatment protocols.
Quality of life, patient lifestyle, medication use, and comorbidities should be assessed prior to the development of a wound treatment plan.
Wound assessment and treatment should focus on wound debridement, control of infection/inflammation, providing appropriate moisture balance, and wound edge preparation and wound depth.
Wound healing should be regularly reevaluated to ensure current treatment plans are promoting healing.
The US Wound Registry has a number of wound care–related quality measures that are absent in the Physician Quality Reporting System measures.
Footnotes
Acknowledgments: The authors thank Julie M Robertson, PhD (Acelity), for manuscript preparation and editorial assistance. There was no funding source for this manuscript.
REFERENCES
- 1. Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012; 9 Suppl 2: 1- 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sibbald RG, Orsted HL, Coutts PM, Keast DH. Best practice recommendations for preparing the wound bed: update 2006. Adv Skin Wound Care 2007; 20: 390- 405. [DOI] [PubMed] [Google Scholar]
- 3. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009; 17: 763- 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis 2006; 43: 322- 30. [DOI] [PubMed] [Google Scholar]
- 5. Yong PL, Saunders RS, Olsen LA, eds. Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: The National Academies Press, 2010. [PubMed] [Google Scholar]
- 6. Franz MG, Robson MC, Steed DL, et al. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen 2008; 16: 723- 48. [DOI] [PubMed] [Google Scholar]
- 7. Robson MC, Barbul A. Guidelines for the best care of chronic wounds. Wound Repair Regen 2006; 14: 647- 8. [DOI] [PubMed] [Google Scholar]
- 8. Schultz G, Mozingo D, Romanelli M, Claxton K. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen 2005; 13 (4 Suppl): S1- 11. [DOI] [PubMed] [Google Scholar]
- 9. Sibbald RG, Woo KY, Ayello E. Wound bed preparation: DIM before DIME. Wound Healing Southern Africa 2008; 1: 29- 34. [Google Scholar]
- 10. Kirshen C, Woo K, Ayello EA, Sibbald RG. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care 2006; 19: 506- 17. [DOI] [PubMed] [Google Scholar]
- 11. Mat Saad AZ, Khoo TL, Halim AS. Wound bed preparation for chronic diabetic foot ulcers. ISRN Endocrinol 2013; 2013: 608313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panzer RJ, Gitomer RS, Greene WH, Webster PR, Landry KR, Riccobono CA. Increasing demands for quality measurement. JAMA 2013; 310: 1971- 80. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. The Affordable Care Act, 2010. http://www.hhs.gov/healthcare. Last accessed March 17, 2016.
- 14. Bufalino V, Bauman MA, Shubrook JH, et al. Evolution of “The Guideline Advantage”: lessons learned from the front lines of outpatient performance measurement. Diabetes Care 2014; 37: 1745- 50. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. 2013 Physician Quality Reporting System. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html?redirect=/pqri. Last accessed March 17, 2016.
- 16.US Wound Registry. Quality measures in wound care. https://www.uswoundregistry.com/specifications.aspx. Last accessed March 17, 2016.
- 17. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010; 89: 219- 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonder MA, Lazarus GS, Cowan DA, Aronson B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58: 185- 206. [DOI] [PubMed] [Google Scholar]
- 19. Reddy M. Skin and wound care: important considerations in the older adult. Adv Skin Wound Care 2008; 21: 424- 36. [DOI] [PubMed] [Google Scholar]
- 20. Minimas DA. Ageing and its influence on wound healing. Wounds UK 2007; 3: 42,46, 8-50. [Google Scholar]
- 21. Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg 1998; 176 (2A Suppl): 39S- 47S. [DOI] [PubMed] [Google Scholar]
- 22. Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007; 25: 19- 25. [DOI] [PubMed] [Google Scholar]
- 23. Sibbald RG, Snyder RJ, Botros M, et al. Role of a point-of-care protease activity diagnostic test in Canadian clinical practice: a Canadian expert consensus. Adv Skin Wound Care 2012; 25: 267- 75. [DOI] [PubMed] [Google Scholar]
- 24. Snyder RJ, Cullen B, Nisbet LT. An audit to assess the perspectives of US wound care specialists regarding the importance of proteases in wound healing and wound assessment. Int Wound J 2013; 10: 653- 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996; 4: 321- 5. [DOI] [PubMed] [Google Scholar]
- 26. Snyder RJ, Driver V, Fife CE, et al. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage 2011; 57 (12): 36- 46. [PubMed] [Google Scholar]
- 27. Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg 1990; 17: 485- 92. [PubMed] [Google Scholar]
- 28. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997; 77: 637- 50. [DOI] [PubMed] [Google Scholar]
- 29. Gardner SE, Frantz RA, Troia C, et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage 2001; 47: 40- 7. [PubMed] [Google Scholar]
- 30. Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen 2000; 8: 347- 52. [PubMed] [Google Scholar]
- 31. Enoch S, Harding K. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003; 15: 213- 29. [Google Scholar]
- 32. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003; 49: 24- 51. [PubMed] [Google Scholar]
- 33. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003; 11: S1- 28. [DOI] [PubMed] [Google Scholar]
- 34. Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg 2006; 117 (7 Suppl): 72S- 109S. [DOI] [PubMed] [Google Scholar]
- 35. Halim AS, Khoo TL, Mohd Yussof SJ. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg 2010; 43 (Suppl): S23- S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Snyder RJ, Richter D, Hill ME. A retrospective study of sequential therapy with advanced wound care products versus saline gauze dressings: comparing healing and cost. Ostomy Wound Manage 2010; 56 (11A): 9- 15. [Google Scholar]
- 37. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003; 26: 1879- 82. [DOI] [PubMed] [Google Scholar]
- 38. Snyder RJ, Cardinal M, Dauphinee DM, Stavosky J. A post-hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010; 56: 44- 50. [PubMed] [Google Scholar]
- 39. Warriner RA, Snyder RJ, Cardinal MH. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int Wound J 2011; 8: 632- 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962; 193: 293- 4. [DOI] [PubMed] [Google Scholar]
- 41. Sibbald RG, Williamson D, Orsted HL, et al. Preparing the wound bed—debridement, bacterial balance, and moisture balance. Ostomy Wound Manage 2000; 46: 14- 35. [PubMed] [Google Scholar]
- 42. Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of TIME. Int Wound J 2004; 1: 19- 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Attinger CE, Bulan EJ. Debridement. The key initial first step in wound healing. Foot Ankle Clin 2001; 6: 627- 60. [DOI] [PubMed] [Google Scholar]
- 44. Ramundo J, Gray M. Enzymatic wound debridement. J Wound Ostomy Continence Nurs 2008; 35: 273- 80. [DOI] [PubMed] [Google Scholar]
- 45. Tan J, Abisi S, Smith A, Burnand KG. A painless method of ultrasonically assisted debridement of chronic leg ulcers: a pilot study. Eur J Vasc Endovasc Surg 2007; 33: 234- 8. [DOI] [PubMed] [Google Scholar]
- 46. Stone P, Prigozen J, Hofeldt M, Hass S, DeLuca J, Flaherty S. Bolster versus negative pressure wound therapy for securing split-thickness skin grafts in trauma patients. Wounds 2004; 16: 219- 23. [Google Scholar]
- 47. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006; 44: 1029- 38. [DOI] [PubMed] [Google Scholar]
- 48. Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg 2014; 133: 709- 16. [DOI] [PubMed] [Google Scholar]
- 49. Fluieraru S, Bekara F, Naud M, et al. Sterile-water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care 2013; 22: 293- 9. [DOI] [PubMed] [Google Scholar]
- 50. Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost-effectiveness. Eplasty 2014; 14: e41. [PMC free article] [PubMed] [Google Scholar]
- 51. Kaplan M. Negative pressure wound therapy in the management of abdominal compartment syndrome. Ostomy Wound Manage 2004; 50 (11A Suppl): 20S- 25S. [PubMed] [Google Scholar]
- 52. Bradley M, Cullum N, Sheldon T. The debridement of chronic wounds: a systematic review. Health Technol Assess 1993; 3 (17 Pt 1): 1- 78. [PubMed] [Google Scholar]
- 53. Molan PC. Re-introducing honey in the management of wounds and ulcers—theory and practice. Ostomy Wound Manage 2002; 48 (11): 28- 40. [PubMed] [Google Scholar]
- 54. Hiro ME, Pierpont YN, Ko F, Wright TE, Robson MC, Payne WG. Comparative evaluation of silver-containing antimicrobial dressings on in vitro and in vivo processes of wound healing. Eplasty 2012; 12: e48. [PMC free article] [PubMed] [Google Scholar]
- 55. Khan MN, Naqvi AH. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J Tissue Viability 2006; 16 (4): 6- 10. [DOI] [PubMed] [Google Scholar]
- 56. Jensen JL, Seeley J, Gillin B. Diabetic foot ulcerations. A controlled, randomized comparison of two moist wound healing protocols: Carrasyn Hydrogel Wound dressing and wet-to-moist saline gauze. Adv Wound Care 1998; 11 (7 Suppl): 1- 4. [PubMed] [Google Scholar]
- 57. Thomas DR, Goode PS, LaMaster K, Tennyson T. Acemannan hydrogel dressing versus saline dressing for pressure ulcers. A randomized, controlled trial. Adv Wound Care 1998; 11: 273- 6. [PubMed] [Google Scholar]
- 58. Belmin J, Meaume S, Rabus MT, Bohbot S. Sequential treatment with calcium alginate dressings and hydrocolloid dressings accelerates pressure ulcer healing in older subjects: a multicenter randomized trial of sequential versus nonsequential treatment with hydrocolloid dressings alone. J Am Geriatr Soc 2002; 50: 269- 74. [DOI] [PubMed] [Google Scholar]
- 59. Ramundo J, Gray M. Collagenase for enzymatic debridement: a systematic review. J Wound Ostomy Continence Nurs 2009; 36 (6 Suppl): S4- 11. [DOI] [PubMed] [Google Scholar]
- 60. Chiu T, Pang P, Ying SY, Burd A. Porcine skin: friend or foe? Burns 2004; 30: 739- 41. [DOI] [PubMed] [Google Scholar]
- 61. Martin BR, Sangalang M, Wu S, Armstrong DG. Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: an initial experience. Int Wound J 2005; 2: 161- 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J 2006; 3: 181- 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol 1998; 134: 293- 300. [DOI] [PubMed] [Google Scholar]
- 64. Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns 2007; 33: 946- 57. [DOI] [PubMed] [Google Scholar]
- 65. Snyder RJ. Treatment of nonhealing ulcers with allografts. Clin Dermatol 2005; 23: 388- 95. [DOI] [PubMed] [Google Scholar]
- 66. Winters CL, Brigido SA, Liden BA, Simmons M, Hartman JF, Wright ML. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care 2008; 21: 375- 81. [DOI] [PubMed] [Google Scholar]
- 67. Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009; 6: 196- 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gibbs S, van den Hoogenband HM, Kirtschig G, et al. Autologous full-thickness skin substitute for healing chronic wounds. Br J Dermatol 2006; 155: 267- 74. [DOI] [PubMed] [Google Scholar]
- 69. Stacey DH. Use of an acellular regenerative tissue matrix over chronic wounds. Eplasty 2013; 13: e61. [PMC free article] [PubMed] [Google Scholar]
- 70. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001; 24: 290- 5. [DOI] [PubMed] [Google Scholar]


