Supplemental Digital Content is Available in the Text.
Keywords: Algorithm, Pressure ulcer, Pressure injury, Prevention, Support surface, Treatment
Abstract
Support surfaces are an integral component of pressure ulcer prevention and treatment, but there is insufficient evidence to guide clinical decision making in this area. In an effort to provide clinical guidance for selecting support surfaces based on individual patient needs, the Wound, Ostomy and Continence Nurses Society (WOCN®) set out to develop an evidence- and consensus-based algorithm. A Task Force of clinical experts was identified who: 1) reviewed the literature and identified evidence for support surface use in the prevention and treatment of pressure ulcers; 2) developed supporting statements for essential components for the algorithm, 3) developed a draft algorithm for support surface selection; and 4) determined its face validity. A consensus panel of 20 key opinion leaders was then convened that: 1.) reviewed the draft algorithm and supporting statements, 2.) reached consensus on statements lacking robust supporting evidence, 3.) modified the draft algorithm and evaluated its content validity. The Content Validity Index (CVI) for the algorithm was strong (0.95 out of 1.0) with an overall mean score of 3.72 (out of 1 to 4), suggesting that the steps were appropriate to the purpose of the algorithm. To our knowledge, this is the first evidence and consensus based algorithm for support surface selection that has undergone content validation.
Introduction
Support surfaces comprise a variety of overlays, mattresses, and integrated bed systems used to redistribute pressure, reduce shearing forces, and control heat and humidity. The use of support surfaces is included in nearly all evidence-based clinical practice guidelines as a component of comprehensive pressure ulcer prevention programs and treatment recommendations.1–5 Although a number of support surfaces have been shown to reduce the incidence of pressure ulcers or facilitate wound healing when compared to standard mattresses, there is insufficient evidence to guide support surface selection to match individual patient needs in many situations. Findings from clinical studies are often of limited use due to inconsistencies in how support surfaces are classified, limitations in research design, and advances in technology since studies were published. Results of 4 high-quality systematic reviews6–9 reveal insufficient evidence to conclude superiority of one type of support surface over another. Evidence concerning optimal selection of a particular support surface for treatment of pressure ulcers is even more limited. Further details of the Study Group findings are available as Supplemental Digital Content (see Supplemental Digital Content 1, http://links.lww.com/JWOCN/A27 and Supplemental Digital Content 2, http://links.lww.com/JWOCN/A28).
In an effort to provide clinical guidance for selecting a support surface based on individual patient needs, the WOCN elected to develop an evidence- and consensus-based algorithm. Society leaders assembled a Task Force of key opinion leaders to: 1) identify and rank levels of evidence for the use of support surfaces for prevention and treatment of pressure ulcers; 2) develop evidence-based statements needed to support the algorithm; 3) develop consensus statements needed to support decisions and pathways not supported by higher level evidence; and 4) determine the face validity of the first draft of the support surface algorithm. Subsequently, a group of 20 key opinion leaders was convened to 1) review the draft algorithm and supporting statements, 2) reach consensus on statements lacking robust supporting evidence, 3) modify the draft algorithm where indicated, and 4) establish its content validity (Box 1).
Box 1.
Support Surface Consensus Panel Members
Task Force
Three WOCN members with clinical expertise in pressure ulcer prevention and treatment were invited to act as a Task Force for generation and validation of a support surface algorithm (CW, DM, LM). They identified search terms for a comprehensive literature search, reviewed the literature and identified key publications, categorized levels of evidence for the use of support surfaces for the prevention and treatment of pressure ulcers, formulated a draft algorithm and evaluated its face validity. Based on recommendation from the Task Force, an experienced moderator (MG) was invited to act in an advisory role to the Task Force and serve as moderator for a consensus conference. The moderator has expertise in facilitating and moderating consensus conferences and is knowledgeable about, but not directly vested in, the issue of support surface selection and did not participate in the voting process. The Task force also sought assistance from an expert in algorithm development (JB) who also has extensive knowledge of support surface selection for prevention and treatment of pressure ulcers. An independent third party (Magellan Medical Technology Consultants, Inc. Minneapolis, MN) was contracted to plan and facilitate the developmental process and consensus conference.
Comprehensive Literature Review
A comprehensive literature search was conducted from December 2013 through April 2014. The following electronic databases were searched: MEDLINE, CINAHL, Agency for Healthcare Research and Quality (AHRQ), Evidence Reports and Technology Assessments, and the Cochrane Database of Systematic Reviews. Additional sources included AHRQ publications and the Blue Cross and Blue Shield Center for Clinical Effectiveness (formerly the Technology Evaluation Center). Search terms identified by the Task Force and Boolean functions were incorporated to capture all pertinent literature. They were: 1) bed OR mattress OR sleep surface OR support surface AND: air-fluidized, active, algorithm, alternating-air/pressure, bariatric, bead, clinical pathways, critical care, decision tree, decubitus ulcer, fluid, foam, gel, high/low air loss, hospital, integrated, interactive, interface pressure, non-powered, overlay, powered, pressure mapping, pressure redistribution, pressure reducing/reduction, pressure relief/relieving, pressure ulcer, reactive, sand, smart, specialty, static air, therapeutic/therapy, tissue interface pressure, tissue tolerance, treatment, and water; 2) prevention AND: friction, heat, humidity, microclimate, pressure, pressure ulcer, shear, friction coefficient, integrated bed system, pressure redistribution, support surface, tissue tolerance. The MeSH (Medical Subject Heading) term “beds” was also combined with the subheading “adverse effects” and the text words “friction” or “shear.” All articles with an English language abstract that were published from 1993 to 2014 were included in the search. An additional search was conducted for relevant clinical practice guidelines or procedures not previously identified. Ancestry searches of key articles were also completed.
The initial search retrieved 1309 citations; they included systematic and integrative reviews, original research reports, preclinical studies (in vitro and in vivo research), technical articles, letters to the editor, and product-related articles. A title review narrowed the search to 342 citations; redundant publications, individual case reports or case series, letters to the editor, single-product evaluations, and publications deemed not relevant to the topic were eliminated.
Because the purpose of this review was generation of an algorithm rather than creation of a systematic review, the Task Force completed an abstract review of the remaining 342 citations and identified 4 high-quality systematic reviews with meta-analysis; 2 from the Cochrane Collaboration Library of Systematic Reviews6,7 and 2 from the AHRQ.8,9 Because the Cochrane Library for Systematic Reviews and US Agency for Health Care Quality are widely accepted as authoritative sources for systematic reviews and meta-analysis, the Task Force elected to use them as primary resources for identification of existing evidence concerning use of support surfaces for pressure ulcer prevention and treatment. In addition, key publications were identified to aid in algorithm development and provide relevant background; they included integrative and comprehensive review articles not discussed in the 4 systematic reviews and clinical research articles not covered in these authoritative resources. Each article was ranked as “keep” or “discard” by Task Force members. Seventy-two key publications were ranked as “keep” by 3 of 3 members and an additional 70 publications were ranked as “keep” by 2 of 3 members.
Supporting Statements for the Algorithm
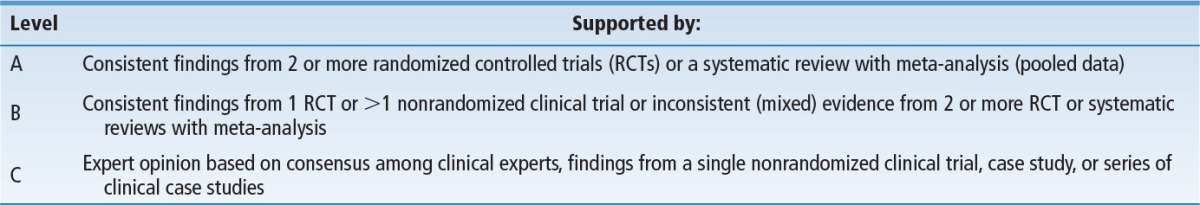
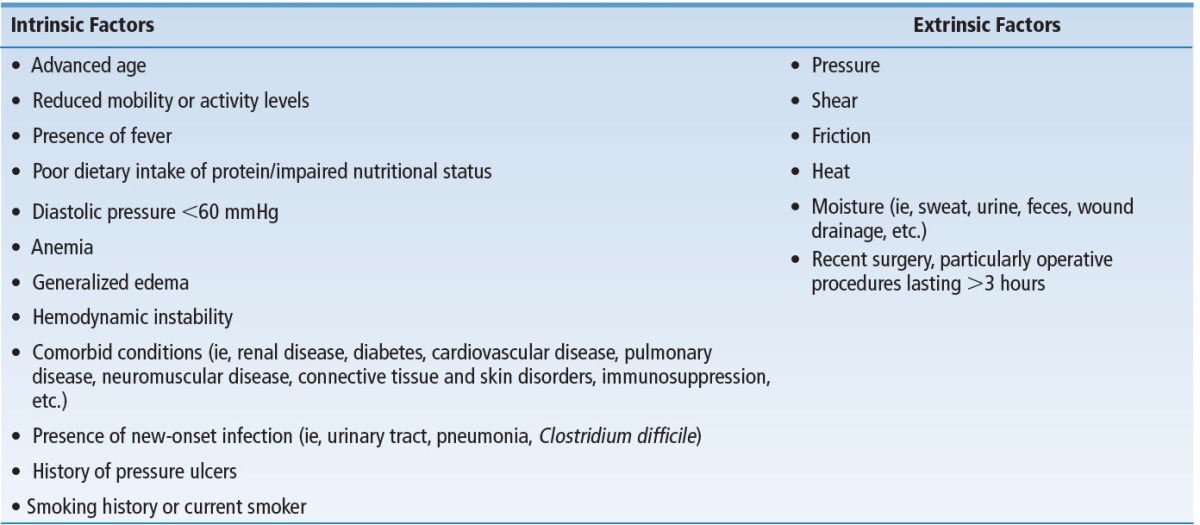
The task force then generated statements from the 4 systematic reviews and key publications described above that supported elements of the algorithm including clinical decision points and various pathways within the algorithm. The strength of evidence from these statements were ranked using a 3-point ordinal scale adapted from the Level of Evidence Rating found in the WOCN Clinical Practice Guideline for Prevention and Management of Pressure Ulcers and the Strength of Recommendations Taxonomy (SORT) from the American Academy of Family Physicians10,11 (Table 1). Statements supported by A- or B-level evidence were deemed “evidence-based” and were used to support elements of the algorithm (Box 2). In contrast, statements supported by C-level evidence were deemed “consensus statements”; they were further subjected to formal consensus among a panel of 20 experts before incorporation into the algorithm (Box 3). The Task Force further acknowledged that skin and pressure ulcer risk assessments and consideration of other risk factors would be incorporated into the algorithm (Table 2). General principles supporting use of these instruments were derived from existing clinical practice guidelines from the WOCN, National Pressure Ulcer Advisory Panel (NPUAP), and Association for the Advancement of Wound Care.2,3
TABLE 1. Levels of Evidence Taxonomy for Supporting Statements.
Box 2.
Evidence-based Statements
Box 3.
Consensus Statements
TABLE 2. Intrinsic and Extrinsic Risk Factors for Pressure Ulcer Development 1–3,20,21.
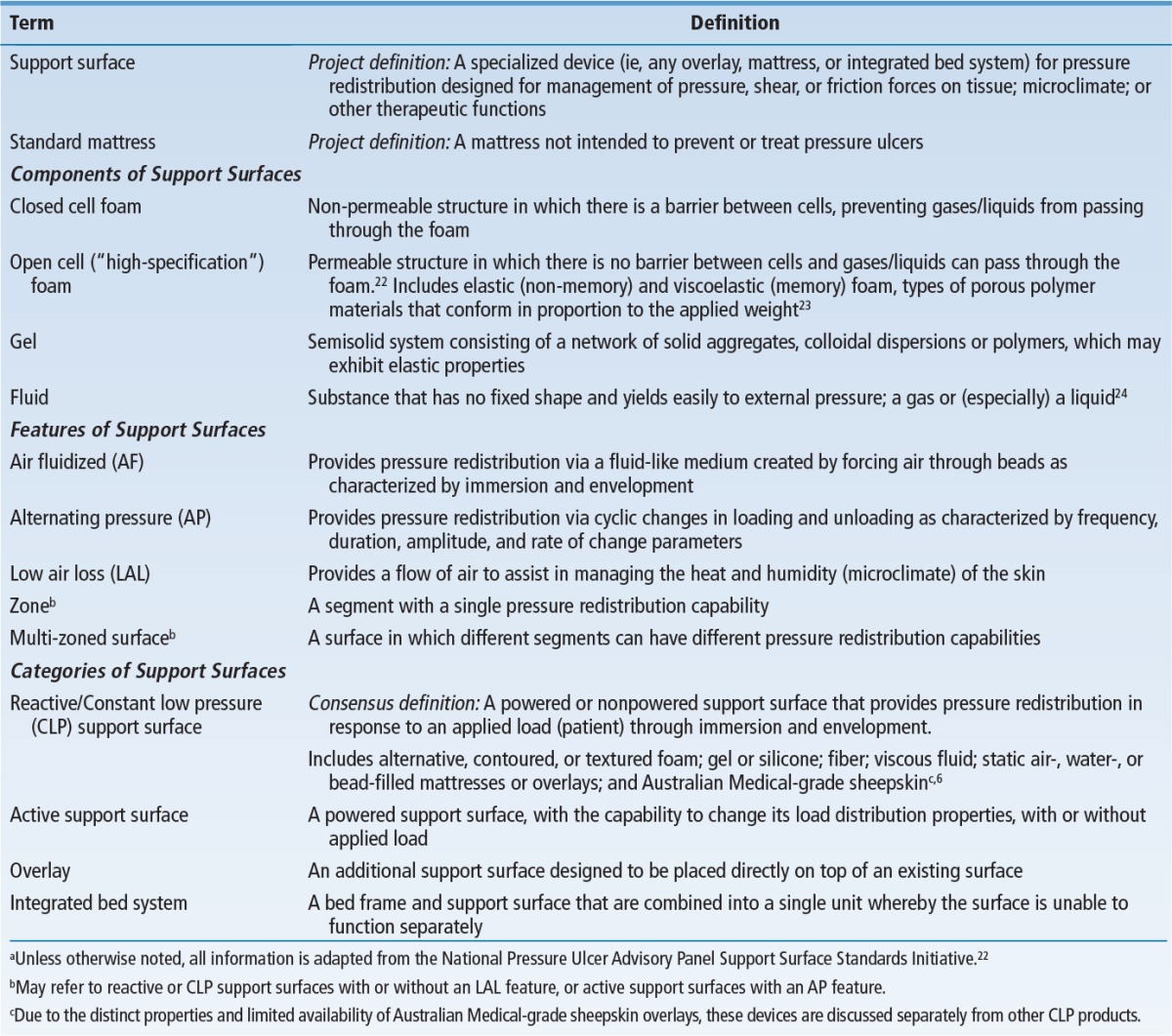
Inconsistencies in support surface terminology were detected during the comprehensive literature review, potentially leading to confusion in use of the algorithm in the clinical setting. Therefore, the Task Force identified and used uniform terms and definitions related to support surfaces developed by the NPUAP Support Surface Standards Initiative (S3I) in 2007 to enhance consistency with existing nomenclature (Table 3).22 Additional terms essential for development of the algorithm are defined in a glossary that serves as supplemental information for the algorithm (Box 4).
TABLE 3. Terminology Related to Support Surfacesa.
Box 4.
Glossary
Development of Draft Algorithm
The Task Force then developed a draft algorithm via a series of web-based conference calls and a single face to face meeting. Members of the Task Force evaluated the face validity of the draft algorithm at multiple points during its development by identifying representative patient scenarios at their facilities and creating hypothetical scenarios and following each patient through the algorithm to ensure that the processes followed (eg, assessments, considerations, reassessments), decision points, interim and end results (eg, recommendations for use of a particular type of support surface, a change in support surface) were comprehensive, feasible, and appropriate.
Following extensive discussion, the Task Force decided that the algorithm was to be designed for selection of specific categories of support surfaces, including overlays, mattresses, and integrated bed systems, for prevention and treatment of pressure ulcers excluding medical device related pressure ulcers. The target audience for the algorithm includes nurses, specialty and advanced practice nurses, physicians, physician assistants, physical therapists and occupational therapists. The algorithm was designed for adult patients (including morbidly obese individuals) in acute care facilities (critical care units, medical-surgical, orthopedic, rehabilitation, units and the emergency department), long-term acute care facilities, long-term care/skilled nursing homes, and home care settings. The algorithm was not designed for use in patients <16 years of age, or selected settings such as the operating room and interventional diagnostic suite where the length of stay is less than 24 hours. Selection of seating surfaces and cushions, continuous lateral rotation mattresses, and other special purpose beds or surfaces, such as those for proning, multiple fractures, and unstable spine, were not incorporated into the algorithm.
Consensus Conference
The Task Force identified potential consensus panel members based on their expertise in support surface technologies and their clinical applications. Additional criteria for participation included membership in relevant professional organizations, geographic location, and practice settings (acute care, long-term acute care, long-term care, and home care). Many potential invitees were responsible for support surface selection and value-based purchasing (VBP) decisions in their respective clinical setting. The panel comprised 20 experts; 9 (45%) were advanced practice nurses, 6 (30%) were registered nurses, 2 (10%) were physical therapists, 1 was an engineer, 2 were researchers, and 1 was a certified expert in prosthetics. The majority (80%) were certified in wound care. More than half (59%) encountered 10 or more patients per week who are at risk for or have a pressure ulcer. Three panel members were researchers; they reported 6 to 25 years experience conducting research in the area of support surface technology.
The 2-day conference began with a presentation summarizing preconference activities and a state-of-the-science presentation on support surface selection. This was followed by a discussion of evidence-based statements; several statements were clarified based on panel member input. For example, panel members recommended adding the comorbid conditions of advanced age, fever, poor dietary intake of protein, diastolic pressure <60 mm Hg, hemodynamic instability, anemia, and generalized edema to intrinsic and extrinsic risk factors for pressure ulcer development. Comments and recommendations related specifically to support surfaces are summarized in Table 4.
TABLE 4. Evidence-based Statements: Panel Comments and Recommendations.


Statements supported by level C evidence were then subjected to a formalized process of consensus validation. An interactive software program and wireless response system (IML ViewPoint Express and IML Click, IML, Minneapolis, MN) allowed anonymous interactive voting by the panel members and Task Force. Consensus on each statement was obtained based on general principles outlined by Murphy and colleagues,26 using 80% agreement as the criterion for consensus. If consensus was not achieved on the first vote, the statement was edited based on panel member input and second, and sometimes third, votes were taken. If consensus could not be reached after 3 rounds of discussion, or the statement deemed irrelevant to algorithm development, consensus regarding deletion of the statement was obtained. The draft algorithm was then reviewed in detail by the panel and modified based on evidence-based and consensus statements and additional discussion.
Support Surface Algorithm
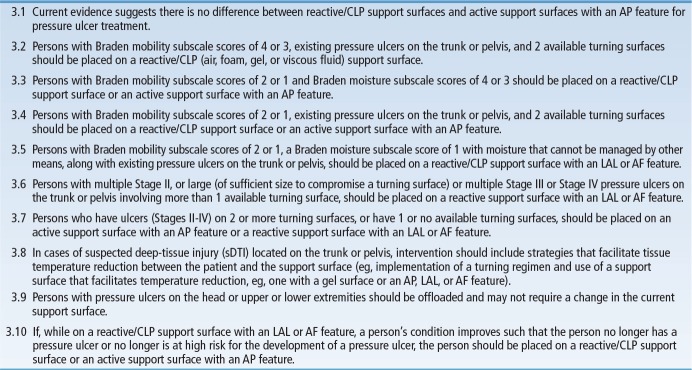
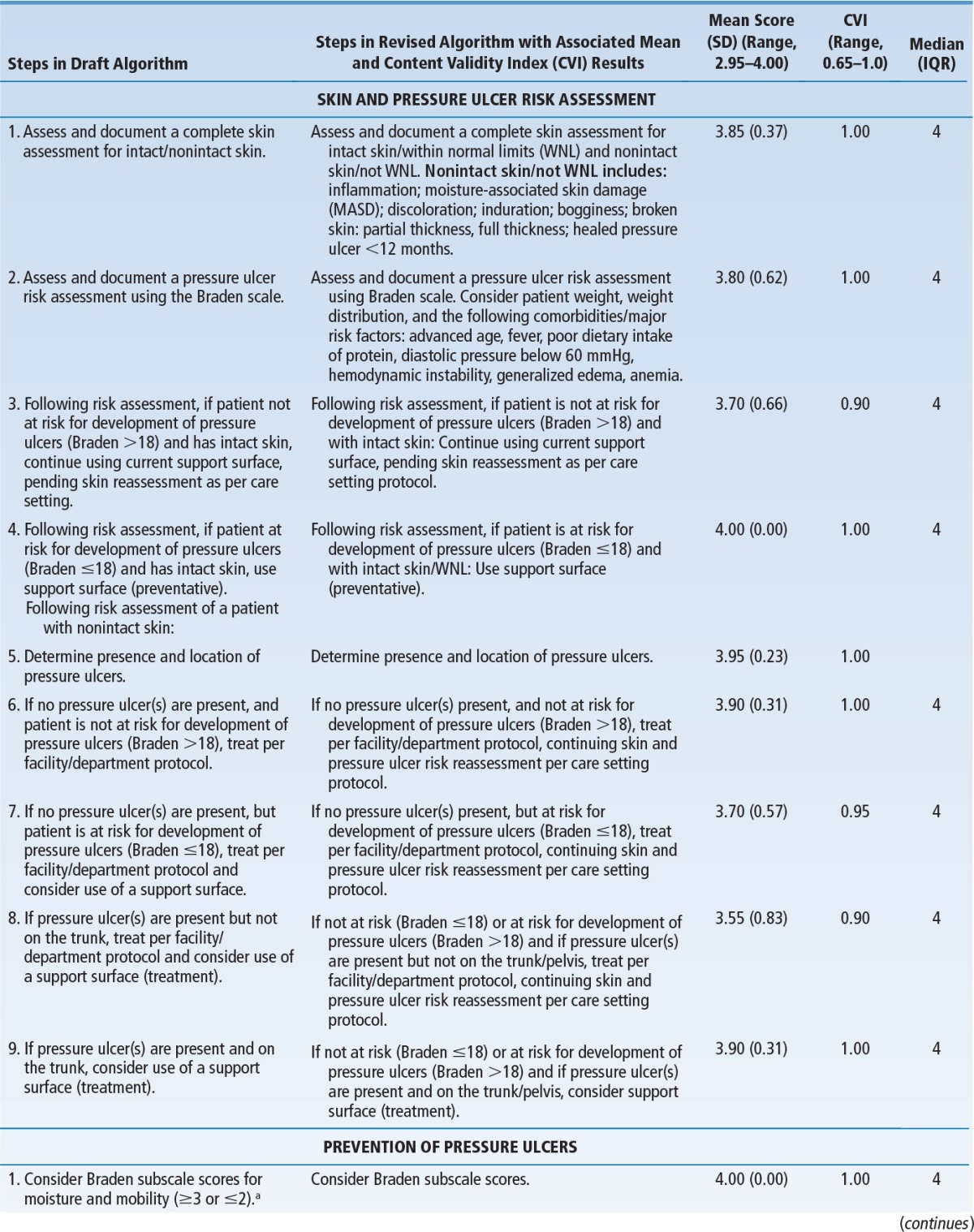
Users enter the algorithm at the point of the initial skin assessment, followed by pressure ulcer risk assessment (Figure 1). Based on the risk for development of pressure ulcers (Braden score cut-off of 18)27 or presence of pressure ulcers, users follow pathways that guide clinical decision making for support surface use for pressure ulcer prevention or treatment. Support surface selections based primarily on Braden moisture and mobility subscale scores are provided, as well as guidance regarding performance of skin and pressure ulcer risk reassessments, determining the need for a change in or removal from a support surface, and support surface considerations and contraindications. Task Force and Consensus Panel members acknowledge the need for individual facilities to adapt the algorithm for their own use by including the specific products used at their facility, along with appropriate staff education.
FIGURE 1.
Algorithm for support surface selection.
Content Validation
Content validation was based on procedures originally proposed by Lynn28 and Waltz & Bausell29 and modified by Grant & Davis.30 A data collection form was developed to evaluate content validity of the algorithm. The form contained 18 questions regarding panel demographic and pertinent professional credential data including gender, age, educational background, wound care certification, years of experience, and practice setting. Twenty nine items representing each pathways and decision points in the algorithm were developed. Following revision of the algorithm during the consensus conference, panel members were asked to rank individual items on scale of 1 to 4 where: 1 = not relevant/appropriate; 2 = unable to assess relevance without revision, 3 = relevant but needs minor alteration, or 4 = very relevant and appropriate. Panel members were also asked to provide qualitative feedback (written comments and suggestions) on the comprehensiveness of the algorithm, omissions of essential content, and suggest changes to improve clarity, parsimony, and relevance. All panel members agreed to participate.
Data analysis was conducted using Excel® version 2013 (Microsoft, Seattle, WA). Data were coded and entered into a database, analyzed by the data coordinator, and reviewed by the authors. Descriptive statistics were used to summarize demographic and pertinent professional credential data. Ratings of 29 algorithm decision statements/steps were entered and mean scores were calculated. A Content Validity Index (CVI) was calculated using processes described by Polit and Beck.31 Qualitative comments regarding decision statements/steps were transcribed and thematically analyzed using qualitative data reduction techniques.
Quantitative Analysis
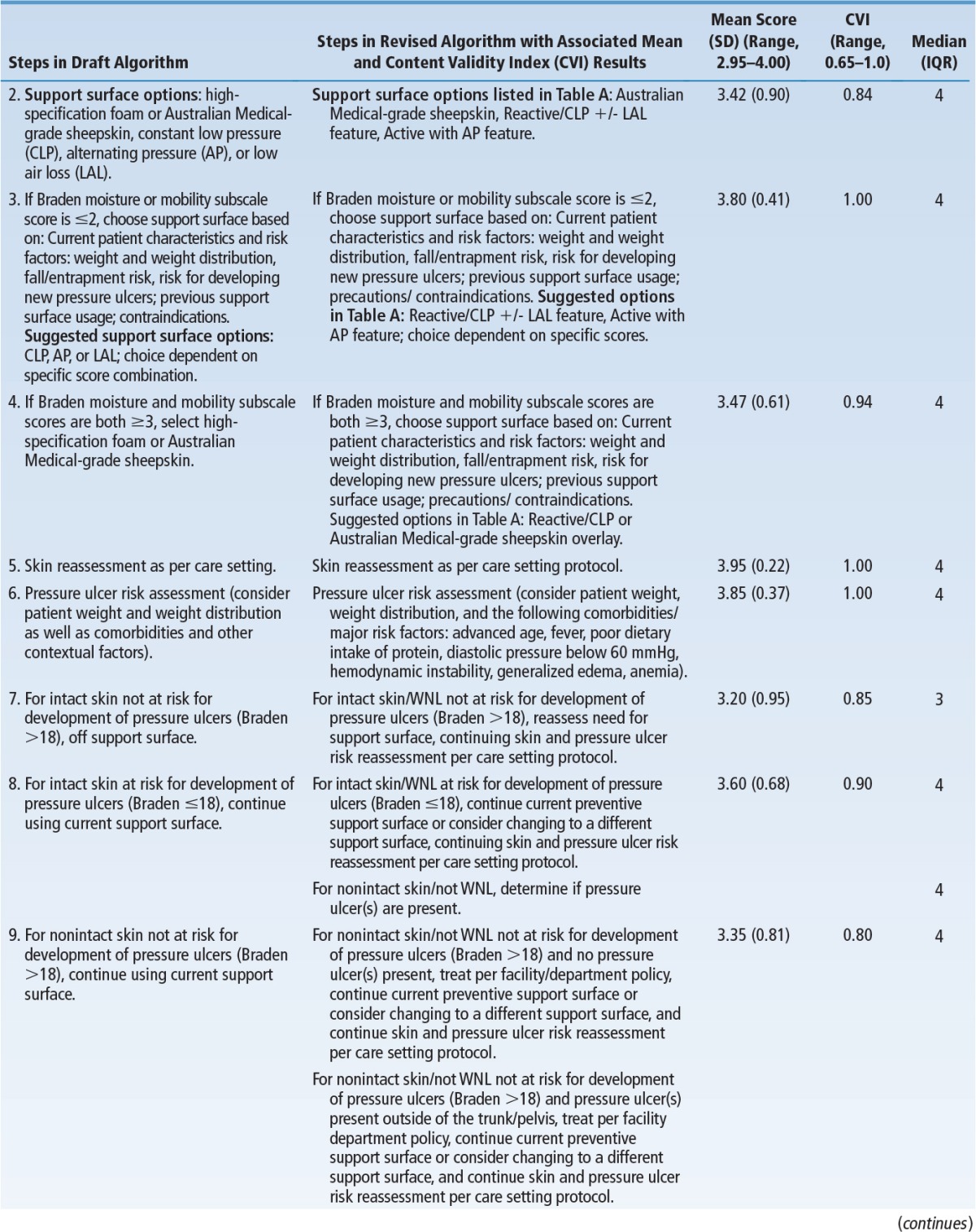
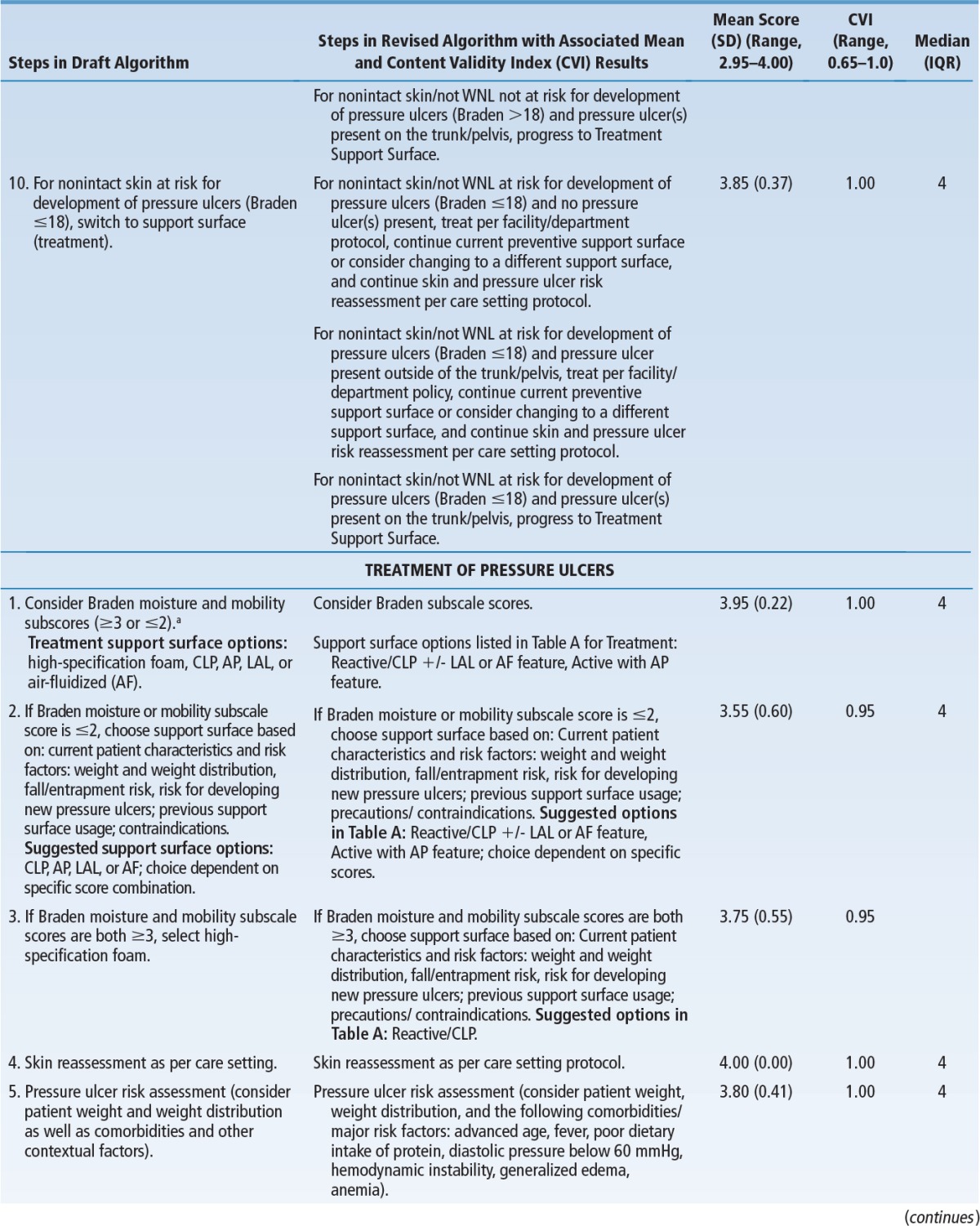
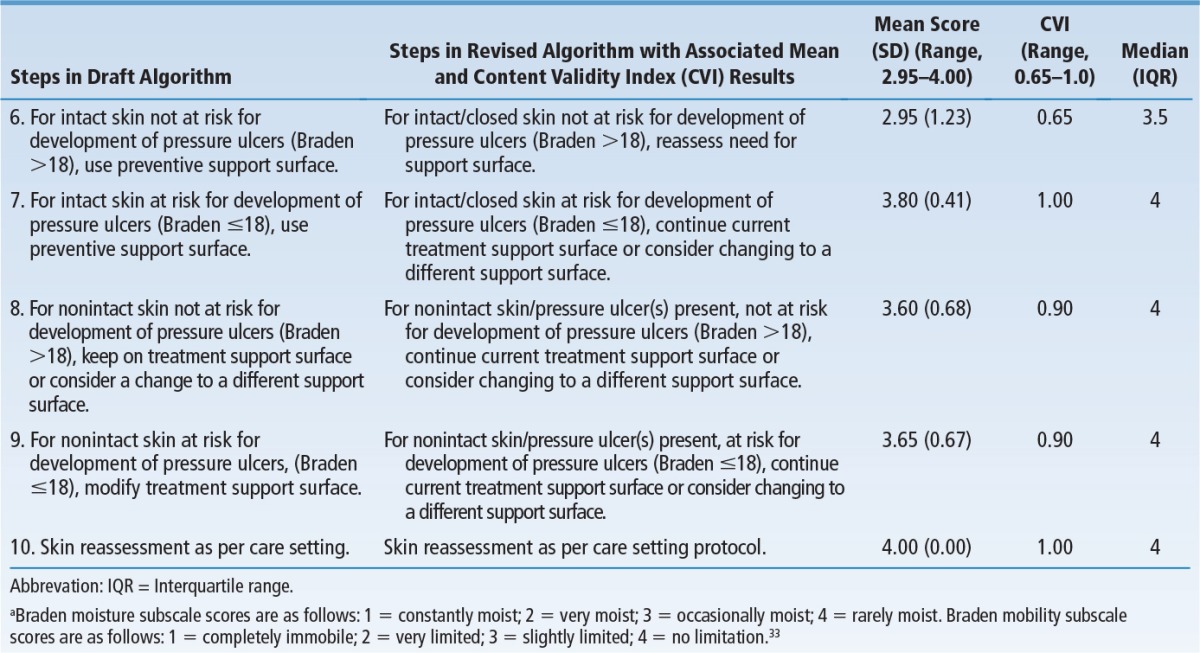
Table 5 summarizes changes incorporated into the final algorithm, mean scores, and the CVI for decision points and pathways in the algorithm. The overall mean score was 3.72 ± 0.48 out of 4 (mean ± SD), indicating components of the algorithm were ranked as “very relevant and appropriate” or “relevant and needed only minor alteration.” The CVI for the entire algorithm was 0.95, well above the minimum (0.70 or 0.80) considered acceptable.28,31,32 All decision statements/pathways were above this minimum except for Treatment of Pressure Ulcers, Step 6, “For intact/closed skin not at risk for development of pressure ulcers (Braden >18), reassess need for support surface.” The CVI for this item was 0.65 out of 1.00. Review of qualitative data revealed that the lower CVI on this item reflected disagreement with language included in the draft algorithm; it was subsequently clarified.
TABLE 5. Changes Incorporated into Final Algorithm and Quantitative Analysis.
Qualitative Analysis
All comments entered into the data collection form were collated and reviewed by the Task Force. Respondents' comments reflected concern about: 1) exclusive use of the Braden Scale for pressure ulcer risk assessment and the limited number of comorbid conditions listed for consideration; 2) the need to provide definitions for each of the categories of support surfaces, particularly Australian Medical-grade sheepskin, as well as a desire for inclusion of examples of support surfaces in each category; 3) the desire to provide more specific guidance with regard to support surface recommendations; 4) possible inclusion of patient preference as a consideration for support surface selection; and 5) a desire to compress the algorithm presented during the conference for efficiency and ease of use. In a few instances, respondents felt that instructions for the user to “consider” use of a support surface were too soft and should be replaced with “should.” Modifications to were made to the algorithm's wording to improve clarity or appropriateness based on this qualitative feedback.
Discussion
An evidence- and consensus-based algorithm for support surface selection was created and its content validity analyzed. The CVI for the algorithm was strong (0.95 out of 1.0), with an overall mean score of 3.72 (out of 1 to 4), suggesting that the steps were appropriate to the purpose of the algorithm. Only one validation score was below 3.0, and this statement was revised. Consensus panel member comments reflected concern about exclusive use of the Braden Scale for Pressure Sore Risk Assessment, but they also acknowledged the instrument is widely used in North America and has undergone extensive validation. Panel members also noted the limited number of comorbid conditions listed for consideration. Other issues discussed were the need to provide definitions for categories of support surfaces, a desire for inclusion of examples, a desire to provide more specific guidance with regard to support surface recommendations, and a desire to compress the algorithm for efficiency and ease of use.
Support surface terminology generated considerable discussion when drafting the algorithm and during the Consensus Conference. Agreement was reached to use the convention of a respective support surface category with added features as applicable. Definitions of these terms were provided for algorithm users. The use of this convention is adaptable to addition of new support surface features or combinations in the future. Despite higher level clinical evidence supporting the effectiveness of Australian Medical-grade sheepskin for prevention of pressure ulcers, inclusion of these support surfaces generated considerable discussion due to their limited availability and usage in the United States. Since this product is now available through online suppliers, this category of support surface was included in the algorithm as a suggested option for pressure ulcer prevention, although it was considered separately from other reactive/CLP products.
Unique to this algorithm is the use of 2 Braden subscale scores, mobility and moisture, to guide support surface selection. While research is limited, Task Force members believed that these subscale scores are indicative of clinically relevant risk for development of pressure ulcers, even when the overall risk score indicates minimal risk. The cumulative Braden Scale score is a valid and reliable predictor of pressure ulcer risk, but its application does not reduce the risk of pressure ulcers to zero.27,34,35 As a result, there has been increasing interest in investigating whether patient outcomes may be improved by tailoring pressure ulcer prevention strategies based on individual subscale scores in addition to a cumulative score.36–39 Bergquist40 analyzed risk factors for pressure ulcer development in older adults receiving home health care and found that mobility and moisture subscale scores predicted pressure ulcer development, but they also noted that the cumulative Braden Scale score was more strongly related to pressure ulcer development than were these subscale scores. Tescher and colleagues41 reported findings from a large, retrospective study (N = 12,566) that examined risk factors associated for pressure ulcer development in patients cared for in intensive and progressive care units. They found that low scores on the friction/shear, moisture, sensory perception, and mobility subscales were more predictive than the cumulative Braden score alone. Results of a comprehensive literature review on pressure ulcer risk assessment in the critical care population suggests that sensory perception, mobility, moisture, and friction/shear subscale scores are predictive of pressure ulcer development.42 A study examining the relationship of individual Braden subscale scores to pressure ulcer prevalence in obese and non-obese hospitalized patients found high-risk total Braden and Braden subscale scores, except for moisture, to be significantly related to the occurrence of pressure ulcers in both groups.43 However, high-risk total Braden score and mobility and friction/shear subscale scores were much more strongly related to ulcer occurrence in obese patients. Results of a retrospective review of hospitalized Brazilian patients deemed at risk of pressure ulcers (cumulative Braden Scale score ≤13) suggests that score stratification by subscale may extend and specify the total Braden score to better direct interventions to prevent pressure ulcers.37 Gadd39 reported results of a retrospective review of 20 patients with hospital-acquired pressure ulcers identified patients deemed at low risk of pressure ulcer development based on cumulative Braden score. Analysis of these cases revealed that these patients may have benefitted from interventions based on suboptimal Braden score on the sensory perception, activity, and mobility subscales.
The relative contributions of the cumulative Braden scale score, subscale scores, clinical judgment and experience in clinical decision-making are not known. Magnan & Maklebust44 evaluated relationships between Braden subscale scores and nurses' selection of 10 commonly used pressure ulcer preventive interventions. Findings suggest that subscale scores influence nurses' endorsement of various preventive interventions in 2 ways; participants used unique combinations of subscale scores to assess risk, and they were more likely to implement preventive interventions as these scores decreased and risk increased. Additional research is needed to determine the efficacy of preventive strategies based on Braden Scale subscores alone or in combination.
Limitations
The support surface selection algorithm was designed for use in adult and bariatric patients in care settings with a length of stay > 24 hours. It does not address use of seating surfaces and cushions, continuous lateral rotation mattresses, and other special purpose beds or surfaces. High-level evidence regarding comparative efficacy of support surfaces and their optimal usage in specific patient populations and in conjunction with other therapeutic modalities is lacking, particularly for individuals with existing pressure ulcers. Clinical evidence regarding the use of the combination of Braden moisture and mobility subscale scores as predictors of pressure ulcer risk or as a means to tailor prevention strategies is also lacking. In each of these cases, decisions supported in the algorithm relied on lower level evidence (consensus among members of an expert panel). In some instances, consensus on more specific recommendations for support surface selection could not be achieved, suggesting that multiple support surface options may be appropriate under specific circumstances.
Conclusions
Support surfaces are one of a bundle of interventions used for pressure ulcer prevention and treatment. Nevertheless, their role is critical. Multiple factors come into play when selecting a support surface, but limited guidance supported by high-level evidence for choice of a specific type of support surface over another is available. This content validated support surface selection algorithm and the accompanying consensus statements were developed in response to the critical need for this type of information for use in clinical practice. To our knowledge, this is the first support surface selection algorithm based on a comprehensive literature review that has been content validated. In the algorithm, support surface selection is largely driven by Braden mobility and moisture subscale scores. Facilities are encouraged to adapt this algorithm for their own use by including the specific products used at their facility and incorporate appropriate staff education for optimal implementation.
KEY POINTS
In an effort to provide clinical guidance for selecting support surfaces to match individual patient needs, an evidence- and consensus-based algorithm for support surface selection that largely utilizes Braden mobility and moisture subscale scores to drive selection was developed and content validated.
Consensus was obtained for statements supporting decision points in the draft algorithm not supported by high-level evidence and/or providing ancillary information.
Health care facilities may adapt this algorithm for their own use by including the specific products used at their institutions.
ACKNOWLEDGEMENTS
The Support Surface Consensus Panel would like to thank the following individuals for their special contributions to the project: the WOCN for conceiving this important initiative; Laurie McNichol, Dianne Mackey, and Carolyn Watts for their leadership, dedication, and time commitment in making this project a reality; Hill-Rom (Batesville, Indiana) for educational grant support; Magellan Medical Technology Consultants for their role in planning and facilitating the Conference; Mikel Gray for his advisory role; Janice Beitz for her expertise in algorithm development and content validation; Evan Call for providing recommendations for support surface inspection; Marie Sabo Recine, MS, MT(ASCP), for providing medical writing assistance; Jeffrey M. Williams for conducting the search of electronic databases for the medical literature; and Charlotte Gasperlin for conducting data analysis for content validation.
Footnotes
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jwocnonline.com).
References
- 1.Wound, Ostomy and Continence Nurses Society (WOCN). Guideline for Prevention and Management of Pressure Ulcers. Mount Laurel, NJ: Wound, Ostomy and Continence Nurses Society (WOCN); 2010:1–108. [DOI] [PubMed] [Google Scholar]
- 2.National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Osborne Park, Western Australia: Cambridge Media; 2014. [Google Scholar]
- 3.Association for the Advancement of Wound Care (AAWC). AAWC Guideline of Pressure Ulcer Guidelines. Malvern, PA: Association for the Advancement of Wound Care (AAWC); 2010;14:1–57. [Google Scholar]
- 4.National Clinical Guideline Centre. Pressure Ulcers: Prevention and Management of Pressure Ulcers. National Institute for Clinical Excellence (NICE) Clinical guideline 179. http://www.nice.org.uk/guidance/CG179. Published April 2014. Accessed July 30, 2014.
- 5.International NPUAP/EPUAP Pressure Ulcer Guideline. In press, 2014.
- 6.McInnes E, Dumville JC, Jammali-Blasi A, Bell-Syer SEM. Support surfaces for treating pressure ulcers (review). Cochrane Database Syst Rev. 2011;(12):CD009490. [DOI] [PubMed] [Google Scholar]
- 7.McInnes E, Jammali-Blasi A, Bell-Syer SEM, Dumville JC, Cullum NA. Support surfaces for pressure ulcer prevention (review). Cochrane Database Syst Rev. 2011;(4):CD001735. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Dana T, Bougatsos C, et al. Pressure Ulcer Risk Assessment and Prevention: Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; 2013. May. [PubMed] [Google Scholar]
- 9.Saha S, Smith MEB, Totten A, et al. Pressure Ulcer Treatment Strategies: Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; 2013. May. [PubMed] [Google Scholar]
- 10.Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, Bowman M. Strength of Recommendation Taxonomy: A patient centered approach to rating evidence in the medical literature. Am Fam Physicians. 2004;69:548–556. [PubMed] [Google Scholar]
- 11.Wound, Ostomy and Continence Nurses Society. Guideline for Prevention and Management of Pressure Ulcers. WOCN Clinical Practice Guideline Series. Glenview, IL: WOCN; 2003. [Google Scholar]
- 12.Sprigle S, Sonenblum S. Assessing evidence supporting redistribution of pressure for pressure ulcer prevention: a review. JRRD. 2011;48:203–214. [DOI] [PubMed] [Google Scholar]
- 13.DeFloor T, Grypdonck MFH. Pressure ulcers: validation of two risk assessment scales. J Clin Nurs. 2005;14(3):373–82. [DOI] [PubMed] [Google Scholar]
- 14.Commonwealth Scientific and Industrial Research Organisation (CSIRO). Australian Medical Sheepskins. http://www.csiro.au/Organisation-Structure/Divisions/CMSE/Fibre-Science/MedicalSheepskinsBrochure.aspx. Published September 15, 2008. Accessed July 11, 2014.
- 15.Nixon J, Cranny G, Iglesias C, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ. 2006;332:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarré L, Beeckman D, Vanderwee K, et al. Multi-stage versus single-stage inflation and deflation cycle for alternating low pressure air mattresses to prevent pressure ulcers in hospitalised patients: a randomised-controlled clinical trial. Int J Nurs Stud. 2012;49:416–426. [DOI] [PubMed] [Google Scholar]
- 17.Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age Ageing. 2005;34:261–267. [DOI] [PubMed] [Google Scholar]
- 18.Demarré L, Verhaeghe S, Hecke AV, et al. The effectiveness of three types of alternating pressure air mattresses in the prevention of pressure ulcers in Belgian hospitals. Res Nurs Health. 2013;36:439–52. [DOI] [PubMed] [Google Scholar]
- 19.Unal S, Ozmen S, Demir Y, et al. The effect of gradually increased blood flow on ischemia reperfusion injury. Ann Plast Surg. 2001;47:412–416. [DOI] [PubMed] [Google Scholar]
- 20.Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehab Nurs. 1987;12:8–12. [DOI] [PubMed] [Google Scholar]
- 21.Primiano M, Friend M, McClure C, et al. Pressure ulcer prevalence and risk factors during prolonged surgical procedures. AORN J. 2011;94:555–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Pressure Ulcer Advisory Panel Support Surface Standards Initiative. Terms and definitions related to support surfaces. Ver. 01/29/2007. http://www.npuap.org/wp-content/uploads/2012/03/NPUAP_S3I_TD.pdf. Published January 29, 2007. Accessed June 25, 2014.
- 23.Nix DP, Mackey DM. Support surfaces. In: Bryant RA, Nix DP, eds. Acute & Chronic Wounds: Current Management Concepts. 4th ed. St. Louis, MO: Elsevier Mosby; 2012:154–167. [Google Scholar]
- 24.International Organization for Standardization. Wheelchairs —Part 26: Vocabulary. ISO 7176–26–2007 E, 4.8.15. https://www.iso.org/obp/ui/#iso:std:iso:7176:-26:ed-1:v1:en. Published April 15, 2007. Accessed September 3, 2014.
- 25.Huang HY, Chen HL, Xu XJ. Pressure-redistribution surfaces for prevention of surgery-related pressure ulcers: a meta-analysis. Ostomy Wound Manage. 2013;59:36–48. [PubMed] [Google Scholar]
- 26.Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assessment. 1998; 2:i–iv, 1–88. [PubMed] [Google Scholar]
- 27.Bergstrom N, Braden B, Kemp M, Champagne M, Rube E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the Braden Scale. Nurs Res. 1998;47:261–269. [DOI] [PubMed] [Google Scholar]
- 28.Lynn M. Determination and quantification of content validity. Nurs Res. 1986;35:19–26. [PubMed] [Google Scholar]
- 29.Waltz CW, Bausell RB. Nursing Research: Design, Statistics and Computer Analysis. Philadelphia, PA: FA Davis; 1981. [Google Scholar]
- 30.Grant JS, Davis LL. Focus on quantitative methods: selection and use of content experts for instrument development. Res Nurs Health. 1997;20:269–274. [DOI] [PubMed] [Google Scholar]
- 31.Polit DF, Beck CJ. The content validity index: are you sure you know what's being reported? Critique and recommendations. Res Nurs Health. 2006;29:489–497. [DOI] [PubMed] [Google Scholar]
- 32.Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health. 2007;30:459–467. [DOI] [PubMed] [Google Scholar]
- 33.Braden B, Bergstrom N. Braden scale for predicting pressure sore risk. Prevention Plus, home of the Braden scale web site. http://bradenscale.com/images/bradenscale.pdf. Copyright 1988. Accessed August 8, 2014.
- 34.Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs. 2006;54:94–110. [DOI] [PubMed] [Google Scholar]
- 35.Anthony D, Parboteeah S, Saleh M, Papanikolaou P. Norton, Waterlow and Braden scores: a review of the literature and a comparison between the scores and clinical judgment. J Clin Nurs. 2008;17:646–653. [DOI] [PubMed] [Google Scholar]
- 36.Gadd MM. Preventing hospital-acquired pressure ulcers: improving quality of outcomes by placing emphasis on the Braden subscale scores. JWOCN. 2012;39:292–294. [DOI] [PubMed] [Google Scholar]
- 37.Menegon DB, Bercini RR, Santos CT, Lucerna AF, Pereira AGS, Scain SF. Braden subscales analysis as indicative of risk for pressure ulcer. Texto Contexto - Enfermagem. 2012;21:854–861. [Google Scholar]
- 38.Gadd MM. Braden Scale cumulative score versus subscale scores: are we missing opportunities for pressure ulcer prevention? JWOCN. 2014;41:86–89. [DOI] [PubMed] [Google Scholar]
- 39.Gadd MM, Adkins SM. Use of the Braden Scale for pressure ulcer risk assessment in a community hospital setting. JWOCN. 2014;41: In press. [DOI] [PubMed] [Google Scholar]
- 40.Bergquist S. Subscales, subscores, or summative score: evaluating the contribution of Braden Scale items for predicting pressure ulcer risk in older adults receiving home health care. JWOCN. 2001;28:279–289. [DOI] [PubMed] [Google Scholar]
- 41.Tescher AN, Branda ME, Byrne TJ, Naessens JM. All at-risk patients are not created equal: analysis of Braden pressure ulcer risk scores to identify specific risks. JWOCN. 2012;39:282–291. [DOI] [PubMed] [Google Scholar]
- 42.Cox J. Predictive power of the Braden scale for pressure sore risk in adult critical care patients: a comprehensive review. JWOCN. 2012;39:613–621. [DOI] [PubMed] [Google Scholar]
- 43.Swanson MS, Rose MA, Baker G, et al. Braden subscales and their relationship to the prevalence of pressure ulcers in hospitalized obese patients. Bariatric Nurs Surg Patient Care. 2011;6:21–23. [Google Scholar]
- 44.Magnan MA, Maklebust J. Braden Scale risk assessments and pressure ulcer prevention planning: what's the connection? JWOCN. 2009;36:622–634. [DOI] [PubMed] [Google Scholar]