This longitudinal, multisite study reports a high prevalence of norovirus infection and norovirus-positive diarrhea and describes patterns of age acquisition, disease severity, genogroup-specific immunity, and relationships between norovirus and undernutrition in the first 2 years of life.
Keywords: norovirus, diarrhea, immunity
Abstract
Background. Norovirus is an important cause of childhood diarrhea. We present data from a longitudinal, multicountry study describing norovirus epidemiology during the first 2 years of life.
Methods. A birth cohort of 1457 children across 8 countries contributed 7077 diarrheal stools for norovirus testing. A subset of 199 children contributed additional asymptomatic samples (2307) and diarrheal stools (770), which were used to derive incidence rates and evaluate evidence for acquired immunity.
Results. Across sites, 89% of children experienced at least 1 norovirus infection before 24 months, and 22.7% of all diarrheal stools were norovirus positive. Severity of norovirus-positive diarrhea was comparable to other enteropathogens, with the exception of rotavirus. Incidence of genogroup II (GII) infection was higher than genogroup I and peaked at 6–11 months across sites. Undernutrition was a risk factor for symptomatic norovirus infection, with an increase in 1 standard deviation of length-for-age z score associated with a 17% reduction (odds ratio, 0.83 [95% confidence interval, .72–.97]; P = .011) in the odds of experiencing diarrhea when norovirus was present, after accounting for genogroup, rotavirus vaccine, and age. Evidence of acquired immunity was observed among GII infections only: Children with prior GII infection were found to have a 27% reduction in the hazard of subsequent infection (hazard ratio, 0.727; P = .010).
Conclusions. The high prevalence of norovirus across 8 sites in highly variable epidemiologic settings and demonstration of protective immunity for GII infections provide support for investment in vaccine development.
(See the Editorial Commentary by Lopman and Grassly on pages 1218–20.)
Norovirus is a common cause of childhood diarrhea [1, 2], which remains the second leading cause of under-5 morbidity and mortality globally [3]. Despite this, few large studies have described the burden, epidemiology, and natural history of this enteropathogen in the community, particularly in low-resource settings.
Human noroviruses are genetically diverse RNA viruses, with at least 25 genotypes, predominantly from genogroup I (GI) and genogroup II (GII), causing disease in humans [4]. This diversity has led to skepticism that vaccine development is a plausible disease control strategy [5–7]; nonetheless, others argue that an effective vaccine could significantly reduce diarrheal hospitalizations and deaths [1, 5]. The need for cohort studies describing infection, disease presentation, and immunity has been highlighted as crucial to addressing gaps in knowledge and informing ongoing vaccine efforts [8, 9]. This longitudinal multisite study describes the epidemiology of asymptomatic and symptomatic norovirus infection among children 0–24 months of age in 8 low- and middle-income countries (LMICs).
METHODS
Study Population
The Etiology, Risk Factors, and Interactions of Enteric Infection and Malnutrition and the Consequences for Child Health and Development (MAL-ED) study was conducted in 8 countries with high rates of diarrheal disease and malnutrition [10]. Sites were located in Bangladesh, Brazil, Pakistan, Peru, South Africa, Tanzania, Nepal, and India [10]. Healthy infants were enrolled within 17 days of birth and followed longitudinally until age 24 months, between November 2009 and February 2012. All sites received ethical approval and informed consent. Details of the study design are discussed elsewhere [10].
Stool Collection and Microbiology
Routine stool collection was conducted monthly from age 1 to 12 months, and at 15, 18, 21, and 24 months of age. Homes were visited twice weekly for active surveillance of diarrhea and other symptoms, including fever, vomiting, cough, and appetite, to generate a continuous illness history. Diarrhea was defined as ≥3 loose stools in a 24-hour period and separated by at least 2 diarrhea-free days. Children experiencing moderate to severe diarrhea were referred to local health services.
Nucleic acid extraction was performed with the QIAamp viral RNA (Qiagen, Valencia, California), and real-time reverse transcriptase polymerase chain reaction was used to detect genogroups [11]. The presence of other enteropathogens was assessed per previously reported microbiologic methods [11].
Norovirus testing was performed on all diarrheal stools collected in the study. Additionally, 10% of children at each site were randomly selected by identification number to have their routine stool samples tested for norovirus. Longitudinal analyses were performed using this subsample, whose symptomatic and asymptomatic history of infection was captured. Additional analyses were performed on diarrheal stools from the full study population to describe coinfection and disease severity.
Data Description and Definitions
Birthdate, sex, birth weight, and anthropometrics were recorded at enrollment. Data on anthropometrics and breastfeeding were collected monthly. Length-for-age z score (LAZ) was calculated according to World Health Organization's Multicentre Growth Reference Study Group guidelines [12]. Duration of exclusive breastfeeding (EBF) was defined as time from birth until age at which any clear liquid, solid food, milk, or milk formula was first given. Rotavirus vaccination in national immunization programs was included as a binary variable by country.
Other factors considered were diarrheal severity, incidence, cycle time as a surrogate for norovirus load, and coinfection with >40 other pathogens (full list available in [2, 10, 11]). Severity was calculated using a Community Diarrheal Assessment (CODA) score [13], with episodes assigned a severity score out of 15 points based on maternally reported presence and duration of fever, vomiting, anorexia, liquid stools, and the maximum number of stools in a 24-hour period at any time during the episode.
In the subsample of children, a new episode was defined as a positive stool sample separated by ≥1 negative sample from the previous episode. For wider analyses of only diarrheal stools, distinct norovirus episodes were defined as positive samples preceded by either a norovirus-negative diarrheal stool sample or a 14-day lag from the previous norovirus-positive sample. Viral load was estimated in each genogroup using the cycle time, with lower values denoting greater pathogen loads. Coinfection was considered a nominal categorical variable describing infection with neither, either, or both pathogens.
Statistical Analyses
Longitudinal analyses were conducted on the subsample of infants selected for routine and diarrheal norovirus testing. Kaplan–Meier survival analysis was conducted to estimate time to first GI/GII infection. Incidence rates were calculated using survival analysis allowing for multiple failures per child. A Cox proportional hazards model was used to model immunity against infection, using the Breslow method for ties and a robust variance estimate to account for within-child clustering. We used the adjusted attributable fraction to incorporate differences in norovirus prevalence and associations between detection and diarrheal symptoms across sites and to estimate the norovirus-specific burden of diarrhea in the population [2, 14, 15]. To model associations between norovirus detection and diarrheal symptoms at each site, we used generalized estimating equations specifying a binomial distribution to obtain odds ratios (ORs) adjusted for age and sex. The relationship between norovirus infection and subsequent child growth was explored using mixed-effects linear regression, specifying a random effect at the child level to account for within-child correlations. Growth models were adjusted for age and age squared, LAZ at the time of infection, and site.
Analysis of diarrheal samples was conducted to estimate proportions of pathogen-negative diarrhea, GI and GII norovirus, and coinfections. Mixed-effects linear regression was used to explore associations between age, coinfection, pathogen load, genogroup, LAZ, and diarrheal severity score. Age, EBF, LAZ, rotavirus vaccination, and norovirus load were included in adjusted models if associated with the outcome at P < .1. All analyses were conducted using Stata software, version 13 (StataCorp, College Station, Texas).
RESULTS
Longitudinal Analysis
A total of 1457 infants participated in the study, 199 of whom were randomly selected for routine norovirus testing. This subsample contributed 2598 surveillance stools and 770 diarrheal stools. Norovirus status was available for 3077 of the 3368 samples. Quality assurance review resulted in exclusion of anthropometrics from Pakistan. The numbers of participants and samples in the cohort are shown in Table 1.
Table 1.
Participants, Person-time, and Stool Samples Included in Analysis, by Country
| Longitudinal Subsample |
Full Cohort |
||||||
|---|---|---|---|---|---|---|---|
| Country | No. of Children | Child-months Contributed | Stool Samples |
No. of Children | Diarrhea Stool Samples Collected | ||
| Diarrhea | Surveillance | Total | |||||
| Asia | |||||||
| Bangladesh | 25 | 488.75 | 146 | 296 | 442 | 239 | 1525 |
| India | 24 | 530.95 | 81 | 318 | 399 | 195 | 615 |
| Nepal | 24 | 535.76 | 82 | 325 | 407 | 214 | 860 |
| Pakistan | 27 | 574.01 | 227 | 272 | 499 | 259 | 1793 |
| Africa | |||||||
| South Africa | 26 | 451.81 | 18 | 250 | 268 | 106 | 172 |
| Tanzania | 25 | 524.18 | 14 | 324 | 338 | 110 | 171 |
| South America | |||||||
| Brazil | 21 | 361.42 | 7 | 188 | 195 | 71 | 98 |
| Peru | 27 | 551.32 | 195 | 334 | 529 | 263 | 1843 |
| Total | 199 | 4018.19 | 770 | 2307 | 3077 | 1457 | 7077 |
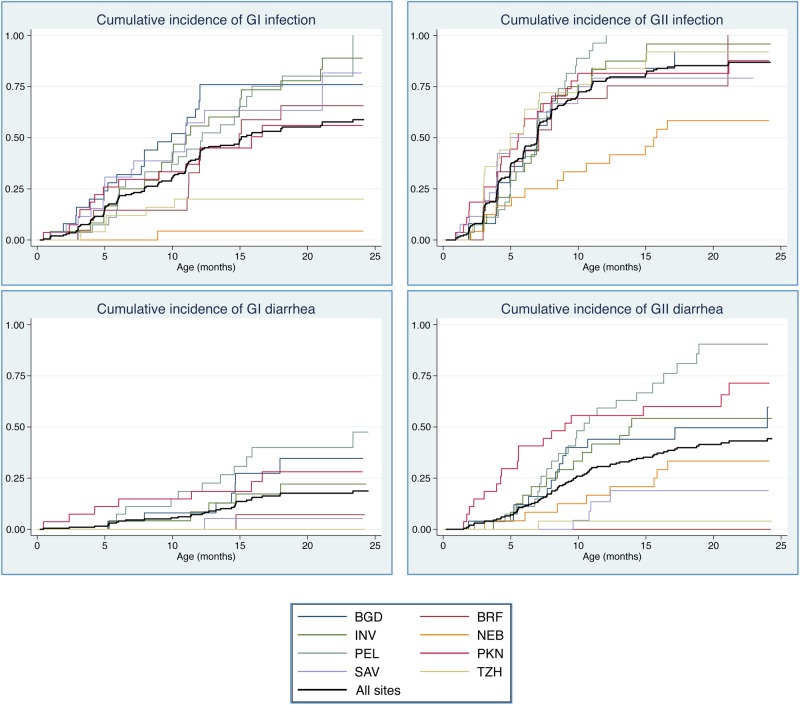
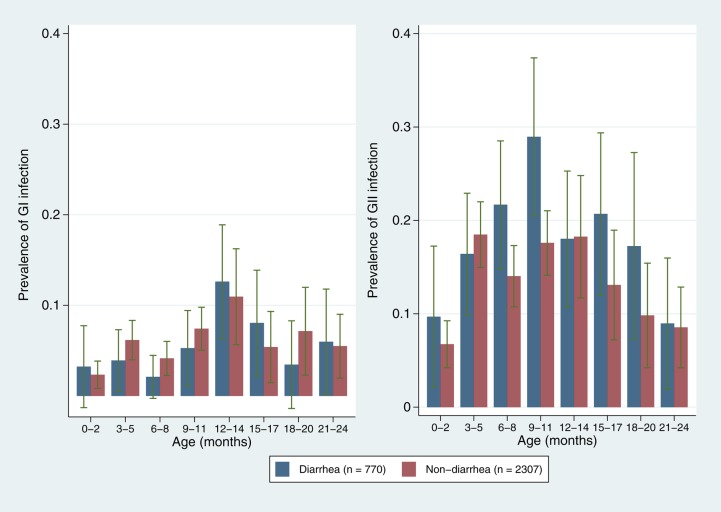
Eighty-six percent of children in the subsample had least 1 norovirus infection by 12 months, increasing to 89% by the end of the second year. Of these 199 children, 107 (53.8%) had at least 1 GI infection during follow-up, and 31 (15.6%) experienced at least 1 diarrheal episode with GI present. Likewise, 168 (84.4%) children had at least 1 GII infection and 72 (36.2%) experienced at least 1 GII diarrheal episode in the first 2 years of life. Across sites, 71.1% of infections and 81.8% of all norovirus-positive diarrhea occurred after 6 months of age. Cumulative incidence and prevalence were higher for GII across sites and age groups (Figure 1 and Figure 2). Incidence rates for GI and GII in routine and diarrheal stools peaked at 6–12 months of age (Table 2). Patterns of carriage and disease presentation varied markedly between sites. Overall prevalence of asymptomatic carriage was 19.0%, ranging from 2.2% in Nepal to 30.4% in South Africa. Norovirus was detected in 23.5% of diarrheal stools, ranging from 7.1% in Tanzania to 32.8% in Peru. The adjusted attributable fraction was 5.1 (95% confidence interval [CI], 1.2–8.3).
Figure 1.
Cumulative incidence of norovirus infection, by genogroup (GI and GII) and country: Abbreviations: BGD, Bangladesh; BRF, Brazil; INV, India; NEB, Nepal; PEL, Peru; PKN, Pakistan; SAV, South Africa; TZH, Tanzania.
Figure 2.
Prevalence of norovirus genogroup I (GI) and genogroup II (GII) in routine and diarrheal stool samples, by month of age. Blue: diarrheal stools; red: surveillance stools.
Table 2.
Incidence Rates of Norovirus Genogroup I and II Detection Among 199 Children, by Age Group and Site
| Age Group and Site | Total Episodes With a GI Sample Associated | Total Episodes With a GII Sample Associated | GI/GII Coinfections | GI Incidence (95% CI) | GII Incidence (95% CI) |
|---|---|---|---|---|---|
| Age group | |||||
| 0–5 mo | 41 | 98 | 6 | 3.86 (2.77–5.11) | 8.99 (7.38–10.96) |
| 6–11 mo | 57 | 153 | 10 | 5.01 (3.86–6.49) | 13.43 (11.47–15.74) |
| 12–17 mo | 39 | 61 | 6 | 4.88 (3.57–6.68) | 7.64 (5.94–9.82) |
| 18–24 mo | 20 | 34 | 1 | 2.02 (1.30–3.13) | 3.43 (2.45–4.80) |
| Country | |||||
| Asia | |||||
| Bangladesh | 34 | 49 | 7 | 6.96 (4.97–9.74) | 10.03 (7.58–13.27) |
| India | 31 | 54 | 1 | 5.84 (4.11–8.30) | 10.17 (7.79–13.28) |
| Nepal | 1 | 16 | 1 | 1.87 (.03–1.33) | 2.99 (2.83–4.88) |
| Pakistan | 18 | 52 | 1 | 3.14 (1.98–4.98) | 9.06 (6.90–11.89) |
| Africa | |||||
| South Africa | 23 | 43 | 5 | 5.09 (3.38–7.66) | 9.52 (7.06–12.83) |
| Tanzania | 6 | 40 | 0 | 1.14 (.51–2.55) | 7.63 (5.60–10.40) |
| South America | |||||
| Brazil | 14 | 21 | 1 | 3.87 (2.29–6.54) | 5.81 (3.79–8.91) |
| Peru | 30 | 71 | 7 | 5.44 (3.81–7.78) | 12.88 (10.21–16.25) |
Incidence expressed as number of detections per 100 child-months. All detections, whether asymptomatic or diarrheal, were considered episodes for the calculation of incidence rates. Incidence rates for GI and GII groups were inclusive of coinfection.
Abbreviations: CI, confidence interval; GI, norovirus genogroup I; GII, norovirus genogroup II.
Viral load, genogroup, anthropometry, and coinfection with any other of the 40 enteropathogens were assessed as risk factors for symptomatic infection. Viral load was higher in GII samples than GI samples, but was not associated with the presence (GI: P = .176; GII: P = .695) or severity (GI: P = .691; GII: P = .813) of diarrhea. After adjusting for EBF, rotavirus vaccine, LAZ, and age, GII-positive samples had 85% increased odds of being symptomatic relative to GI (OR, 1.85 [95% CI, 1.12–3.08]; P = .017), and 42% increased odds relative to other pathogen-positive samples (OR, 1.42 [95% CI, 1.09–1.86]; P = .010).
Undernutrition was a risk factor for symptomatic infection. An increase in one LAZ was associated with a 17% reduction (OR, 0.83 [95% CI, .72–.97]; P = .011) in the odds of experiencing diarrhea when norovirus was present, accounting for genogroup, rotavirus vaccine, and age. The relationship between undernutrition and symptomatic infection was not affected by genogroup (P = .531). Of 619 stools in which norovirus was detected, 464 (75%) were coinfected with other enteropathogens. Most common coinfections included Campylobacter (37.6%), enteroaggregative Escherichia coli (30.9%), and Giardia (13.6%). However, no difference in the odds of symptomatic infection was seen comparing coinfected stools to those with norovirus alone (P = .767).
Protection associated with prior infection was adjusted for duration of EBF. Prior GI infection was not associated with subsequent infection (hazard ratio [HR], 0.973 [95% CI, .682–1.388]; P = .880) or clinical episodes (HR, 1.25 [95% CI, .703–2.22]; P = .447). Children with any prior GII infection had a 27% reduction in the hazard of subsequent infection (HR, 0.727 [95% CI, .571–.926]; P = .010) and a 26% reduction in the hazard of subsequent GII diarrhea (HR, 0.761 [95% CI, .504–1.150]; P = .195). Although not statistically significant, there was a decreasing trend in hazards of subsequent symptomatic infections among children with 1 or more prior infections (HR, 0.798 [95% CI, .524–1.215]; P = .293) and ≥2 prior infections (HR, 0.668 [95% CI, .381–1.172]; P = .159) (Table 3).
Table 3.
Protection Associated With Genogroup II Norovirus
| Genogroup II | Prior Detection | Hazard Ratio (95% Confidence Interval) |
|
|---|---|---|---|
| Subsequent Episodes | Subsequent Diarrheal Episodes | ||
| Prior detection | None (Reference) | ||
| Any | 0.727 (.571–.926) P = .010 |
0.761 (.504–1.150) P = .195 |
|
| No. of prior episodes | 0 (Reference) | ||
| 1 | 0.820 (.628–1.069) P = .142 |
0.798 (.524–1.215) P = .293 |
|
| ≥2 | 0.830 (.600–1.147) P = .259 |
0.668 (.381–1.172) P = .159 |
|
Adjusted for duration of exclusive breastfeeding. Items in bold were statistically significant at a threshold of P < .05.
The association between norovirus and subsequent growth was explored adjusting for age squared, study site, and LAZ at the time of infection. No significant impact on growth was detected 3, 6, or 9 months postinfection for GI (P = .792, P = .552, and P = .724, respectively) or GII (P = .531, P = .219, and P = .848, respectively) infection.
Analysis of Symptomatic Stools
Further analyses were conducted on 7707 diarrheal stools collected from 1457 children in the subsample and the wider cohort (Table 1). Campylobacter species (41.1%), enterovirulent E. coli (39.7%), and norovirus (22.7%) were the most prevalent pathogen detected in diarrheal stools across sites. The proportion of stools with detectable norovirus is shown by country and genogroup in Figure 3. Of 1607 stools with norovirus present, 1248 (77.7%) also tested positive for other viral, bacterial, or parasitic enteropathogens (Table 4).
Figure 3.
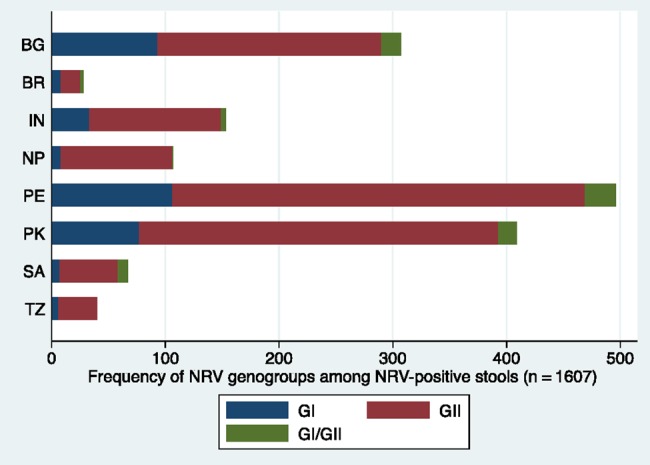
Proportion of norovirus genogroup I (GI) and genogroup II (GII)–positive stools. Blue: GI; red: GII; green: GI/GII coinfection. Abbreviations: BG, Bangladesh; BR, Brazil; IN, India; NP, Nepal; NRV, norovirus; PE, Peru; PK, Pakistan; SA, South Africa; TZ, Tanzania.
Table 4.
Norovirus Coinfections in Diarrheal Stools Across Sitesa
| Pathogen | Norovirus-Positive Stools(n = 1607) | All Stools(N = 7077) |
|---|---|---|
| Rotavirus | 56 (3.5) | 435 (6.2) |
| Adenovirus | 71 (4.4) | 295 (4.2) |
| Astrovirus | 98 (6.1) | 394 (5.6) |
| Aeromonas | 53 (3.3) | 224 (3.2) |
| Campylobacter | 692 (43.1) | 2838 (40.1) |
| EAEC | 390 (24.3) | 1664 (23.5) |
| Atypical EPEC | 92 (5.7) | 343 (4.9) |
| Typical EPEC | 74 (4.6) | 307 (4.3) |
| ETEC | 187 (11.6) | 751 (10.6) |
| Cryptosporidium | 112 (7.0) | 487 (6.9) |
| Giardia | 300 (18.7) | 1291 (18.2) |
Data are presented as No. (%).
Abbreviations: EAEC, enteroaggregative Escherichia coli; EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic Escherichia coli.
a Enteropathogens with >3% prevalence in norovirus-positive stools included in table.
Severity was measured using a continuous score with 15 points denoting maximum diarrheal severity. Scores were calculated for 5568 stool samples. Mean severity associated with norovirus episodes was 3.1, equal to the mean severity across all pathogen-associated episodes in the population. The final model included terms for norovirus infection, age, rotavirus vaccination, and EBF. Each additional month of life was associated with a mean reduction in diarrheal severity of 0.05 points (95% CI, −.054 to −.035; P < .001) and in norovirus-positive diarrheal severity of 0.06 points (95% CI, −.081 to −.033; P < .001). Severity of all diarrhea was found to decrease significantly by 0.004 points with each additional month of EBF (95% CI, −.005 to −.002; P < .001) in the adjusted model. This association was similar in magnitude, but the effect was not significant among norovirus-positive diarrheal stools (β = −.003 [95% CI, −.005 to .000]; P = .075). Children in countries with rotavirus campaigns experienced mean severity scores 0.87 lower points in diarrheal episodes, and 0.79 points lower in norovirus-positive episodes after adjustment. No univariate associations between LAZ and diarrheal severity score were observed (P = .153), which remained consistent after adjustment for age, EBF, and vaccination.
Age, rotavirus vaccine, and EBF were included in multivariable models to evaluate associations between norovirus, coinfection, and diarrheal severity. No difference was observed in severity between genogroups (P = .925 and P = .224 for GII and GI/GII coinfection, relative to GI infection). Norovirus was associated with a mean increase of 0.44 points relative to pathogen-negative diarrheal stools (95% CI, .24–.64; P < .001) in the adjusted severity model. While increased severity was observed in norovirus/rotavirus coinfection, this was explained by the increased severity associated with rotavirus alone. Mean severity during coinfection (4.32) was not significantly greater than rotavirus (4.19) in the adjusted model (P = .678).
DISCUSSION
This study highlights the significant burden of norovirus at 8 sites in variable epidemiologic settings using a unified protocol and sensitive diagnostic methods. Norovirus was the third most prevalent pathogen in 7077 diarrheal stools collected from 1457 children worldwide. More than half of the 199 children in the longitudinal cohort experienced at least 1 GI infection and >80% experienced at least 1 GII infection in the first 2 years of life. This adds to the growing evidence of the high prevalence of norovirus in the community among children living in poverty.
We observed heterogeneity between countries with regard to infection and disease presentation. Norovirus diarrhea incidence rates ranged from 3 episodes per 100 child-months in Nepal to 18 per 100 child-months in Peru. Factors determining asymptomatic or symptomatic disease presentation also differed by site. We report an overall attributable fraction of 5.1%, but in some sites norovirus detection in stool was not significantly associated with diarrheal disease. For example, although asymptomatic carriage was highest in South Africa, only 4 children in this cohort experienced norovirus-positive diarrhea in their first 2 years of life. In contrast, nearly all children in Peru experienced at least 1 episode of norovirus-positive diarrhea before 2 years of age. This heterogeneity was also noted in the GEMS study, and suggests important differences in either circulating norovirus genotypes, host susceptibility among populations, or both. Evidence suggests that specific binding to histo-blood group antigens [16] and expression of α(1,2) fucosyltransferase “secretor status” [17] alters the susceptibility of individuals to some but not all genotypes. Lopman et al reported that in Ecuador, GII.4 infections were only observed in secretor-positive children; however, secretor-negative children actually had a higher incidence of other genotypes, such that the overall incidence of norovirus infection was the same in both groups [7]. Viral genotyping (ongoing) and the characterization of HBGA polymorphisms thus warrant more attention in future studies of norovirus epidemiology and immunity in endemic settings. Nevertheless, it is important that in this study, nutritional status of the host was also a significant determinant of disease, demonstrating that improving nutrition among children in developing countries can have an important impact on disease burden reduction.
Our findings are consistent with other recent work in LMICs emphasizing the ubiquity of norovirus in the community setting and its considerable contribution to endemic childhood diarrhea [5, 7, 18]. Furthermore, this work contributes to the evidence refuting the notion that norovirus causes mild disease [1, 19]. Using a well-validated severity score and a broad diagnostic platform, we found no evidence that norovirus-positive diarrhea caused milder disease than other enteropathogens, with the exception of rotavirus. This contrasts findings in the GEMS study [20], which did not point to norovirus as a key contributor to moderate to severe diarrhea. The difference in these findings may be somewhat explained by study design; whereas the GEMS study relied on health centers for the recruitment of cases, we captured symptomatic disease in the household and therefore detected more mild to moderate cases of disease. Thus, it is possible that norovirus diarrhea in the hospital setting is indeed rare, but that the cumulative burden of norovirus causes significant morbidity. Considering that severity of norovirus-positive diarrhea in the community was comparable to that of other important enteropathogens, but the prevalence was considerably higher, we argue that it be prioritized accordingly.
This work builds on that of birth cohorts conducted by O'Ryan et al in Chile [6], Saito et al in Peru [5], and Lopman et al in Ecuador [7] describing norovirus carriage and associated disease in young children. O'Ryan et al observed increased incidence of infection in children aged 7–12 month, at a rate of 8.4 per 100 child-months. Similarly, a peak incidence of 6.3 among 6- to 11-month-olds was reported in Ecuador, and an even higher incidence of 15.7 was observed in Peru. We report a considerable range of incidence rates in this age group, with 5 of our 8 sites vastly exceeding these previous estimates. We did not observe any evidence of growth insults at 3, 6, or 9 months after infection with GI or GII. This finding conflicts with those elsewhere in Peru, although we when reproduced Saito et al's methods, we did observe an association between cumulative infections and reduced mean LAZ at 6 and 18 months of age. Our approach controls for LAZ at the time of each infection to allow for the temporality of the infection and growth to be resolved for improved causal inference.
We observed protective immunity within the GII genogroup, similar to work in Peru and Chile. Although there was a trend toward a decrease in the incidence of symptomatic disease following prior infection (HR, 0.76 [95% CI, .50–1.15]), the protection was not significant. Evidence suggested a dose-response in acquired immunity, as children with ≥2 prior infections exhibited greater protection to symptomatic episodes (33%) compared with children with only 1 prior infection (20%). However, these findings were not statistically significant. Across sites, there was no evidence of protective immunity conferred by GI infection. Importantly, 81% of all symptomatic infections occurred after 6 months of age. Given that peak incidence of infection and disease is occurring at 6–11 months, this work supports the recent finding by Shioda et al that immunization schedules completed by 6 months would be preferable to 12-month schedules [21]. Using the attributable fraction of 5.7% among children aged >6 months, and based on the estimate by Walker et al [22] of 1.731 billion episodes of diarrhea annually, we estimate that an effective vaccine could avert approximately 99 million episodes of diarrhea among children aged 6–24 months. However, the attributable fraction risks underestimating this figure in high-transmission settings [23], and metrics assigning diarrheal disease to a single pathogen should be treated with caution when estimating the impacts of specific interventions [2].
We report a high prevalence of coinfection, illustrating the challenge of attributing diarrhea to a single pathogen in studies employing broad diagnostic platforms with sensitive detection of multiple enteropathogens. However, there was no evidence of interaction between pathogens in determining symptoms or disease severity; therefore, this should not discredit our findings.
This work is an important contribution to the limited number of studies longitudinally describing norovirus infection and disease, particularly outside of clinical trials or hospital settings. To our knowledge, the data presented here comprise the most extensive multisite description of norovirus epidemiology among children in countries with high diarrheal morbidity and mortality. Incidence, asymptomatic carriage, and disease presentation exhibited vast heterogeneity by country and region, underscoring the need for more community-based epidemiologic studies in LMICs to inform appropriate prevention and control. Nonetheless, this work demonstrates the clear burden of norovirus in early childhood, particularly GII, and evidence for natural immunity, thus providing strong impetus for ongoing vaccine development.
Notes
Acknowledgments. The authors thank the staff and participants of the Malnutrition and the Consequences for Child Health and Development (MAL-ED) Network for their important contributions, and Larry Moulton for critical review of the manuscript.
Financial support. The Etiology, Risk Factors, and Interactions of Enteric Infections and MAL-ED Project is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health (NIH), and the Fogarty International Center, NIH.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
The MAL-ED Investigators are as follows (numbers correspond to the institutional affiliations listed at the end of the Appendix): Maribel Paredes Olotegui,1 Cesar Banda Chavez,1 Dixner Rengifo Trigoso,1 Julian Torres Flores,1 Angel Orbe Vasquez,1 Silvia Rengifo Pinedo,1 Angel Mendez Acosta,1 Imran Ahmed,2 Didar Alam,2 Asad Ali,2 Zulfiqar A. Bhutta,2 Shahida Qureshi,2 Muneera Rasheed,2 Sajid Soofi,2 Ali Turab,2 Aisha K Yousafzai,2 Anita K. M. Zaidi,2 Ladaporn Bodhidatta,3 Carl J. Mason,3 Sudhir Babji,4 Anuradha Bose,4 M. Steffi Jennifer,4 Sushil John,4 Gagandeep Kang,4 Shiny Kaki,4 Beena Koshy,4 Jayaprakash Muliyil,4 Mohan Venkata Raghava,4 Anup Ramachandran,4 Anuradha Rose,4 Srujan L. Sharma,4 Rahul J. Thomas,4 William Pan,5,6 Ramya Ambikapathi,6 Danny Carreon,6 Vivek Charu,6 Leyfou Dabo,6 Viyada Doan,6 Jhanelle Graham,6 Christel Hoest,6 Stacey Knobler,6 Dennis Lang,6,7 Benjamin McCormick,6 Monica McGrath,6 Mark Miller,6 Archana Mohale,6 Gaurvika Nayyar,6 Stephanie Psaki,6 Zeba Rasmussen,6 Stephanie A. Richard,6 Jessica C Seidman,6 Vivian Wang,6 Rebecca Blank,7 Michael Gottlieb,7 Karen H. Tountas,7 Caroline Amour,8 Estomih Mduma,8 Buliga Mujaga Swema,8 Ladislaus Yarrot,8 Rosemary Nshama,8 Tahmeed Ahmed,9 A. M. Shamsir Ahmed,9 Fahmida Tofail,9 Rashidul Haque,9 Iqbal Hossain,9 Munirul Islam,9 Mustafa Mahfuz,9 Dinesh Mondal,9 Ram Krishna Chandyo,10 Prakash Sunder Shrestha,10 Rita Shrestha,10 Manjeswori Ulak,10 Robert Black,11 Laura Caulfield,11 William Checkley,11,6 Ping Chen,11,6 Margaret Kosek,11 Gwenyth Lee,11 Pablo Peñataro Yori,11 Laura E. Murray-Kolb,12 Barbara Schaefer,12,6 Laura Pendergast,13 Cláudia Abreu,14 Alexandre Havt,14 Hilda Costa,14 Alessandra Di Moura,14 Jose Quirino Filho,14,6 Álvaro Leite,14 Aldo Lima,14 Noélia Lima,14 Ila Lima,14 Bruna Maciel,14 Milena Moraes,14 Francisco Mota,14 Reinaldo Oriá,14 Josiane Quetz,14 Alberto Soares,14 Crystal L Patil,16 Pascal Bessong,17 Cloupas Mahopo,17 Angelina Maphula,17 Cebisa Nesamvuni,17 Emanuel Nyathi,17 Amidou Samie,17 Leah Barrett,18 Jean Gratz,18 Richard Guerrant,18 Eric Houpt,18 William Petri,18 Rebecca Scharf,18 James Platts-Mills,18 Binob Shrestha,19 Sanjaya Kumar Shrestha,19 Tor Strand,19,15 Erling Svensen20,8.
Institutions: 1A.B. PRISMA, Iquitos, Peru; 2Aga Khan University, Naushahro Feroze, Pakistan; 3Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand; 4Christian Medical College, Vellore, India; 5Duke University, Durham, North Carolina; 6Fogarty International Center/National Institutes of Health, Bethesda, Maryland; 7Foundation for the National Institutes of Health, Bethesda, Maryland; 8Haydom Lutheran Hospital, Haydom, Tanzania; 9icddr,b, Dhaka, Bangladesh; 10Institute of Medicine, Tribhuvan University, Kathmandu, Nepal; 11Johns Hopkins University, Baltimore, Maryland; 12Pennsylvania State University, University Park; 13Temple University, Philadelphia, Pennsylvania; 14Universidade Federal do Ceará, Fortaleza, Brazil; 15University of Bergen, Norway; 16University of Illinois at Chicago; 17University of Venda, Thohoyandou, South Africa; 18University of Virginia, Charlottesville; 19Walter Reed/Armed Forces Research Institute of Medical Sciences Research Unit, Kathmandu, Nepal; and 20Haukeland University Hospital, Bergen, Norway.
Contributor Information
Collaborators: for the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) Network Investigators, Maribel Paredes Olotegui, Cesar Banda Chavez, Dixner Rengifo Trigoso, Julian Torres Flores, Angel Orbe Vasquez, Silvia Rengifo Pinedo, Angel Mendez Acosta, Imran Ahmed, Didar Alam, Asad Ali, Zulfiqar A. Bhutta, Shahida Qureshi, Muneera Rasheed, Sajid Soofi, Ali Turab, Aisha K Yousafzai, Anita K. M. Zaidi, Ladaporn Bodhidatta, Carl J. Mason, Sudhir Babji, Anuradha Bose, M. Steffi Jennifer, Sushil John, Gagandeep Kang, Shiny Kaki, Beena Koshy, Jayaprakash Muliyil, Mohan Venkata Raghava, Anup Ramachandran, Anuradha Rose, Srujan L. Sharma, Rahul J. Thomas, William Pan, Ramya Ambikapathi, Danny Carreon, Vivek Charu, Leyfou Dabo, Viyada Doan, Jhanelle Graham, Christel Hoest, Stacey Knobler, Dennis Lang, Benjamin McCormick, Monica McGrath, Mark Miller, Archana Mohale, Gaurvika Nayyar, Stephanie Psaki, Zeba Rasmussen, Stephanie A. Richard, Jessica C Seidman, Vivian Wang, Rebecca Blank, Michael Gottlieb, Karen H. Tountas, Caroline Amour, Estomih Mduma, Buliga Mujaga Swema, Ladislaus Yarrot, Rosemary Nshama, Tahmeed Ahmed, A. M. Shamsir Ahmed, Fahmida Tofail, Rashidul Haque, Iqbal Hossain, Munirul Islam, Mustafa Mahfuz, Dinesh Mondal, Ram Krishna Chandyo, Prakash Sunder Shrestha, Rita Shrestha, Manjeswori Ulak, Robert Black, Laura Caulfield, William Checkley, Ping Chen, Margaret Kosek, Gwenyth Lee, Pablo Peñataro Yori, Laura E. Murray-Kolb, Barbara Schaefer, Laura Pendergast, Cláudia Abreu, Alexandre Havt, Hilda Costa, Alessandra Di Moura, Jose Quirino Filho, Álvaro Leite, Aldo Lima, Noélia Lima, Ila Lima, Bruna Maciel, Milena Moraes, Francisco Mota, Reinaldo Oriá, Josiane Quetz, Alberto Soares, Crystal L Patil, Pascal Bessong, Cloupas Mahopo, Angelina Maphula, Cebisa Nesamvuni, Emanuel Nyathi, Amidou Samie, Leah Barrett, Jean Gratz, Richard Guerrant, Eric Houpt, William Petri, Rebecca Scharf, James Platts-Mills, Binob Shrestha, Sanjaya Kumar Shrestha, Tor Strand, and Erling Svensen
References
- 1.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 2008; 14:1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Babji S, Bodhidatta L et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Heal 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Johnson H, Cousens S. Global, regional, and national causes of child mortality in 2000–2010: an updated systematic analysis. Lancet 2012; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 4.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med 2009; 361:1776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, Goel-Apaza S, Espetia S et al. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis 2014; 58:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Ryan ML, Lucero Y, Prado V et al. Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr Infect Dis J 2009; 10:879–84. [DOI] [PubMed] [Google Scholar]
- 7.Lopman B, Trivedi T, Vicuna Y et al. Norovirus infection and disease in an Ecuadorian birth cohort: association of certain norovirus genotypes with host FUT2 secretor status. J Infect Dis 2015; 211:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atmar RL, Bernstein DI, Harro CD et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopman B, Kang G. Editorial commentary: in praise of birth cohorts: norovirus infection, disease, and immunity. Clin Infect Dis 2014; 58:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MAL-ED Network Investigators. The MAL-ED project: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, growth, cognitive development and immune responses in infants/children in resource poor environments. Clin Infect Dis 2014; 59:S193–206. [DOI] [PubMed] [Google Scholar]
- 11.Houpt E, Gratz J, Kosek M et al. Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis 2014; 59:S225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization, Multicentre Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 2006; 450:76–85. [DOI] [PubMed] [Google Scholar]
- 13.Lee G, Peñataro Yori P, Paredes Olortegui M et al. An instrument for the assessment of diarrhoeal severity based on a longitudinal community-based study. BMJ Open 2014; 4:e004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackwelder WC, Biswas K, Wu Y et al. Statistical methods in the Global Enteric Multicenter Study (GEMS). Clin Infect Dis 2012; 55:S246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 1985; 122:904–14. [DOI] [PubMed] [Google Scholar]
- 16.Lindesmith L, Moe C, Marionneau S et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med 2003; 9:548–53. [DOI] [PubMed] [Google Scholar]
- 17.Thorven M, Grahn A, Hedlund K-O et al. A homozygous nonsense mutation (428G→A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J Virol 2005; 79:15351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.My PVT, Thompson CN, Phuc HL et al. Endemic norovirus infections in children, Ho Chi Minh City, Vietnam, 2009–2010. Emerg Infect Dis 2013; 19:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DWG. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin Infect Dis 2004; 39:318–24. [DOI] [PubMed] [Google Scholar]
- 20.Kotloff KL, Blackwelder WC, Nasrin D et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis 2012; 55:S232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shioda K, Kambhampati A, Hall AJ, Lopman BA. Global age distribution of pediatric norovirus cases. Vaccine 2015:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker CL, Rudan I, Liu L et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopman B, Simmons K, Gambhir M, Vinjé J, Parashar U. Epidemiologic implications of asymptomatic reinfection: a mathematical modeling study of norovirus. Am J Epidemiol 2014; 179:507–12. [DOI] [PubMed] [Google Scholar]