Delayed appropriate antibiotic therapy for enterococcal bacteremia >48.1 hours was independently associated with increased mortality. Vancomycin resistance strongly predicted delayed therapy, underscoring the importance of rapid diagnostic testing in optimizing the therapy of enterococcal bacteremia.
Keywords: appropriate antibiotic therapy, vancomycin-resistant enterococci, Enterococcus faecium, Enterococcus faecalis, bacteremia
Abstract
Background. With increasing prevalence of vancomycin-resistant enterococci (VRE), appropriate antibiotic therapy for enterococcal bloodstream infections (EBSI) can be delayed. Data regarding the impact of delayed therapy on EBSI outcomes are conflicting, and the time delay most strongly associated with poor outcomes has not been defined.
Methods. This was a single-center, retrospective cohort study of adult, nonneutropenic patients with hospital-onset EBSI from 2010 to 2014. Classification and regression tree (CART) analysis was used to determine the delay in appropriate therapy most predictive of 30-day mortality. Appropriate therapy was defined as antibiotic therapy to which the enterococci and copathogen, where applicable, were susceptible. Outcomes and clinical characteristics were compared between patients receiving early or delayed therapy, defined by CART timepoint. Poisson regression was employed to determine the independent association of delayed therapy on 30-day mortality and predictors of delayed therapy.
Results. Overall, 190 patients were included. A breakpoint in time to appropriate therapy was identified at 48.1 hours, where 30-day mortality was substantially increased (14.6% vs 45.3%; P < .001). Patients receiving appropriate therapy after 48.1 hours also experienced higher in-hospital mortality and longer EBSI duration. After adjustment for severity of illness and comorbidity, delayed therapy ≥48.1 hours was associated with a 3-fold increase in 30-day mortality (risk ratio, 3.16 [95% confidence interval, 1.96–5.09]). Vancomycin resistance was the only independent predictor of delayed therapy.
Conclusions. In patients with hospital-onset EBSI, receipt of appropriate therapy within the first 48 hours was associated with reduced mortality, underscoring the potential role of rapid diagnostic testing for early identification of VRE.
Once regarded as an innocuous constituent of human gastrointestinal flora, enterococci have emerged as a significant clinical and epidemiologic threat [1, 2]. Enterococci are among the most commonly isolated nosocomial pathogens in the United States, responsible for serious and complicated infections including urinary tract infection, intra-abdominal infection, bloodstream infection (BSI), and infective endocarditis [2–5]. Collectively, Enterococcus faecalis and Enterococcus faecium represent the second leading cause of central line–associated BSI [2]. Enterococcal bloodstream infections (EBSIs) are associated with significant morbidity and mortality, with mortality rates estimated between 20% and 50% [6–9]. Treatment of EBSI has become increasingly difficult due to emergence of multidrug-resistant enterococci [1]. Vancomycin-resistant enterococci (VRE) prevalence has rapidly increased in the United States over the past decade, especially among E. faecium with 80%–95% of isolates vancomycin-resistant [10–12]. Coupled with rising VRE prevalence, resistance to the few viable VRE BSI treatment options, including linezolid and daptomycin, has emerged [13, 14].
The rise in VRE is troubling considering vancomycin resistance has been shown to independently predict mortality among patients with EBSI [7, 8, 15]. While patients with VRE BSI typically have a higher degree of comorbidity and disease severity relative to those with vancomycin-susceptible infections, delayed appropriate antibiotic therapy is thought to contribute to their deleterious outcomes [8]. However, data examining the outcomes associated with delayed antibiotic therapy in EBSI are conflicting, raising questions regarding the true impact of delayed therapy in this population [16–19]. Differences in observed results could be explained by limitations of previous studies, which were not specifically designed to examine time to appropriate therapy and outcomes in EBSI. There are also no data describing the delay in appropriate therapy most strongly associated with poor outcomes, determining a critical time period in which EBSI patients should receive appropriate therapy [16, 18]. This analysis sought to determine the impact of delayed appropriate antibiotic therapy on outcomes of patients with hospital-onset EBSI and quantify the time to appropriate therapy most strongly associated with poor outcomes.
MATERIALS AND METHODS
Study Design and Population
This was a retrospective, observational cohort study of adult patients with hospital-onset EBSI from 2010 to 2014 at the Detroit Medical Center (DMC). Patients aged ≥18 years with 1 or more positive blood cultures for E. faecalis or E. faecium meeting Centers for Disease Control and Prevention (CDC) criteria for BSI [20] were eligible for inclusion. Patients with community-onset EBSI (index bloodstream isolate collected <48 hours from hospital admission [20]) and those with neutropenia (absolute neutrophil count <500 cells/µL) were excluded. Only initial EBSI episodes from patients with multiple episodes during the study period were included [21]. This study was approved by the institutional review board at Wayne State University, and a waiver of informed consent was granted.
Patient Data Elements and Collection
Enterococcal bloodstream infections for inclusion were identified through a list of all positive enterococcal blood cultures at DMC during the study period. Patient data were extracted from the medical record by trained reviewers using a structured data collection form within the REDCap (Research Electronic Data Capture, Vanderbilt University) data capture tool hosted at Wayne State University [22]. Data elements included demographics, past medical history, comorbid conditions, prior hospitalization (1 year), prior systemic antibiotics (90 days), surgery, chemotherapy/radiation therapy, or receipt of immunosuppressive medications (each 30 days). The degree of comorbidity was quantified using the Charlson comorbidity index [23]. Severity of illness was quantified using the Pitt bacteremia score calculated using the worst clinical parameters in the 48 hours preceding index culture [24, 25]. Source of EBSI was based on treating physicians' notes and available clinical/diagnostic data. Microbiologic data including antibiotic susceptibilities by Microscan and/or Etest were collected from the medical record. Polymicrobial bacteremia was defined as isolation of an additional pathogen satisfying CDC criteria for BSI [20] within 24 hours of index enterococcal isolate. Treatment data including infectious diseases (ID) consult, pursuit of source control, and antimicrobial treatment including associated laboratory data were documented. Empiric antibiotic therapy was defined as therapy employed prior to release of antibiotic susceptibility results, whereas definitive therapy was defined as therapy given after release of antibiotic susceptibility results [21].
Outcomes
The primary outcome was 30-day mortality, defined as mortality from any cause within 30 days of index enterococcal blood culture. Secondary outcomes included all-cause in-hospital mortality, EBSI duration, and hospital length of stay after EBSI onset.
Data Analysis
In the primary analysis, outcomes were compared between patients classified as having received early or delayed appropriate antibiotic therapy for EBSI. Appropriate therapy was defined as an antibiotic regimen to which the index enterococcal isolate and copathogen (when applicable) were susceptible in vitro based on Clinical and Laboratory Standards Institute guidelines [21, 26]. Time to appropriate therapy was calculated in hours between the time the index culture was drawn and receipt of the first dose of appropriate therapy. Classification and regression tree (CART) analysis [27] was used to discover a breakpoint in time to appropriate therapy, modeled continuously in hours, where the incidence of 30-day morality was most disproportionate. The remaining outcome measures were then compared between subjects who received appropriate therapy before (early appropriate therapy) and after (delayed appropriate therapy) this breakpoint. The predictive performance of time to appropriate therapy, modeled continuously in hours, for 30-day mortality was assessed through receiver operating characteristic (ROC) curves. Performance of CART-derived and other a priori–defined time thresholds (24, 36, 48, 60, and 72 hours) was also assessed through ROC curves. The relationship between time to appropriate therapy, modeled ordinally in days, and 30-day mortality was also assessed through the χ2 test and χ2 test for linear trend. Secondary subgroup analyses were conducted to examine the impact of disease severity, by Pitt bacteremia score and intensive care unit (ICU) level of care at index culture, and organism phenotype, based on enterococcal species and vancomycin susceptibility, on the relationship between delayed therapy and 30-day mortality using stratification and CART analysis. Secondary analyses to determine risk factors for delayed appropriate therapy were also conducted.
Bivariate comparisons of outcomes and clinical characteristics between patients who received early and delayed therapy as well as clinical characteristics between 30-day survivors and nonsurvivors were conducted using the Pearson χ2 test or Fisher exact test and Student t test or Mann–Whitney U test. Following bivariate analyses, multivariable analyses were conducted using Poisson regression with robust variance estimation to quantify the independent association between delayed therapy and 30-day mortality as well as clinical factors associated with delayed therapy. Poisson regression was selected in favor of logistic regression to generate accurate estimates of adjusted risk ratios given that the dependent variables of interest (30-day mortality and delayed therapy) had estimated incidences >10% [28]. Variables associated with the outcome/exposure of interest at a P value <.2 in bivariate analyses with clinical relevance/biologic plausibility were entered into the Poisson models as potential confounders and removed in a backward, stepwise fashion, retained if the confidence interval (CI) of the risk ratio (RR) remained significant. Model fit was assessed through the χ2 test of deviance; only models with a nonsignificant result were considered adequate. Factors associated with time to appropriate therapy were also assessed through Cox proportional hazards regression using a backward, stepwise approach. All calculations were performed using IBM SPSS Statistics software, version 22.0 (IBM SPSS, Armonk, New York).
RESULTS
Five hundred eighty-one E. faecalis or E. faecium EBSI episodes occurred in adult patients at the DMC during the study period. One hundred ninety patients were included; 355 were excluded for community-onset EBSI, 19 for neutropenia, and 17 as repeat EBSI episodes during the study period. A complete description of demographic and clinical characteristics is shown in Supplementary Table 1. Patients were predominantly African American (77.4%), with a mean age of 63.4 (SD, 16.4) years; 54.2% were female. The median Charlson comorbidity index and Pitt bacteremia score were 7 (interquartile range [IQR], 5–8) and 3 (IQR, 3–5), respectively. Enterococcus faecium accounted for 89 (46.8%) of the 190 index enterococcal isolates. A vancomycin-resistant phenotype was present in 119 (62.6%) of these enterococcal isolates. Of 124 enterococcal isolates tested against daptomycin and 110 tested against linezolid, 9 (7.3%) and 1 (0.9%) were daptomycin-nonsusceptible and linezolid-intermediate, respectively. Copathogens isolated in the 61/190 (32.1) polymicrobial BSIs are listed in Supplementary Table 2. The most common copathogens were Staphylococcus aureus (5.8%), coagulase-negative staphylococci (5.3%), and Klebsiella species (4.7%). Vancomycin was the empiric gram-positive active agent in most instances (76.3%). The most common definitive therapies were linezolid (32.1%), daptomycin (30.5%), and ampicillin (25.8%); 10.5% of patients received some form of combination therapy. The median time to appropriate therapy was 31.6 (IQR, 20.3–51.2) hours. Thirty-day mortality occurred in 44 of the 190 (23.2%) subjects.
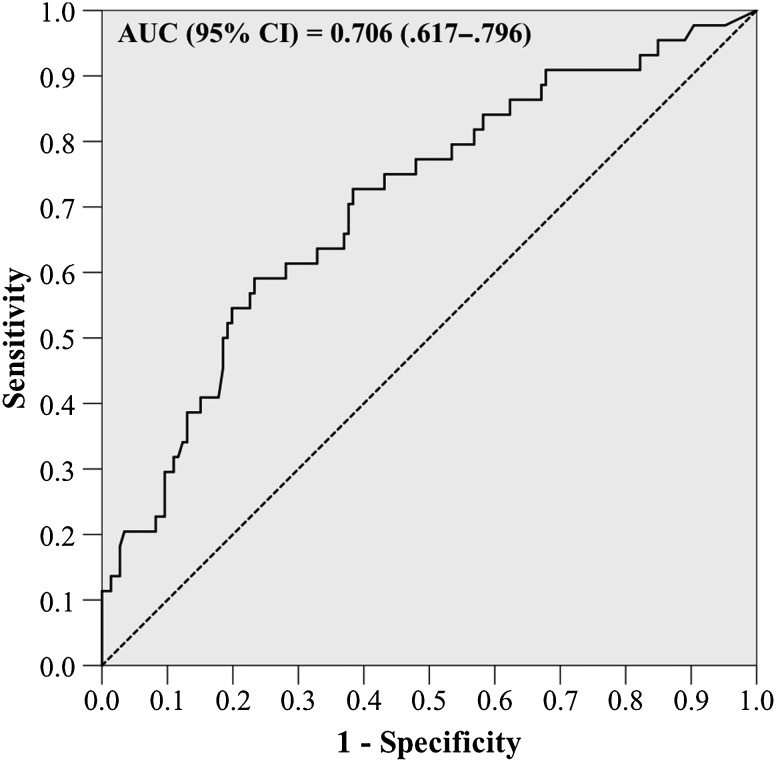
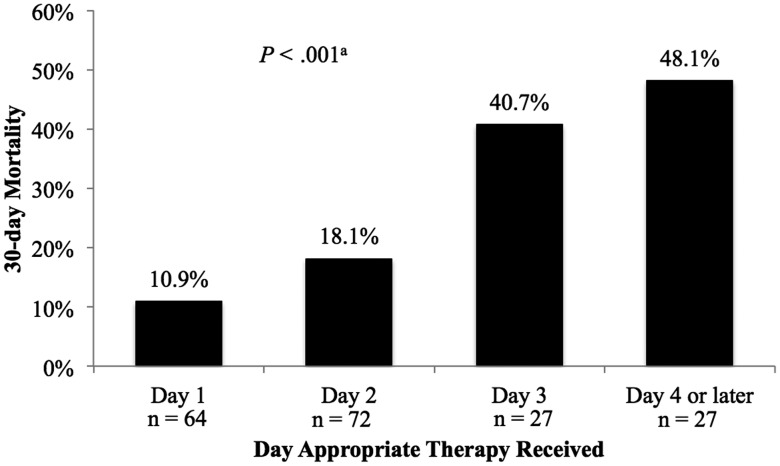
In the primary CART analysis, a breakpoint in time to appropriate therapy was discovered at 48.1 hours. The incidence of 30-day mortality among patients receiving appropriate therapy prior to 48.1 hours was 14.6% compared with 45.3% among patients receiving appropriate therapy after 48.1 hours (P < .001). In ROC curve analysis, time to appropriate therapy significantly predicted 30-day mortality, with fair performance (Figure 1). The CART-derived timepoint had the best performance (sensitivity, 54.5%; specificity 80.1%; area under the curve, 0.673 [95% CI, .577–.770]), although the 24-, 36-, and 60-hour thresholds also significantly predicted 30-day mortality (Supplementary Table 3). Patients who received delayed therapy also had a higher incidence of in-hospital mortality (35.8% vs 13.1%; P < .001) and longer median EBSI duration (4 [IQR, 2–5.5] vs 3 [IQR, 2–4] days; P = .01). No difference in median hospital length of stay after EBSI onset between delayed and early therapy was observed (13 [IQR, 8–20] vs 11 [IQR, 8–17.5] days; P = .61). When 30-day morality was examined by day appropriate therapy was received, a significant relationship was noted in the χ2 test and χ2 test for linear trend (P < .001 for both). The proportion of patients experiencing 30-day mortality increased each day appropriate therapy was delayed (Figure 2).
Figure 1.
Receiver operating characteristic curve of time to appropriate therapy for prediction of 30-day mortality. Abbreviations: AUC, area under the curve; CI, confidence interval.
Figure 2.
Relationship between day appropriate therapy was received and 30-day mortality. aP value for χ2 and χ2 test for linear trend.
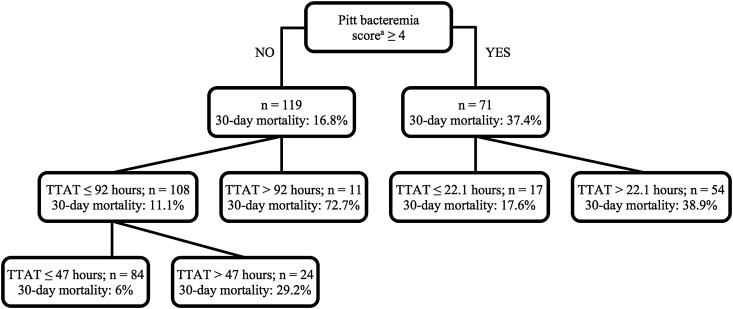
The results of the secondary subgroup analysis are displayed in Supplementary Table 4. When stratified by ICU level of care, enterococcal species, and vancomycin susceptibility, 30-day mortality was consistently higher among patients receiving delayed appropriate therapy. This was also true in the subgroup of patients with a Pitt bacteremia score <4. In contrast, no difference in 30-day mortality between early and delayed appropriate therapy was observed in the Pitt bacteremia score ≥4 subgroup. Given the lack of association between delayed therapy, defined by the CART-derived timepoint, and 30-day mortality in patients with a Pitt bacteremia score ≥4, additional CART analysis was conducted to explore for Pitt bacteremia score–specific breakpoints (Figure 3). After identifying 11 outliers in the Pitt bacteremia score <4 subgroup with a time to appropriate therapy >92 hours, where 30-day mortality was 72.3%, a breakpoint of 47 hours was discovered in the remaining 108 patients in this subgroup, after which 30-day mortality was significantly higher (29.2% vs 6%; P = .004). In patients with a Pitt bacteremia score ≥4, a breakpoint was found at 22.1 hours, after which 30-day mortality was numerically higher (38.9% vs 17.6%; P = .145).
Figure 3.
Classification and regression tree analysis results for 30-day mortality stratified by Pitt bacteremia score. aWorst score in 48 hours preceding index culture. Abbreviation: TTAT, time to appropriate therapy.
Bivariate comparisons between 30-day survivors and nonsurvivors are listed in Table 1. Delayed appropriate therapy along with age, presence of prosthetic device/hardware, chronic hemodialysis, chemotherapy/radiation therapy in the past 30 days, malignancy, mechanical ventilation at index culture, Charlson comorbidity index >4, unknown EBSI source, Pitt bacteremia score ≥4, pursuit of source control, and ampicillin-resistant phenotype were entered into the Poisson regression model. The results of the Poisson regression analysis for 30-day mortality are shown in Table 2. After adjusting for confounding variables, the relationship between delayed appropriate therapy and 30-day mortality persisted (RR, 3.16 [95% CI, 1.96–5.09]). Charlson comorbidity index >4, presence of prosthetic device/hardware, and Pitt bacteremia score ≥4 also remained in the final model as predictors of 30-day mortality.
Table 1.
Bivariate Comparisons of Baseline Demographics, Clinical Characteristics, and Outcomes Between 30-Day Survivors and Nonsurvivors and Patients Receiving Early and Delayed Therapy
| Characteristic | 30-Day Mortality |
P Value | Delayed Therapy |
P Value | ||
|---|---|---|---|---|---|---|
| No (n = 146) | Yes (n = 44) | No (n = 137) | Yes (n = 53) | |||
| Demographics | ||||||
| Age, y, mean (SD) | 62.4 (17.1) | 66.6 (13.4) | .14 | 62.9 (17.1) | 64.6 (14.5) | .54 |
| Female sex | 79 (54.1) | 24 (54.5) | .96 | 75 (54.7) | 28 (52.8) | .81 |
| Race/ethnicity | .59 | .60 | ||||
| African American | 113 (77.4) | 34 (77.3) | … | 105 (76.6) | 42 (79.9) | … |
| White | 20 (13.7) | 5 (11.4) | … | 17 (12.4) | 8 (15.1) | … |
| Hispanic/Latino | 3 (2.1) | 0 | … | 2 (1.5) | 1 (1.9) | … |
| Other | 10 (6.8) | 5 (11.4) | … | 13 (9.5) | 2 (3.8) | … |
| Comorbidities/past medical history | ||||||
| Prior hospitalization (1 y) | 108 (74.0) | 32 (72.7) | .87 | 95 (69.3) | 45 (84.9) | .03 |
| Prior surgery (30 d) | 55 (37.7) | 12 (27.3) | .21 | 49 (35.8) | 18 (34.0) | .82 |
| VRE infection (1 y) | 5 (3.4) | 2 (4.5) | .66 | 4 (2.9) | 3 (5.7) | .40 |
| IVDU | 12 (8.2) | 1 (2.3) | .31 | 9 (6.6) | 4 (7.5) | .76 |
| Prosthetic device/hardware | 4 (2.7) | 5 (11.4) | .03 | 6 (4.4) | 3 (5.7) | .71 |
| Chronic kidney disease | 74 (50.7) | 27 (61.4) | .21 | 72 (52.6) | 29 (54.7) | .79 |
| Chronic hemodialysis | 46 (31.5) | 19 (43.2) | .15 | 49 (35.8) | 16 (30.2) | .47 |
| Liver disease | 24 (16.4) | 9 (20.5) | .54 | 21 (15.3) | 12 (22.6) | .23 |
| Diabetes mellitus | 65 (44.5) | 21 (47.7) | .71 | 64 (46.7) | 22 (41.5) | .52 |
| Cerebrovascular accident | 31 (21.2) | 11 (25.0) | .60 | 28 (20.4) | 14 (26.4) | .37 |
| Hemiplegia/paraplegia | 9 (6.2) | 2 (4.5) | 1 | 7 (5.1) | 4 (7.5) | .50 |
| HIV/AIDS | 8 (5.5) | 1 (2.3) | .69 | 8 (5.8) | 1 (1.9) | .45 |
| Immunosuppression (30 d) | 15 (10.3) | 7 (15.9) | .31 | 16 (11.7) | 6 (11.3) | .95 |
| Bone marrow transplant | 0 | 1 (2.3) | .23 | 0 | 1 (1.9) | .28 |
| Solid organ transplant | 0 | 1 (2.3) | .23 | 1 (0.7) | 0 | 1 |
| Chemotherapy/radiation therapy (30 d) | 11 (7.5) | 7 (15.9) | .10 | 12 (8.8) | 6 (11.3) | .59 |
| Urinary cathetera | 18 (12.3) | 10 (22.7) | .09 | 23 (16.8) | 5 (9.4) | .20 |
| Acute kidney injurya | 44 (30.1) | 16 (36.4) | .44 | 45 (32.8) | 15 (28.3) | .55 |
| Mechanical ventilationa | 21 (14.4) | 13 (29.5) | .02 | 26 (19.0) | 8 (15.1) | .53 |
| Decubitus ulcer | 19 (13.0) | 13 (29.5) | .02 | 15 (10.9) | 9 (17.0) | .26 |
| Malignancy | 21 (14.4) | 11 (25.0) | .10 | 23 (16.8) | 9 (17.0) | .98 |
| Receiving TPNa | 10 (6.8) | 2 (4.5) | .74 | 9 (6.6) | 3 (5.7) | 1 |
| Prior antibiotics (90 d) | 75 (51.4) | 29 (65.9) | .09 | 71 (51.8) | 33 (62.3) | .20 |
| Charlson comorbidity indexa, median (IQR) | 7 (4–9) | 7.5 (5–9) | .05 | 7 (5–8) | 7 (5–8) | .88 |
| EBSI characteristics | ||||||
| Hospital LOS pre-EBSI, d, median (IQR) | 10 (5–21.5) | 13.5 (4.3–28) | .22 | 10 (5–25) | 8 (3–22) | .22 |
| Primary source | .10 | .32 | ||||
| Intra-abdominal | 22 (15.1) | 8 (18.2) | .62 | 19 (13.9) | 11 (20.8) | … |
| Infective endocarditis | 4 (2.7) | 3 (6.8) | .20 | 4 (2.9) | 3 (5.7) | … |
| Intravenous catheter | 76 (52.1) | 18 (40.9) | .20 | 71 (51.8) | 23 (43.4) | … |
| Skin/soft tissue | 16 (11.0) | 2 (4.5) | .25 | 12 (8.8) | 6 (11.3) | … |
| Urinary tract | 16 (11.0) | 4 (9.1) | 1 | 17 (12.4) | 3 (5.7) | … |
| Unknown | 12 (8.2) | 8 (18.2) | .06 | 14 (10.2) | 6 (11.3) | … |
| Enterococcal species | ||||||
| Enterococcus faecium | 65 (44.5) | 24 (54.5) | .24 | 61 (44.5) | 28 (52.8) | .30 |
| Polymicrobial BSI | 50 (34.2) | 11 (25.0) | .25 | 44 (32.1) | 18 (32.1) | 1 |
| Intensive care unita | 47 (32.2) | 22 (50.0) | .03 | 48 (35.0) | 21 (39.6) | .56 |
| Pitt bacteremia scoreb, median (IQR) | 3 (3–4) | 4 (3–5) | .01 | 3 (3–5) | 3 (3–5) | .68 |
| Susceptibility phenotypes | ||||||
| Ampicillin-resistant | 63 (43.2) | 24 (54.5) | .18 | 59 (43.1) | 28 (52.8) | .23 |
| Vancomycin-resistant | 89 (61.0) | 30 (68.2) | .39 | 75 (54.7) | 44 (83.0) | <.001 |
| Daptomycin-nonsusceptible | 5 (11.1) | 4 (19.0) | .38 | 3 (2.2) | 6 (11.3) | .06 |
| Linezolid-intermediate | 1 (1.7) | 0 | 1 | 0 | 1 (1.9) | .28 |
| Treatment data | ||||||
| Antimicrobial therapy | ||||||
| Empiric | ||||||
| Vancomycin | 111 (76.0) | 30 (68.2) | .30 | 100 (73.0) | 41 (77.4) | .54 |
| Linezolid | 33 (33.6) | 7 (15.9) | .34 | 36 (26.3) | 4 (7.5) | .005 |
| Daptomycin | 19.9 (29) | 7 (15.9) | .56 | 29 (21.2) | 7 (13.2) | .20 |
| Ampicillin | 15 (10.3) | 6 (13.6) | .53 | 15 (10.9) | 6 (11.3) | .94 |
| Piperacillin/tazobactam | 7 (4.8) | 2 (4.5) | 1 | 7 (5.1) | 2 (3.8) | 1 |
| Other | 4 (2.7) | 3 (6.8) | .20 | 6 (4.4) | 1 (1.9) | .68 |
| Definitive | ||||||
| Vancomycin | 27 (18.5) | 5 (11.4) | .27 | … | … | … |
| Linezolid | 48 (32.9) | 13 (29.5) | .68 | … | … | … |
| Daptomycin | 42 (28.8) | 16 (36.4) | .34 | … | … | … |
| Ampicillin | 37 (25.3) | 12 (27.2) | .80 | … | … | … |
| Piperacillin/tazobactam | 10 (6.8) | 2 (4.5) | .74 | … | … | … |
| Other | 10 (6.8) | 3 (6.8) | 1 | … | … | … |
| Combination therapy | 13 (8.9) | 7 (15.9) | .18 | … | … | … |
| Vancomycin trough, mg/dL, mean (SD) | 19.0 (5.9) | 18.8 (7.0) | .89 | 18.8 (6.1) | 19.4 (6.3) | .70 |
| Daptomycin dose, mg/kg, median (IQR) | 9 (7–10) | 8 (6.9–9.9) | .46 | 9.1 (7–10) | 8.6 (6.9–9.5) | .33 |
| Time to appropriate therapy, h, mean (SD) | 33.1 (25.6) | 56.9 (37.4) | <.001 | … | … | … |
| Time to appropriate therapy, h, median (IQR) | 28.7 (14.6–44) | 50.7 (30.3–76.3) | <.001 | … | … | … |
| Other treatment information | ||||||
| Source control intervention pursued | 87 (59.6) | 18 (40.9) | .03 | 82 (59.9) | 23 (43.4) | .04 |
| ID consult within 24 h after index | 76 (52.1) | 22 (50.0) | .81 | 80 (58.4) | 18 (34.0) | .003 |
| Outcomes | ||||||
| Hospital LOS post-EBSI, d, median (IQR) | … | … | … | 11 (8–17.5) | 13 (8–20) | .61 |
| Duration EBSI, d, median (IQR) | 3 (2–4) | 3 (2–4) | .46 | 3 (2–4) | 4 (2–5.5) | .01 |
| 30-day mortality | … | … | … | 18 (13.1) | 19 (35.8) | <.001 |
| In-hospital mortality | … | … | … | 20 (14.6) | 24 (45.3) | <.001 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: BSI, bloodstream infection; EBSI, enterococcal bloodstream infection; HIV, human immunodeficiency virus; ID, infectious diseases; IQR, interquartile range; IVDU, intravenous drug use; LOS, length of stay; SD, standard deviation; TPN, total parenteral nutrition; VRE, vancomycin-resistant enterococci.
a At time of index culture.
b Worst score within 48 hours preceding index culture.
Table 2.
Results of Poisson Regression Analysis of Variables Associated With 30-Day Mortality and Delayed Appropriate Therapy
| Variable | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
|
| 30-Day Mortality | ||||
| Delayed appropriate therapy (≥48.1 h) | 3.10 (1.88–5.12) | <.001 | 3.16 (1.96–5.09) | <.001 |
| Charlson comorbidity index >4a | 3.10 (1.17–8.2) | .009 | 3.00 (1.16–7.80) | .024 |
| Prosthetic device or hardware | 2.58 (1.35–4.93) | .032 | 2.50 (1.19–5.24) | .016 |
| Pitt bacteremia score ≥4b | 2.01 (1.20–3.37) | .021 | 1.76 (1.09–2.84) | .021 |
| Age ≥54 y | 2.56 (1.07–6.12) | .018 | … | … |
| Unknown primary EBSI source | 1.89 (1.03–3.48) | .059 | … | … |
| Chronic hemodialysis | 1.46 (.87–2.45) | .152 | … | … |
| Chemotherapy/radiation therapy (30 d) | 1.81 (.95–3.45) | .096 | … | … |
| Mechanical ventilationa | 1.92 (1.13–3.27) | .021 | … | … |
| Malignancy | 1.65 (.93–2.90) | .099 | … | … |
| Ampicillin-resistant phenotype | 1.42 (.84–2.39) | .184 | … | … |
| Source control pursued | 0.56 (.33–.95) | .029 | … | … |
| Delayed Therapy | ||||
| Vancomycin-resistant phenotype | 2.92 (1.52–5.61) | <.001 | 3.44 (1.86–6.35) | <.001 |
| ID consult within 24 h following index culture | 0.48 (.30–.79) | .003 | 0.41 (.26–.64) | <.001 |
| Daptomycin-nonsusceptible phenotypec | 2.23 (1.22–4.11) | .055 | … | … |
| Prior hospitalization (1 y) | 2.01 (1.02–3.96) | .029 | … | … |
Abbreviations: CI, confidence interval; EBSI, enterococcal bloodstream infection; ID, infectious diseases; RR, risk ratio.
a At time of index culture.
b Worst score in 48 hours preceding index culture.
c Withheld from final model due to Hessian matrix singularity.
Poisson regression was also conducted to explore predictors of delayed appropriate therapy based on the bivariate analysis of early vs delayed therapy shown in Table 1. Hospitalization in the preceding year, vancomycin-resistant phenotype, daptomycin-nonsusceptible phenotype, and ID consult within 24 hours following index culture were candidates for model inclusion. Daptomycin nonsusceptibility was withheld from the final model, as it was perfectly collinear with vancomycin resistance. In the final model (Table 2), vancomycin-resistant phenotype was associated with increased risk of delayed therapy (RR, 3.44 [95% CI, 1.86–6.35]), whereas ID consult within 24 hours of index culture was associated with a lower risk for delayed therapy (RR, 0.41 [95% CI, .26–.64]). The results of the Cox proportional hazards model were consistent with the Poisson regression, with vancomycin resistance and ID consult within 24 hours of index culture significantly associated with time to appropriate therapy (Table 3).
Table 3.
Results of Cox Proportional Hazards Regression Model of Clinical Factors Associated With Time to Appropriate Therapy
| Clinical Factor | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Vancomycin-resistant phenotype | 2.915 (2.111–4.026) | <.001 |
| ID consult within 24 h following index culture | 0.593 (.436–.805) | .001 |
| Daptomycin-nonsusceptible phenotypesa | … | … |
| Prior hospitalization (1 y) | … | … |
Abbreviations: CI, confidence interval; ID, infectious diseases.
a Withheld from final model due to Hessian matrix singularity.
DISCUSSION
This study sought to examine the impact of delayed appropriate antibiotic therapy on the outcomes of patients with hospital-onset EBSI and determine the time to appropriate therapy most strongly associated with poor outcomes. The results suggest delayed appropriate therapy is associated with increased mortality and longer EBSI durations in this population. The relationship between delayed therapy and mortality persisted when accounting for confounding variables in Poisson regression, where delayed appropriate therapy ≥48.1 hours was associated with a 3-fold increased risk of 30-day mortality. The increased 30-day mortality among patients receiving delayed therapy was also observed in the majority of subgroups when patients were stratified by disease severity, measured by ICU level of care and Pitt bacteremia score, enterococcal species, and vancomycin susceptibility. The only subgroup where no difference was observed between those receiving appropriate therapy before and after 48.1 hours was patients with a Pitt bacteremia score ≥4. In this subgroup, a threshold of 22.1 hours was identified, although this finding was not statistically significant, potentially due to the limited size of this subgroup.
Although multiple previous studies have demonstrated increased adverse clinical outcomes in patients with EBSI who experience delays in receiving appropriate antibiotic therapy, this has not been consistently observed [16–19, 29]. There are 2 noteworthy differences between the current and previous studies to consider. This study excluded patients with community-onset EBSI to mitigate difficulty in estimating the time from EBSI onset to receipt of appropriate antibiotics in this population. Inclusion of community-onset EBSI introduces substantial misclassification bias to this crucial parameter. This study also excluded neutropenic patients to provide a more homogenous population and remove highly immunocompromised patients who historically experience poor outcomes despite early broad-spectrum antibiotic therapy [30, 31].
This is not the first analysis demonstrating the importance of appropriate antibiotic therapy in the first 2 days of BSI in optimizing outcomes. Receipt of appropriate therapy, defined as in vitro active therapy within 48 hours of EBSI onset, was strongly associated with 14-day survival in a study by Vergis and colleagues [18]. However, this study included community-onset EBSI and did not explore the relationship between time to appropriate therapy, modeled continuously, and outcome to determine the value of other time thresholds. Using a similar approach to the present study, Lodise and colleagues found comparable breakpoints in the time to appropriate therapy for S. aureus and Pseudomonas aeruginosa BSI of approximately 48 hours, after which the risk of mortality was increased [32, 33].
Not surprisingly, antibiotic-resistant phenotypes, primarily vancomycin resistance, were associated with delayed appropriate therapy. The majority of patients in this study received an empiric regimen consisting of vancomycin, frequently leading to a delay in appropriate therapy given the high prevalence of vancomycin resistance. These results have implications for EBSI treatment approaches and antimicrobial stewardship. While early appropriate therapy is the goal of any clinician, this study suggests that practitioners should strive to ensure optimal EBSI therapy within 48 hours of onset. This is problematic, considering that antibiotic sensitivity results from standard clinical microbiology laboratory procedures are not available for 48–72 hours [34]. This highlights the critical role of rapid diagnostic testing (RDT) technologies in optimization of BSI outcomes and antimicrobial stewardship. Rapid identification of Enterococcus species through peptide nucleic acid fluorescent in situ hybridization (PNA FISH) has been shown to significantly reduce time to appropriate therapy and mortality of patients with hospital-onset EBSI [35]. Moreover, use of a rapid molecular diagnostic microarray assay to detect enterococcal species and presence of vancomycin resistance genes (VanA, VanB) in blood cultures resulted in a mean time to appropriate therapy of approximately 24 hours, accompanied by a reduction in hospital cost [19]. Although RDT appears to be the optimal approach, settings without these capabilities must rely on preliminary culture results, such as Gram stain, along with local VRE prevalence and individual patient factors to guide empiric therapy.
There are multiple considerations to note when interpreting these results. First, this was a retrospective study and the findings are subject to the caveats associated with this design. The primary concern is missing data due to incomplete documentation in the medical record. To address this, we selected objective, easily measureable outcomes, such as all-cause mortality and EBSI duration. Although some patients experiencing 30-day mortality following hospital discharge may have been missed, these missed outcomes would have been nondifferentially distributed between the early and delayed therapy groups, thus having little impact on the findings. This was an urban, single-center analysis restricted to adult, nonneutropenic patients with hospital-onset EBSI in an attempt to maximize internal validity in addressing our study question. As isolates were not collected, we were unable to complete genotypic testing to control for institution-specific strains. The high VRE prevalence is not reflective of all settings in the United States or worldwide. However, considering that increased mortality was associated with delayed therapy in both vancomycin-resistant and -susceptible infections, it appears the high VRE prevalence did not substantially influence these findings. Nonetheless, it is uncertain whether these results are directly applicable to other settings and populations, and further study is required to confirm these findings.
In conclusion, delayed appropriate antibiotic therapy for patients with hospital-onset EBSI was associated with poor outcomes including increased 30-day and in-hospital mortality, as well as longer EBSI duration. Delayed therapy >48.1 hours was most strongly associated with 30-day mortality, with a 3-fold increase in 30-day mortality after adjustment for confounding variables. It is important to note that these findings do not necessarily indicate or support incorporation of anti-VRE therapy into all initial empiric regimens for patients with suspected sepsis. These findings highlight the importance of techniques for early identification of patients infected with or at risk for infection with resistant organisms, including RDT and individual patient risk assessment tools to optimize antibiotic therapy of BSIs.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Note
Potential conflicts of interest. S. L. D. has received grants from Allergan and Merck & Co; has served on advisory boards for Allergan, Merck & Co, and Melinta; and has acted as a consultant for Pfizer Pharmaceuticals. M. J. R. has received grants from, has consulted for, has been an advisory board member of, and is on the speaker's bureau for Allergan, Bayer, Cempra Inc, Merck & Co, The Medicines Company, Sunovian, and Theravance, and is supported in part by the National Institutes of Health (grant numbers R21 AI109266-01 and RO1 AI121400-01). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sievert DM, Ricks P, Edwards JR et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14. [DOI] [PubMed] [Google Scholar]
- 3.Osmon DR, Berbari EF, Berendt AR et al. . Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 4.Kallen AJ, Mu Y, Bulens S et al. . Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010; 304:641–8. [DOI] [PubMed] [Google Scholar]
- 5.Reyes K, Zervos M. Endocarditis caused by resistant Enterococcus: an overview. Curr Infect Dis Rep 2013; 15:320–8. [DOI] [PubMed] [Google Scholar]
- 6.Noskin GA, Peterson LR, Warren JR. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin Infect Dis 1995; 20:296–301. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, McKinnon PS, Tam VH, Rybak MJ. Clinical outcomes for patients with bacteremia caused by vancomycin-resistant enterococcus in a level 1 trauma center. Clin Infect Dis 2002; 34:922–9. [DOI] [PubMed] [Google Scholar]
- 8.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis 2005; 41:327–33. [DOI] [PubMed] [Google Scholar]
- 9.Billington EO, Phang SH, Gregson DB et al. . Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis 2014; 26:76–82. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey AM, Zilberberg MD. Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States, 2000–2006. Infect Control Hosp Epidemiol 2009; 30:184–6. [DOI] [PubMed] [Google Scholar]
- 11.Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray BE. Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. Clin Infect Dis 2012; 54(suppl 3):S233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidron AI, Edwards JR, Patel J et al. . NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008; 29:996–1011. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa K, Marchaim D, Pogue JM et al. . Predictors and outcomes of linezolid-resistant vancomycin-resistant Enterococcus: a case-case-control study. Am J Infect Control 2012; 40:e261–3. [DOI] [PubMed] [Google Scholar]
- 14.Judge T, Pogue JM, Marchaim D et al. . Epidemiology of vancomycin-resistant enterococci with reduced susceptibility to daptomycin. Infect Control Hosp Epidemiol 2012; 33:1250–4. [DOI] [PubMed] [Google Scholar]
- 15.Prematunge C, MacDougall C, Johnstone J et al. . VRE and VSE bacteremia outcomes in the era of effective VRE therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2016; 37:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheah AL, Spelman T, Liew D et al. . Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect 2013; 19:E181–9. [DOI] [PubMed] [Google Scholar]
- 17.Han SH, Chin BS, Lee HS et al. . Vancomycin-resistant enterococci bacteremia: risk factors for mortality and influence of antimicrobial therapy on clinical outcome. J Infect 2009; 58:182–90. [DOI] [PubMed] [Google Scholar]
- 18.Vergis EN, Hayden MK, Chow JW et al. . Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Ann Intern Med 2001; 135:484–92. [DOI] [PubMed] [Google Scholar]
- 19.Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J Clin Microbiol 2013; 51:4008–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 21.McGregor JC, Rich SE, Harris AD et al. . A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis 2007; 45:329–37. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 24.Korvick JA, Bryan CS, Farber B et al. . Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 1992; 36:2639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu CL, Chuang YC, Chang HC, Chen YC, Wang JT, Chang SC. Microbiological and clinical characteristics of vancomycin-resistant Enterococcus faecium bacteraemia in Taiwan: implication of sequence type for prognosis. J Antimicrob Chemother 2012; 67:2243–9. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. M100-S24: performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S20 Wayne, PA: CLSI, 2014. [Google Scholar]
- 27.Zhang H, Singer B. Recursive partitioning in the health sciences. New York: Springer, 1999. [Google Scholar]
- 28.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003; 157:940–3. [DOI] [PubMed] [Google Scholar]
- 29.Britt NS, Potter EM, Patel N, Steed ME. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of Veterans Affairs patients. Clin Infect Dis 2015; 61:871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavadze M, Rybicki L, Mossad S et al. . Risk factors for vancomycin-resistant Enterococcus bacteremia and its influence on survival after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2014; 49:1310–6. [DOI] [PubMed] [Google Scholar]
- 31.Dubberke ER, Hollands JM, Georgantopoulos P et al. . Vancomycin-resistant enterococcal bloodstream infections on a hematopoietic stem cell transplant unit: are the sick getting sicker? Bone Marrow Transplant 2006; 38:813–9. [DOI] [PubMed] [Google Scholar]
- 32.Lodise TP Jr, Patel N, Kwa A et al. . Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 2007; 51:3510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 2003; 36:1418–23. [DOI] [PubMed] [Google Scholar]
- 34.Bauer KA, Perez KK, Forrest GN, Goff DA. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis 2014; 59(suppl 3):S134–45. [DOI] [PubMed] [Google Scholar]
- 35.Forrest GN, Roghmann MC, Toombs LS et al. . Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother 2008; 52:3558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.