Interferon gamma secretion following incubation of patient blood with Borrelia antigens may be a useful marker for the laboratory diagnosis of early Lyme disease. Also, the response appears to be short lived, decreasing following antibiotic therapy.
Keywords: Borrelia burgdorferi, IFN-γ, Lyme disease, T cell, cytokine release assay
Abstract
Background. Current serodiagnostics for Lyme disease lack sensitivity during early disease, and cannot determine treatment response. We evaluated an assay based on QuantiFERON technology utilizing peptide antigens derived from Borrelia burgdorferi to stimulate interferon-gamma (IFN-γ) release as an alternative to serodiagnosis for the laboratory detection of Lyme disease.
Methods. Blood was obtained from patients with erythema migrans before (n = 29) and 2 months after (n = 27) antibiotic therapy. IFN-γ release was measured by enzyme-linked immunosorbent assay (ELISA) following overnight stimulation of whole blood with the peptide antigens, and compared to the results of standard serological assays (C6, ELISA, and Western blot).
Results. IFN-γ release was observed in pretreatment blood of 20 of 29 (69%) patients with Lyme disease. Following antibiotic treatment, IFN-γ was significantly reduced (P = .0002), and was detectable in only 4 of 20 (20%) initially positive patients. By contrast, anti-C6 antibodies were detected in pretreatment sera from 17 of 29 (59%) subjects, whereas only 5 of 29 (17%) patients had positive Western blot seroreactivity. Antibody responses persisted and expanded following treatment.
Conclusions. Our findings suggest that measurement of IFN-γ after incubating blood with Borrelia antigens could be useful in the laboratory diagnosis of early Lyme disease. Also, after antibiotic treatment, this response appears to be short lived.
The detection of antibodies to Borrelia burgdorferi is the standard method for the laboratory diagnosis of Lyme disease. Whether using lysates of whole Borrelia species, mixtures of recombinant proteins, or specific peptide antigens (eg, C6, PepC10) as assay targets [1–6], current serological assays rarely exceed a sensitivity of 50% in the positive detection of antibody in early disease. In addition, these antibody detection assays do not provide accurate information concerning treatment response, as antibody levels often remain elevated for years after the infection has been cleared [5–7]. New approaches are therefore needed to overcome the shortcomings of current serologic assays.
Antigen-specific T-cell activation is typically initiated shortly after infection. The expanding cell population secretes cytokines that, among other activities, drives the development of a mature antibody response [8–10]. Following the resolution of infection, the T-cell response wanes, which results in decreased cytokine secretion (or a shift away from proinflammatory cytokine secretion) and rapid contraction of the activated T-cell population. Therefore, a test that monitors T-cell activation might be a useful adjunct to traditional serologic testing methods, especially because the results may provide more accurate information on the presence of active infection compared to antibody responses.
Early attempts to evaluate the utility of monitoring T-cell responses in patients with Lyme disease yielded inconclusive results [11–14]. However, these studies relied prominently on T-cell proliferation as a measurement of T-cell activity, and this approach can suffer from a significant lack of specificity [13]. Furthermore, cytokines, including interferon gamma (IFN-γ), have been shown to inhibit T-cell proliferation under certain conditions [14], which would in turn reduce the usefulness of proliferation as a marker of infection. On the other hand, antigen-induced cytokine release may be a more reliable (albeit indirect) method to confirm T-cell activation [15, 16]. Forsberg et al [15] demonstrated that detection of IFN-γ provided diagnostically relevant information for confirming neurologic Lyme disease, while Jin et al [16] reported that activated T cells from patients with Lyme disease produced IFN-γ following ex vivo stimulation with decorin binding protein A, outer surface protein C (OspC), p100, or vmp-like sequence lipoprotein E.
Despite these findings, the clinical utility of a test that measures T-cell immunity during Lyme disease has not been fully evaluated. We evaluated an assay, based on QuantiFERON technology for Mycobacterium tuberculosis infection, to detect IFN-γ secretion in whole blood from patients with early Lyme disease after overnight incubation with a cocktail of peptides derived from the Borrelia antigens p66, decorin binding protein B (DbpB), OspC, and flagellin (41 kDa). We compared the results, before and after appropriate antibiotic therapy, to those obtained using a standard C6 enzyme-linked immunosorbent assay (ELISA) and commercially available Lyme disease Western blot.
MATERIALS AND METHODS
Peptide Antigens
Full-length sequences of p66, DbpB, flagellin, and OspC from the B31 strain of B. burgdorferi sensu stricto were aligned against other Borrelia species using the protein basic local alignment search tool (BLASTp) on the National Center Biotechnology Information website. These antigens were selected because they are expressed in early disease. OspC is a major B. burgdorferi surface in early Lyme disease [4], and the other proteins are expressed constitutively [4, 7, 8] by the spirochete. In addition, preliminary findings from patients with well-documented Lyme disease generated encouraging results. A proprietary combination of 33 peptides (ranging from 15 to 25 amino acids) was generated from regions of the 4 antigens that were highly conserved among Borrelia species (>80% identity) and also distinct from non-Borrelia proteins (<50% identity). The peptides were dissolved and adjusted to a concentration of 3.3 mg/mL prior to preparing a stock cocktail that contained 100 µg/mL of each peptide in phosphate-buffered saline (pH 7.2).
Patients and Controls
Whole blood was collected from adult (≥18 years old) patients with physician-diagnosed early Lyme disease characterized by a history of tick exposure and 1 or more (≥5-cm annular lesion) erythema migrans (EM) skin lesions. As a control, blood was also obtained within 1–2 weeks after completing antibiotic treatment from patients with Anaplasma phagocytophilum infection (anaplasmosis), confirmed by positive polymerase chain reaction blood test at the Gundersen Health System. The subjects with early Lyme disease or anaplasmosis were each treated with 100 mg of doxycycline twice daily for a minimum of 10 days [17, 18]. Healthy adult (≥18 years old) volunteers who resided in Lyme disease foci [19, 20] in Wisconsin (n = 18) or Maryland (n = 169) and had no prior history of Lyme disease were also included as controls. Informed consent was obtained prior to enrollment, and protocols were approved by the Gundersen Health System Institutional Review Board. Each Lyme disease subject completed a questionnaire that documented epidemiology, history of tick bite, and duration and severity of clinical manifestations. Each Lyme disease subject was also queried immediately prior to the convalescent blood draw to confirm satisfactory resolution of Lyme disease–related symptoms.
Collection and Processing of Blood
Blood samples from the subjects with EM, early Lyme disease, were collected at presentation (pretreatment) and approximately 60 days after completing a course of doxycycline therapy. Blood samples from the healthy volunteers or from patients with anaplasmosis served as controls. All blood samples were drawn into 2 lithium-heparin tubes; 1 tube was aliquoted into 1-mL volumes that were then transferred to separate sterile pyrogen-free tubes that were either empty (nil), preloaded with mitogen, or preloaded with a 10-µL volume of the peptide pool (1 µg/mL of each peptide final concentration). The tubes were then mixed thoroughly and incubated for 16–24 hours at 37°C. The remaining tube of blood was centrifuged (3000g for 15 minutes), and the separated plasma was stored at −80°C until tested.
Detection of IFN-γ
After incubation, the blood plasma from the blood collection tubes was harvested by centrifugation at 3000g for 15 minutes, and the amount of IFN-γ in the plasma was determined using the QuantiFERON ELISA (Qiagen, Germantown, Maryland) as instructed by the manufacturer. Results in the test samples were reported in international units (IU)/mL relative to a standard curve prepared by testing dilutions of a recombinant human IFN-γ standard. The nil sample was used to adjust for background reactivity caused by heterophile antibody or nonspecific IFN-γ; nil tube reactivity was subtracted from the IFN-γ value obtained using the subject's blood sample. The mitogen sample was used as a positive control to ensure the integrity of the lymphocytes in the blood sample.
C6 ELISA
The C6 peptide (CMKKDDQIAAAMVLRGMAKDGQFALK) was synthesized (>95% purity) with biotinylated amine terminal ends (GenScript, Inc, Piscataway, New Jersey), and the ELISA was performed as described previously [21]. Significant cutoff values were determined by testing archived serum samples from normal volunteers with no previous suspicion of Lyme disease (n = 30), sera that contained rheumatoid factor (n = 10) or antinuclear antibodies (n = 10), and sera from patients with Epstein-Barr virus (n = 10), cytomegalovirus (n = 10), syphilis (n = 10), Rocky Mountain spotted fever (n = 4), Borrelia hermsii relapsing fever (n = 14), or anaplasmosis (n = 13). The mean optical density (OD) value was calculated and OD values ≥2 standard deviations above the mean (immunoglobulin M [IgM] ≥ 0.532, immunoglobulin G [IgG] ≥ 1.206) were considered significant. The cutoff values resulted in detection of IgG antibodies in the serum from 1 patient with antinuclear antibodies and 1 with relapsing fever. Positive and normal serum controls were also included on each ELISA plate.
Western Blotting
Western blot reactivity was detected by a commercially available kit (Marblot, Trinity Biotech, Carlsbad, California) according to the manufacturer's recommendations. The criteria for serodiagnostic confirmation of Lyme disease recommended by the Centers for Disease Control and Prevention (CDC) [22] were used to determine positivity (for IgM: at least 2 of the protein bands 23, 39, or 41 detected; for IgG: at least 5 of the protein bands 18, 23, 28, 30, 39, 41, 45, 58, 66, or 93 detected).
Statistical Analyses
The Wilcoxon rank-sum test was used to examine the results, and P values ≤.05 were considered significant.
RESULTS
Study Participants
A total of 29 adult patients with physician-diagnosed Lyme disease (20 males, 9 females) were enrolled in the study (Table 1). Twenty-seven of 29 patients returned to provide a convalescent blood sample approximately 2 months after completing antibiotic therapy. The subjects had single (n = 23) or multiple (n = 6) EM lesions that met the CDC surveillance criterion (>5-cm red round lesion with or without central clearing), and 19 patients had removed an Ixodes scapularis (n = 12) or unidentified (n = 7) tick within the 3 weeks prior to developing the skin lesion. Two individuals had no additional clinical or constitutional complaints. The most common abnormalities in the remaining patients were headache (n = 23), stiff neck (n = 15), and myalgia (n = 15). Each patient completed a minimum of 10 days of doxycycline therapy, which resulted in a complete cessation of clinical and constitutional abnormalities; no patients reported abnormal symptoms during the convalescent visit.
Table 1.
Epidemiology and Clinical Signs/Symptoms of Patients With Early Lyme Disease
| Subject No. | Sex | Age, y | Documented (<3 wk) Tick Bite | Duration of Illness, d | Erythema Migrans Lesion(s) | Other Complaints/Abnormalities |
|---|---|---|---|---|---|---|
| 1 | M | 54 | Yes (deer tick) | 10 | Single (back) | Headache |
| 2 | F | 52 | No | 3 | Single (back) | Headache, fever, fatigue, myalgia |
| 3 | M | 52 | No | 3 | Single (thigh) | Headache, chills, fatigue, arthralgia |
| 4 | M | 24 | Yes (deer tick) | 4 | Single (thigh) | Headache, stiff neck, myalgia, arthralgia |
| 5 | M | 58 | Yes (deer tick) | 2 | Single (shoulder) | Arthralgia |
| 6 | F | 62 | Yes (deer tick) | 3 | Single (thigh) | None |
| 7 | M | 30 | Yes (deer tick) | 5 | Single (back) | Headache, fever, fatigue, stiff neck |
| 8 | M | 61 | Yes (deer tick) | 11 | Single (back) | Fatigue, myalgia |
| 9 | M | 49 | Yes (deer tick) | 7 | Single (trunk) | Headache, fever, stiff neck, fatigue, myalgia |
| 10 | F | 59 | Yes (deer tick) | 3 | Single (chest) | Headache, fever, stiff neck, myalgia |
| 11 | M | 51 | Yes (deer tick) | 7 | Single (hip) | Headache, stiff neck, myalgia |
| 12 | M | 69 | Yes (unidentified) | 4 | Single (thigh) | None |
| 13 | F | 41 | Yes (unidentified) | 3 | Single (back) | Headache, fever, fatigue, myalgia, arthralgia |
| 14 | M | 74 | Yes (deer tick) | 2 | Single (trunk) | Headache, fever, stiff neck, myalgia, arthralgia |
| 15 | F | 68 | No | 3 | Single (abdomen) | Lymphadenopathy |
| 16 | F | 68 | Yes (unidentified) | 7 | Single (abdomen) | Headache, fever, fatigue, myalgia, arthralgia |
| 17 | M | 71 | Yes (unidentified) | 5 | Single (arm) | Headache, stiff neck, fatigue, arthralgia |
| 18 | M | 71 | Yes (unidentified) | 6 | Single (back) | Headache, stiff neck, fatigue, arthralgia |
| 19 | F | 48 | Yes (unidentified) | 3 | Single (groin) | Headache, fever, fatigue |
| 20 | F | 69 | No | 5 | Single (shoulder) | Headache, stiff neck, fatigue |
| 21 | M | 62 | Yes (deer tick) | 20 | Single (back) | Headache, fever, stiff neck, arthralgia, myalgia |
| 22 | M | 73 | Yes (unidentified) | 7 | Single (shoulder) | Headache, fatigue |
| 23 | F | 35 | No | 14 | Single (back) | Headache, stiff neck, arthralgia, myalgia |
| 24 | M | 59 | No | 4 | Multiple (back) | Headache, fever, stiff neck, myalgia, arthralgia |
| 25 | M | 22 | No | 6 | Multiple (abdomen) | Headache, fever, stiff neck, myalgia, arthralgia |
| 26 | M | 31 | No | 19 | Multiple (back) | Headache, stiff neck, fatigue, myalgia |
| 27 | M | 59 | Yes (deer tick) | 6 | Multiple (trunk) | Fever, fatigue |
| 28 | M | 19 | No | 6 | Multiple (arm, torso) | Headache, fever, stiff neck, arthralgia, myalgia |
| 29 | M | 36 | No | 30 | Multiple (thigh, groin) | Headache, fever, stiff neck, arthralgia, myalgia |
Detection of Anti-C6 Antibodies and Western Blot Reactivity
Acute and convalescent serum samples were tested for anti-C6 antibodies and Western blot reactivity. Anti-C6 antibodies were detected in the pretreatment sera from a total of 18 (59%) patients (Table 2), with 16 (55%) patients positive for IgM antibodies and 9 (31%) patients positive for IgG antibodies. In the 2-month posttreatment sera, anti-C6 IgM remained elevated in 11 patients and decreased to an undetectable level in 4 previously positive patients, and 1 previously negative individual seroconverted to IgM positivity. IgG anti-C6 antibody levels increased in convalescence and were detected in 14 of 27 (51.9%) of posttreatment patients. In contrast, both IgM and IgG Western blot sensitivity was very poor in both pretreatment and convalescent sera (Table 3). Although IgM and IgG Western blots detected antibodies that bound to at least 1 protein in many subjects, only 5 (17%) subjects developed antibody responses of sufficient diversity to fulfill the CDC-recommended criteria [22] (minimum 2 IgM or 5 IgG Western blot bands) for serologic confirmation of Lyme disease prior to the antibiotic therapy, and only 7 (26%) patients could be considered positive at 2 months posttreatment. In the majority of patients, antibody levels detected by either the C6 ELISA or Western blotting remained elevated or expanded despite the appropriate antibiotic therapy and resolution of clinical abnormalities.
Table 2.
Detection of Anti-C6 Antibodies in Early Lyme Disease Sera Collected Pretreatment or 2 Months After Antibiotic Therapy
| Subject | Significanta (+/−) Levels of Anti-C6 Antibodies Detected: |
|||
|---|---|---|---|---|
| Pretreatment |
2 mo Posttreatment |
|||
| IgM | IgG | IgM | IgG | |
| 1 | + | − | − | − |
| 2 | − | − | − | − |
| 3 | + | + | + | + |
| 4 | + | + | + | + |
| 5 | − | − | − | − |
| 6 | − | − | − | − |
| 7 | + | − | − | + |
| 8 | + | − | + | + |
| 9 | + | + | + | + |
| 10 | − | − | + | − |
| 11 | + | − | − | − |
| 12 | − | − | − | − |
| 13 | + | − | − | + |
| 14 | − | − | − | + |
| 15 | − | + | − | + |
| 16 | − | − | − | − |
| 17 | − | − | − | − |
| 18 | − | − | NT | NT |
| 19 | + | − | + | + |
| 20 | + | + | + | + |
| 21 | + | + | + | + |
| 22 | − | − | − | + |
| 23 | + | − | + | − |
| 24 | − | − | − | − |
| 25 | + | − | + | − |
| 26 | + | + | + | + |
| 27 | − | − | − | − |
| 28 | + | + | NT | NT |
| 29 | + | + | + | + |
| Total, No. (%) | 16 (55) | 9 (31) | 12 (44) | 14 (52) |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; NT, not tested.
a Significant refers to ≥2 standard deviations (IgM > 0.532, IgG > 1.206) above mean optical density value of sera from normal volunteers (n = 30), sera with rheumatoid factor (n = 10) or antinuclear antibodies (n = 10), and sera from patients with Epstein-Barr virus (n = 10), cytomegalovirus (n = 10), syphilis (n = 10), Rocky Mountain spotted fever (n = 4), Borrelia hermsii relapsing fever (n = 14), or anaplasmosis (n = 13).
Table 3.
Confirmation of Early Lyme Disease by Western Blotting
| Subject | Western Blot Positivitya (+/−) |
|||
|---|---|---|---|---|
| Pretreatment |
2 mo Posttreatment |
|||
| IgM (Bands) | IgG (Bands) | IgM (Bands) | IgG (Bands) | |
| 1 | – | – | – | − (41) |
| 2 | – | – | – | − (41) |
| 3 | − (41) | − (41) | − (41) | − (41) |
| 4 | – | – (39, 41, 45) | – | − (39, 41) |
| 5 | – | – | – | − (41) |
| 6 | – | – | – | – |
| 7 | − (23) | − (41) | − (23) | − (23, 39, 41, 45) |
| 8 | – | − (41) | – | − (41, 58) |
| 9 | + (23, 41) | − (23, 41, 45) | + (23, 41) | − (23, 41, 45) |
| 10 | – | – | − (23) | − (23, 39, 41) |
| 11 | − (23) | – | + (23, 41) | – |
| 12 | – | − (41) | – | − (41) |
| 13 | – | – | – | – |
| 14 | – | – | − (23) | − (23, 41) |
| 15 | − (23) | − (41, 45, 58) | − (23) | + (18, 41, 45, 58, 66) |
| 16 | − (23) | – | − (23) | – |
| 17 | – | − (41) | – | − (41) |
| 18 | – | − (41) | NT | NT |
| 19 | − (23) | − (41) | − (23) | − (41) |
| 20 | − (23) | − (23, 39, 93) | − (23) | − (23, 39, 41, 93) |
| 21 | + (23, 41) | − (31, 41, 45) | + (23, 41) | − (31, 41, 45) |
| 22 | – | − (18, 23, 39, 41) | − (23) | + (18, 23, 39, 41, 45) |
| 23 | – | – | – | − (41) |
| 24 | − (23) | − (23, 41) | + (23, 39, 41) | − (23, 39, 41) |
| 25 | − (23) | – | + (23, 41) | − (23, 41) |
| 26 | + (23, 39, 41) | − (23, 39, 41) | + (23, 39, 41) | − (23, 39, 41, 45) |
| 27 | – | – | – | – |
| 28 | + (23, 39, 41) | + (23, 39, 41, 45, 93) | NT | NT |
| 29 | + (23, 39, 41) | − (23, 39, 41, 45) | − (23) | − (39, 41) |
| Total, No. (%) | 5 (17) | 1 (3) | 6 (22) | 2 (7) |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; NT, not tested.
a Positive (+) result based on Centers for Disease Control and Prevention criteria for Western blot positivity: IgM at least 2 of the 23, 39, or 41 bands, and IgG at least 5 of 18, 23, 28, 30, 39, 41, 45, 58, 66, or 93 bands.
IFN-γ Responses Before and After Antibiotic Therapy
A baseline value for IFN-γ secretion was first determined by incubating whole blood from healthy volunteers (n = 187) and patients with anaplasmosis (n = 5) (mean IFN-γ = 0.036 IU/mL [range, 0–1.02 IU/mL]; Supplementary Table 1). A positive cutoff value was established by calculating 3 standard deviations above the mean of the control values (≥0.33 IU/mL). We then determined the levels of IFN-γ released after overnight culture of whole blood incubated with the cocktail of Borrelia-specific antigens. Blood from 20 of the 29 patients with early Lyme disease (69%) produced significant (≥0.33 IU/mL) levels of IFN-γ prior to treatment with antibiotics (Table 4). The magnitude of the responses varied widely among the patients (mean, 1.46 IU/mL ± 2.26 IU/mL). Interestingly, the levels of IFN-γ did not closely correlate with either the number of EM lesions (single vs multiple) or severity of the clinical/constitutional manifestations (Tables 1 and 4). Only 6 of 192 (3.2%) control blood samples, all from healthy donors, produced significant levels of IFN-γ (mean, 0.49 IU/mL [range, 0.33–1.02 IU/mL]).
Table 4.
Detection of Interferon Gamma in Blood Samples From Patients With Early Lyme Disease Collected Pretreatment or 2 Months Posttreatment With Doxycycline
| Subject | Levels (IU/mL) of IFN-γ in Blood Samples Collecteda |
|
|---|---|---|
| Pretreatment | 2 mo Posttreatment | |
| 1 | 1.16b | 0 |
| 2 | 0 | 0.06 |
| 3 | 5.03b | 0 |
| 4 | 0 | 0 |
| 5 | 0 | 0 |
| 6 | 3.86b | 2.61b |
| 7 | 1.82b | 0.16 |
| 8 | 0.45b | 0.02 |
| 9 | 1.69b | 1.59b |
| 10 | 0.78b | 0.46b |
| 11 | 0.49b | 0.12 |
| 12 | 0.29 | 0 |
| 13 | 0.33b | 0 |
| 14 | 0 | 0 |
| 15 | 2.58b | 0 |
| 16 | 2.02b | 0.11 |
| 17 | 0 | 0 |
| 18 | 10.61b | 1.12b |
| 19 | 0.42b | 0 |
| 20 | 0 | 0.12 |
| 21 | 0 | 0 |
| 22 | 2.29b | NT |
| 23 | 4.99b | 0.21 |
| 24 | 0.75b | 0.27 |
| 25 | 0.43b | NT |
| 26 | 0.47b | 0.20 |
| 27 | 0.82b | 0.30 |
| 28 | 0.66b | 0.05 |
| 29 | 0.30 | 0 |
Abbreviations: IFN-γ, interferon gamma; NT, not tested.
a Values displayed are the IFN-γ value obtained from peptide stimulation of whole blood samples minus nil tube reactivity (Supplementary Table 2).
b Results are ≥3 standard deviations above the mean value (≥0.33 IU/mL) of normal (n = 187) and anaplasmosis (n = 5) sera.
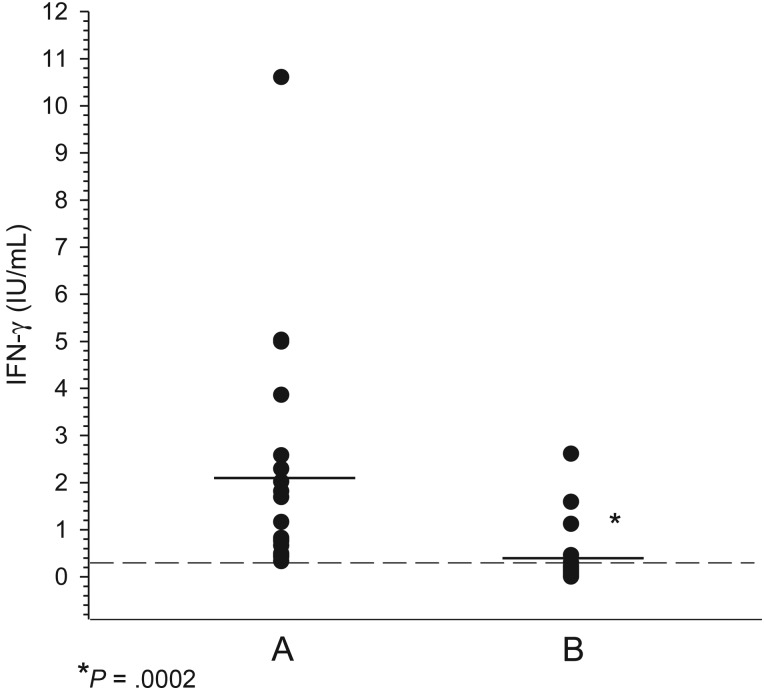
In the EM group providing a convalescent blood sample following antibiotic treatment, the IFN-γ response was decreased (n = 3) or no longer significant (n= 14) in those that were previously positive (P = .0002; Table 4 and Figure 1). The level of IFN-γ remained similar in only 1 returning patient whose pretreatment blood draw yielded a positive result, whereas levels remained undetectable in the 9 (31%) subjects who did not produce significant levels of IFN-γ prior to treatment (Table 4). Therefore, in contrast to the antibody responses detected by the C6 ELISA or Western blotting, decreased levels or inability to detect IFN-γ correlated closely with the resolution of the early Lyme disease symptoms following treatment.
Figure 1.
Detection of interferon gamma (IFN-γ) in blood samples collected approximately 2 months (column B) after treatment from patients with early Lyme disease with positive IFN-γ prior to antibiotic therapy (column A).
DISCUSSION
Traditional antibody tests, the current mainstay of the laboratory diagnosis of Lyme disease, lack sensitivity during early illness. They are also not useful for predicting response to treatment because the antibodies typically persist long after the spirochetes are eliminated by successful antibiotic therapy [5, 6]. This is especially problematic because the long-term seropositivity and the nonspecific abnormalities (eg, arthralgia, myalgia) associated with Lyme disease have continued to cause considerable confusion concerning the success or failure of antibiotic therapy. We therefore performed a preliminary evaluation of the utility of a QuantiFERON technology-based test that quantitates the level of IFN-γ released in blood samples after incubation with Borrelia ssp. specific peptide antigens for confirming Lyme disease and also provides accurate prediction of successful antibiotic therapy.
Our findings showed that incubating whole blood from patients with early Lyme disease and Borrelia species peptides (69%) results in secretion of significant amounts of IFN-γ, thus confirming previous reports [15, 16] of IFN-γ production during infection with B. burgdorferi. As secretion was dependent upon incubation with the peptide antigens, we presume that CD4+ or CD8+ T cells were the primary source of the cytokine, but additional studies are required to confirm this hypothesis. IFN-γ secretion varied widely among patients; however, this is expected from a diverse human population. Interestingly, the levels of IFN-γ also did not correlate closely with either the magnitude of the skin infection (single or multiple EM) or the number and severity of abnormal clinical signs/symptoms. IFN-γ levels in nil tubes were similar for both patients with single and those with multiple EM (Supplementary Table 2); thus, the lack of a difference between the 2 groups is not attributable to differences in baseline IFN-γ. The significance of this is unclear; however, this study only included a small number of patients residing in the same geographic locale.
The IFN-γ detection test and C6 ELISA produced similar results in the detection of early disease, with the IFN-γ release assay being slightly more sensitive (69% vs 59%, respectively). Both assays were significantly more sensitive than Western blotting (17%) for the detection of early disease. Although the overall rate of detection was similar for the C6 ELISA and IFN-γ release assay, analysis of individual patients revealed that the results of the 2 assays often diverged, as 11 patients were positive for only anti-C6 antibodies or only IFN-γ. A simple explanation for this divergence is that the tests used distinct target antigens. This would then also suggest that including additional antigens in the IFN-γ release assay could yield even higher sensitivity. In fact, combining the positive results from the C6 ELISA and IFN-γ release assay increases the sensitivity of confirmation of early Lyme disease in our test subjects to 83% (24 of 29).
In addition, we demonstrated that the antibody responses detected by the C6 ELISA or Western blotting typically persisted or continued to expand, even after the subjects were successfully treated with an appropriate regimen of doxycycline [17, 18]. Kowalski et al [18] showed convincingly in a large clinical study that the course of doxycycline only rarely failed to effectively resolve early Lyme disease. This finding therefore also confirms previous reports [6–8] documenting the inability of serology to provide accurate prediction of successful treatment. More significantly, however, we also showed that the levels of IFN-γ decreased after successful antibiotic therapy and, in fact, the response disappeared entirely in most (78%) of the patients with early Lyme disease within 2 months after the treatment had been completed. Decreases in IFN-γ were not attributable to increased baseline IFN-γ production in convalescent samples, as IFN-γ levels in both pretreatment and posttreatment nil tubes were comparable (Supplementary Table 2). Additional studies are required to more critically evaluate the ability of IFN-γ secretion to provide accurate prediction of active infection. Glickstein et al [23] reported increased levels of IFN-γ secretion by peripheral blood mononuclear cells collected immediately after patients with early Lyme disease had completed antibiotic therapy. However, the methodologies utilized in that study were substantially different from those used here, which may contribute to the difference in observed responses. Therefore, a more comprehensive evaluation that includes earlier time points is required to confirm when the IFN-γ response begins to wane following antibiotic therapy.
Despite these promising results, several shortcomings should also be highlighted. For example, we only tested a small number of patients with Lyme disease, the patients resided in only a single geographic region, the patients were sick for days rather than weeks, and the clinical abnormalities were suggestive of only early skin infection. Moreover, we established a significant cutoff value using only normal sera from patients who resided in only 2 Lyme disease foci and a small number of subjects with anaplasmosis. Patients with other illnesses with clinical manifestations similar to those found in Lyme disease, and patients with potentially cross-reactive bacterial and viral infections should also be evaluated in future studies. In addition, the cocktail of Borrelia-specific peptides was chosen from only 4 proteins; other peptide antigens may be important. Therefore, additional studies to more critically define significant cutoff values, determine the effects of other peptide antigens, and evaluate subjects with localized and disseminated Lyme disease contracted from multiple geographic regions remain necessary.
In summary, the findings in this preliminary study provide strong evidence that detecting IFN-γ secretion after incubating whole blood with Borrelia species antigens offers considerable promise for increasing the sensitivity of laboratory diagnosis of early Lyme disease. The results also provide compelling preliminary evidence that the cytokine response may be short lived after effective antibiotic therapy.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Peter Haviernik and John Wolff for valuable help with collection and processing of normal blood samples; Shelly Clements for assistance with collection and processing of Lyme disease samples and logistics coordination; and Dr Andrew Borgert for statistical analyses.
Financial support. This work was supported by Qiagen, Inc. R. J. D. and P. M. A. were additionally supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number R43 AI102435).
Potential conflicts of interest. M. M. and J. B. are employed by Qiagen, Inc. S. M. C. and R. J. D. provide consulting services for Qiagen, Inc. R. J. D. and P. M. A. have relevant patents that could serve as a source for future research funding. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53:541–7. [DOI] [PubMed] [Google Scholar]
- 2.Ledue TB, Collins MF, Young J, Schriefer ME. Evaluation of recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 2008; 15:1796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porwancher RB, Hagerty CG, Fan J et al. Multiplex immunoassay for Lyme disease using VlsE1-IgG and pepC10-IgM antibodies: improving test performance through bioinformatics. Clin Vaccine Immunol 2011; 18:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jobe DA, Lovrich SD, Asp KE et al. Significantly improved accuracy of diagnosis of early Lyme disease by peptide enzyme-linked immunosorbent assay based on the borreliacidal antibody epitope of Borrelia burgdorferi OspC. Clin Vaccine Immunol 2008; 15:981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peltomaa M, McHugh G, Steere AC. Persistence of the antibody response to the VlsE sixth invariant regions (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis 2003; 187:1178–86. [DOI] [PubMed] [Google Scholar]
- 6.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 2001; 33:780–5. [DOI] [PubMed] [Google Scholar]
- 7.Aguero-Rosenfeld ME, Nowakowski J, Bittker S, Cooper D, Nadelman RB, Wormser GP. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol 1996; 34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dattwyler RJ, Volkman DJ, Halperin JJ, Luft BJ, Thomas J, Golightly MG. Specific immune responses in Lyme borreliosis. Characterization of T cell and B cell responses to Borrelia burgdorferi. Ann NY Acad Sci 1988; 539:93–102. [DOI] [PubMed] [Google Scholar]
- 9.Widhe M, Jarefors S, Ekerfelt C et al. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J Infect Dis 2005; 189:1881–91. [DOI] [PubMed] [Google Scholar]
- 10.Oksi J, Savolainen J, Pene J, Bousquet J, Laippala P, Viljanen MK. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun 1996; 64:3620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benach JL, Coleman JL, Garcia-Monco JC, Deponte PC. Biological activity of Borrelia burgdorferi antigens. Ann N Y Acad Sci 1988; 539:115–25. [DOI] [PubMed] [Google Scholar]
- 12.Shanafelt MC, Anzola J, Soderberg C, Yssel H, Turck CW, Peltz CW. Epitopes on the outer surface protein A of Borrelia burgdorferi recognized by antibodies and T cells of patients with Lyme disease. J Immunol 1992; 48:218–24. [PubMed] [Google Scholar]
- 13.Zoschke DC, Archibald AS, Defosse DL. Lymphoproliferative responses to Borrelia burgdorferi in Lyme disease. Ann Intern Med 1991; 114:285–9. [DOI] [PubMed] [Google Scholar]
- 14.Nurmi L, McKernan L, Blank KJ, Spitanly GL, Muraslo DM. Inhibition of macrophage induced antigen specific T cell proliferation by IFN-gamma. Cell 1988; 114:432–9. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergstrom S. The outer surface proteins of Lyme disease Borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFNγ): diagnostic and pathogenic implications. Clin Exp Immunol 1995; 101:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin C, Roen DR, Lehmann PV, Kellermann GH. An enhanced ELISPOT assay for sensitive detection of antigen-specific T cell responses to Borrelia burgdorferi. Cells 2013; 2:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wormser GP, Dattwyler RJ, Shapiro ED et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski TJ, Tata S, Berth W, Mathiason MA, Agger WA. Antibiotic treatment duration and long-term outcomes of patients with early Lyme disease from a Lyme disease-hyperendemic area. Clin Infect Dis 2010; 50:512–20. [DOI] [PubMed] [Google Scholar]
- 19.Jackson CA, Lovrich SD, Agger WA, Callister SM. Reassessment of a midwestern Lyme disease focus for Borrelia burgdorferi and the human granulocytic ehrlichiosis agent. J Clin Microbiol 2002; 40:2070–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol 2015; 40:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobe DA, Lovrich SD, Asp KE, Mathiason MA, Schell RF, Callister SM. Significantly improved accuracy of diagnosis of early Lyme disease by peptide enzyme-linked immunosorbent assay based on the borreliacidal antibody epitope of Borrelia burgdorferi OspC. Clin Vacc Immunol 2008; 15:981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44:590–1. [PubMed] [Google Scholar]
- 23.Glickstein L, Moore B, Bledsoe T, Damle N, Sikand V, Steere AC. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun 2003; 71:6051–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.