Household spread of Ebola virus is driven by direct physical contact, especially through nursing care. There is no evidence of an increase in the household secondary attack rate since 1976. We estimate that one-quarter of Ebola infections are asymptomatic.
Keywords: Ebola, household, secondary attack rate, asymptomatic infection
Abstract
Factors affecting our ability to control an Ebola outbreak include transmissibility of the virus and the proportion of transmissions occurring asymptomatically. We performed a meta-analysis of Ebola household secondary attack rate (SAR), disaggregating by type of exposure (direct contact, no direct contact, nursing care, direct contact but no nursing care). The estimated overall household SAR is 12.5% (95% confidence interval [CI], 8.6%–16.3%). Transmission was driven by direct contact, with little transmission occurring in its absence (SAR, 0.8% [95% CI, 0%–2.3%]). The greatest risk factor was the provision of nursing care (SAR, 47.9% [95% CI, 23.3%–72.6%]). There was evidence of a decline in household SAR for direct contact between 1976 and 2014 (P = .018). We estimate that 27.1% (95% CI, 14.5%–39.6%) of Ebola infections are asymptomatic. Our findings suggest that surveillance and containment measures should be effective for controlling Ebola.
For emerging infectious agents, such as Ebola virus (EV), effective prophylactic agents, therapeutics, and vaccines have not been available. The preferred strategy of surveillance and containment is achieved through isolation of cases, intense contact tracing, and active monitoring. Such an approach has historically been effective for ending Ebola outbreaks. In contrast, the 2013–2016 outbreak in West Africa was larger than all prior outbreaks combined [1].
Fraser et al propose a framework where the key elements that impact our ability to contain an outbreak are (1) the disease generation time, which is the mean time between infection of an individual and infection of secondary cases, (2) the transmissibility of the virus, and (3) the proportion of asymptomatic transmissions [2]. In addition, (4) availability of prophylactic agents, therapeutics, and vaccines is key. To understand the first 2 elements, household studies are especially useful as contacts are clearly defined and remain fairly constant across cultural settings.
Very little is known about the impact of asymptomatic infection on Ebola outbreaks, including the level of pathogenicity of EV, defined as the proportion of Ebola infections that are symptomatic [3]. The proportion of transmissions occurring asymptomatically has an important bearing on our ability to contain an outbreak, with containment measures being less effective if asymptomatic individuals are infectious.
In this article, we summarize the transmissibility and pathogenicity of EV. We present a meta-analytic summary of transmission within households, disaggregated by type of exposure. We also present estimates of the asymptomatic proportion of the virus from serosurveys. Most of the data described are from earlier outbreaks, as very few data are available on the epidemic in West Africa.
METHODS
Transmissibility is measured by the household secondary attack rate (SAR). The SAR is the probability that an exposed susceptible person develops disease over the duration of infectiousness in a case patient. The denominator of the SAR is the number of exposed contacts, and the numerator is the number of those exposed contacts who develop disease. To estimate the household SAR, searches were conducted in PubMed using the term Ebola plus any of the following: household secondary attack rate, household transmission, contact transmission, contact attack rate, or family transmission. We extracted all articles with original data for estimating the household SAR for Ebola subtypes Sudan or Zaire in African outbreaks. The publication must report a numerator and denominator among household contacts, or at least 2 of numerator, denominator, and SAR. Where denominators (number of exposures) were not reported but the number of case patients and SAR were available, the denominator was calculated acknowledging limits of significant digits (6 case patients and SAR of 0.072 in first generation and 3 case patients and SAR of 0.04 in second generation of transmission in Yamolembia [8]; 24 case patients and SAR of 0.025 overall [15]). Household SAR estimates were recorded by type of exposure (eg, direct contact, nursing care) where such information was available. The last search was conducted on 13 January 2016. For data beyond household SAR, we direct readers to a recent summary of Ebola transmissibility [4].
To estimate the asymptomatic proportion, searches were conducted in PubMed using the term Ebola plus any of the following terms: seroprevalence, serosurvey, asymptomatic, mild, subclinical, or [contact and antibodies]. We extracted all articles that reported either seroprevalence among asymptomatic contacts or prevalence of symptoms among antibody-positive survivors. The last search was conducted on 13 January 2016.
Meta-analyses were performed using a random-effects DerSimonian–Laird model to yield a point estimate and 95% confidence interval (CI) for SAR by exposure type [5]. The Cochran Q-test is reported as a measure of heterogeneity. Limited analyses of trends were conducted by expanding the above model to include fixed-effect moderators, such as generation of transmission, outbreak year to identify time trends, and viral subtype. All tests of significance were at the α = .05 level. All analyses were done in R 3.2.0 using the package metafor [6, 7].
RESULTS
We identified 9 distinct studies/reports with usable results for direct estimation of household SAR, including one from the 2013–2016 West African epidemic (Table 1) [8–18]. Generally, a household contact implies that the contact lives in the same household or contiguous housing, such as a family compound, and shares the same cooking fire or common eating facilities. One study included nonhousehold members who had contact with body fluids or linens/soiled materials [18]. Another study reported SAR among contacts but did not specify the definition of a contact [15]. Studies are often retrospective and reviewed contact tracing records. In some studies, case patients and contacts, or proxies, are interviewed to determine the nature of the contact's relationship with the case patient.
Table 1.
Description of Studies for Review of Household Transmissibility
| Outbreak | Study, Year | Description of Study | Definition of Household Contact |
|---|---|---|---|
| 1976, Zaire Total No. of cases: 318 Ebola subtype: Zaire |
WHO 1978a Breman et al, 1978 [8, 9] | Primarily retrospective studies of households in affected villages. Two studies: (1) Villages near Yambuku (2 independent assessments). Assessed attack rates, gender breakdown, and other basic epidemiological characteristics. (2) Village of Yamolembia, selected for in-depth study because of its high attack rate (24 cases among 415 residents). Conducted a census of the village. Interviews to assess risk factors for person-to-person spread. |
Contact: Face-to-face contact with a probable/proven case (sleeping in same room, sharing meals, caring for patients, preparing a cadaver for burial, touching the body at funeral, etc) in the period between 2 days prior to onset of symptoms and the death or clinical recovery of the patient. Household: All persons using the same kitchen, claiming the same person as family head, living in contiguous dwellings, and sleeping in the village during the time an active case occurred in the family unit. |
| 1976, Sudan Total No. of cases: 318 Ebola subtype: Sudan |
WHO 1978b Francis et al, 1978 [10, 11] | Retrospective studies of households in affected villages. Two studies: (1) Sample of 17 highly infected households for in-depth study of risk factors for person-to-person transmission. (2) Study of 36 families with 38 primary cases to estimate secondary attack rate. |
Household: All persons residing in the same house. |
| 1979, Sudan Total No. of cases: 34 Ebola subtype: Sudan |
Baron et al, 1983 [12] | Concurrent and retrospective study of 5 affected families with chains of secondary spread. | Household contact: Lived in same family compound. |
| 1995, Kikwit, DRCa Total No. of cases: 315 Ebola subtype: Zaire |
Dowell et al, 1999 [13] | Cross-sectional design, assessing risk factors in households of primary cases. Interviewed household members. Exposures subdivided by clinical phase. | Household contact: All those who shared the same cooking fire at the onset of illness in the primary household case. |
| 2000, Uganda Total No. of cases: 425 Ebola subtype: Sudan |
Francesconi et al, 2003 [14] | Retrospective interviews of contacts of cases (or their proxies) concerning nature of exposure. Exploited available data from routine contact tracing. Studied timing of exposure based on case's illness stage. | Household contact: Physical contact, slept in same hut/house during disease period, contact with body fluids during disease, contact with linens or other possible fomites during disease or just after death. Includes family members and neighbors. |
| 2000, Uganda (Gulu only) Total No. of cases: 393 (Gulu only) Ebola subtype: Sudan |
Okware et al, 2002 [15] | Summary of contact tracing activities in Gulu, Uganda. | Contact: Not defined. Likely not restricted to household contacts. |
| 2000, Uganda (Masindi only) Total No. of cases: 26 (Masindi only) Ebola subtype: Sudan |
Borchert et al, 2011 [16] | Retrospective analysis of surveillance records and hospital statistics. Results are from 1 highly infected family (1 initial case, 18 further cases) | Contact: Anyone having had physical contact with a suspected case or his/her remains, body fluids or soiled materials, or having lived in the same house as the case. Household: extended family living in an area of about 2 hectares. |
| 2005, Republic of Congo (fourth outbreak Cuvette Ouest) Total No. of cases: 12 Ebola subtype: Zaire |
Nkoghe et al, 2011 [17] | Retrospective surveillance, detection, and follow-up of contacts. Data inferred from line list table in paper. | Contact: Slept in the same household as a case within the previous month, or who had direct contact with the case (dead or alive), or who touched his/her linen or body fluids. |
| 2014, Firestone compound, Liberia Total No. of cases: ≥28 639 Ebola subtype: Zaire |
Reaves et al, 2014 [18] | Prospective monitoring of contacts. Contacts are not restricted to household contacts and may include contacts from the community. | Contact: Person with no symptoms who had physical contact with any Ebola patient or the body fluids of an Ebola patient within the past 21 d. Physical contact could be proven or highly suspected, such as having the same room or bed, cared for a patient, touched body fluids, or closely participated in a burial. Contacts stratified into high or low risk, where high risk is defined as direct contact with the blood or body fluids of an Ebola patient without personal protective equipment. |
Abbreviation: WHO, world health organization.
a Democratic Republic of the Congo (formerly Zaire).
Household SAR
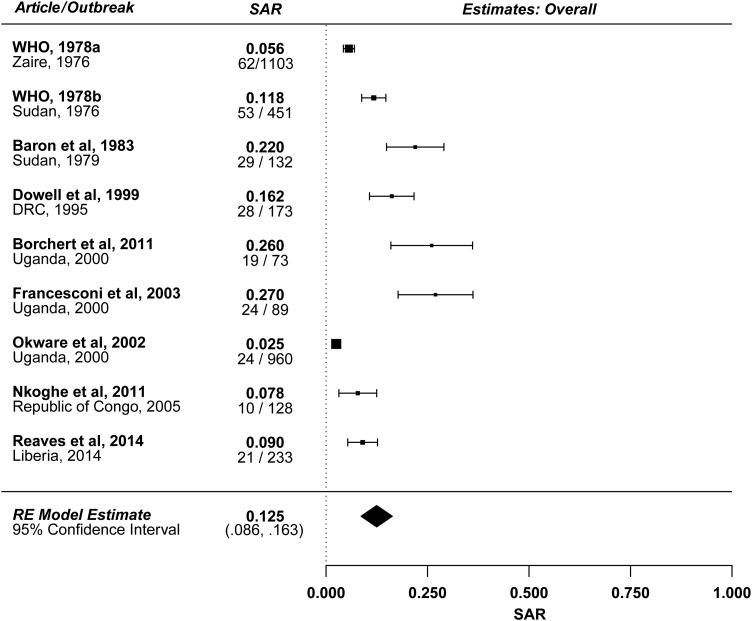
Figure 1 summarizes the estimated SARs from the 9 reports described in Table 1. The estimated mean household SAR is 12.5% (95% CI, 8.6%–16.3%) with significant heterogeneity (P < .001), likely due to variability in study definitions of households and contacts. Excluding the 2 studies that did not restrict to household contacts [15, 18], the estimated mean household SAR is 15.4% (95% CI, 10.0%–20.9%) with significant heterogeneity (P < .001). There was no evidence of a significant relationship between outbreak year and household SAR. There was also no evidence of a significant difference in SAR between subtypes Zaire (outbreaks in Democratic Republic of the Congo [formerly Zaire], Republic of Congo, and Liberia) and Sudan (outbreaks in Sudan and Uganda). Other estimates of household SAR described in the literature yield similar results.
Figure 1.
Forest plot: overall estimate. The denominator is the number of exposed household contacts of infectious Ebola virus disease cases. The numerator is the number of these contacts who develop disease. Abbreviations: DRC, Democratic Republic of the Congo; RE, random-effects; SAR, secondary attack rate; WHO, world health organization.
Direct Physical Contact
General understanding of the Ebola virus suggests that transmission requires close and prolonged contact with an acutely ill patient [11]. Figure 2 summarizes the results of 5 studies reporting household SAR among contacts with direct physical contact with the case patient—for example, touching the ill case patient or his/her bodily fluids. The estimated mean household SAR for individuals with direct contact is 22.9% (95% CI, 11.6%–34.2%) with significant heterogeneity (P < .001). There is a significant inverse relationship between outbreak year and household SAR, with household SAR for direct physical contacts declining between 1976 and 2014 (P = .018), with nonsignificant residual heterogeneity. There was no evidence of a significant difference in SAR for direct physical contacts between viral subtypes.
Figure 2.
Forest plot: direct contact. Abbreviations: DRC, Democratic Republic of the Congo; RE, random-effects; SAR, secondary attack rate; WHO, world health organization.
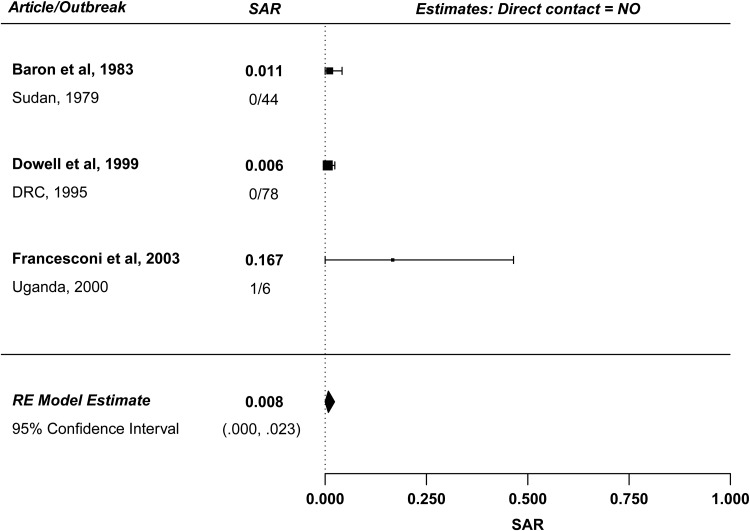
In the absence of direct contact, very little EV transmission occurred. Figure 3 summarizes 3 studies reporting SAR among contacts with no direct contact with the case patient. The estimated mean household SAR for individuals without direct contact is 0.8% (95% CI, 0%–2.3%) with no significant heterogeneity. In all reports, only 1 person was infected without direct contact; in the 2000 outbreak in Uganda, a man was infected from sleeping in the same heavily contaminated blanket as a case patient (his brother) [14]. Documented instances of transmission by touching contaminated inanimate objects (fomites) have occurred, though this type of spread is rare [19]. The low SAR for household contacts without direct physical contact supports the hypothesis that transmission does not occur primarily through fomites.
Figure 3.
Forest plot: no direct contact. Abbreviations: DRC, Democratic Republic of the Congo; RE, random-effects; SAR, secondary attack rate.
Nursing Care
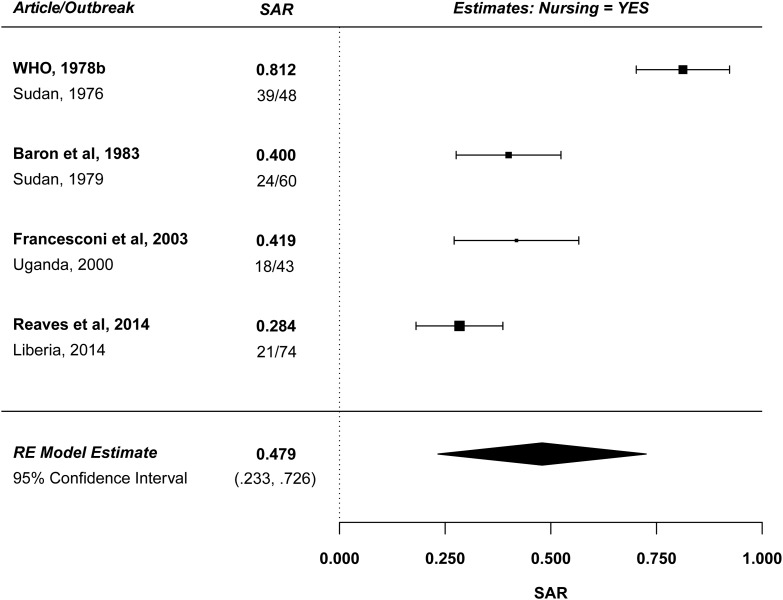
While direct physical contact is an important risk factor for transmission of EV, risk appears to be highest for contacts who take care of the acutely ill patient. Figure 4 summarizes 4 studies reporting SAR for contacts providing nursing care. The estimated mean household SAR for contacts providing nursing care is 47.9% (95% CI, 23.3%–72.6%) with significant heterogeneity (P < .001), primarily because the estimated SAR in the 1976 Sudan outbreak was very high (81%). There was no significant time trend. For comparison, Figure 5 summarizes 3 studies reporting SARs for contacts with direct physical contact who did not provide nursing care. The estimated mean household SAR for these contacts is 2.1% (95% CI, 0%–6.3%) with no significant heterogeneity. Restricting consideration to the 3 studies that report both an SAR for nursing and an SAR for direct contact without nursing [12, 14, 18], the data suggest that the estimated SAR is nearly 17 times higher for contacts providing nursing care, adjusting for direct contact. Similarly, in the 1979 Sudan outbreak, nursing care carried a 5.1-fold increased odds of developing disease compared to less intense physical contact (95% CI, 1.31- to 15.48- fold) [12].
Figure 4.
Forest plot: nursing care. For Reaves et al [18], the authors assumed that a high-risk contact was equivalent to providing nursing care as it is defined as “percutaneous or mucous membrane exposure to, or direct skin contact with blood or body fluids of an Ebola patient or a corpse … without appropriate personal protective equipment.” Abbreviations: RE, random-effects; SAR, secondary attack rate; WHO, world health organization.
Figure 5.
Forest plot: direct contact but no nursing care provided. Abbreviations: RE, random-effects; SAR, secondary attack rate.
Other Epidemiological Factors
Close contacts of index cases are at greater risk than other household members. In the 1976 Zaire outbreak, SAR was 27.3% among spouses, brothers, sisters, parents, and children of case patients, but only 8% among all other relatives in the household [8]. In the 1995 DRC outbreak, the SAR was 45% (10/22) among spouses of case patients, compared with 12% (18/151) among nonspouse household contacts [13]. Evidence is not consistent regarding transmission and gender. In the 1976 Zaire outbreak, higher SARs were observed when the female was the case patient; authors suggested this was due to increased intimate family contact, including sexual intercourse [9]. Women may also be at greater risk of being infected because they are more likely to provide nursing care, a high-risk activity for EV disease. In the 1995 DRC outbreak, female household contacts had an SAR of 21%, whereas males had an SAR of 10% (no numerators, denominators, or CIs provided) [13]. In the 2000 Uganda outbreak, twice as many women (63%) as men (37%) were infected [15]. In contrast, within that same outbreak, restricting to Masindi only, two-thirds of the cases were male with no obvious cause [16]. In the ongoing West African epidemic, males and females have been similarly affected (48.9% male as of 3 February 2016) [1].
Children tend to be spared in Ebola epidemics [8, 11, 13, 15, 16]. In the 1995 DRC outbreak, the SAR observed among contacts aged >18 years was 30% (24/81) vs 4% (4/92) for contacts aged ≤18 years [13]. Similarly, in the 2000 Uganda outbreak in Masindi, the SAR among contacts aged 15–49 years was 53% (16/30) compared with 7% (3/43) among all other ages. In the West African epidemic, significantly lower incidence has been observed in children aged <16 years in all 3 primarily affected countries [20]. Children do not typically provide nursing care and are also less likely to experience close contact with case patients [11]. Nonetheless, in one study, the decreased risk among children remained after adjusting for direct exposure to ill family members [13], suggesting that children may be less susceptible to infection or severe disease [21].
Generation of Transmission
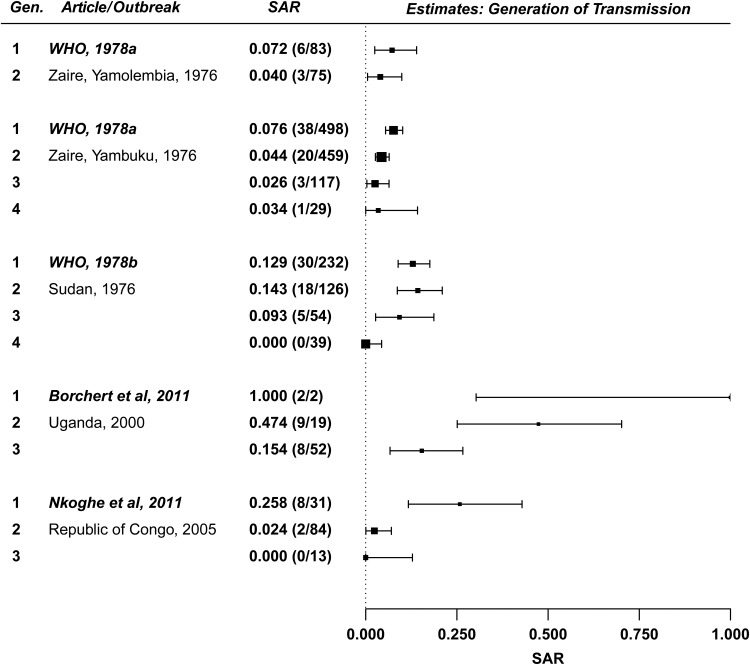
SAR decreases as a function of generation of transmission within a household (Figure 6). Using a meta-analytic regression with generation number as a moderator, a significant inverse relationship was observed between SAR and generation (P = .031). Family members may take more caution to avoid contact with body fluids after the first generation of transmission, as was observed in the 1995 DRC outbreak [19]. Similarly, case patients may be identified more quickly and taken to a hospital for care.
Figure 6.
Forest plot: generation of transmission. Abbreviations: SAR, secondary attack rate; WHO, world health organization.
Transmission and Stage of Illness
The proportion of transmissions occurring asymptomatically is an important factor for determining how easily an outbreak can be controlled. It is believed that individuals are not infectious during the incubation period (ie, prior to developing symptoms) [22]. One possible exception is an afebrile, infected pregnant woman with high viral load, although her presentation with premature membrane rupture suggests that she experienced symptoms [23]. After the case patient becomes febrile, infectiousness increases over time, maximizing during late-stage infection [14]. The increase in infectiousness is likely explained by (1) an increase in viral load over the course of infection [24] and (2) an increase in bodily fluid output resulting from disease symptoms, as late-stage illness is characterized by high output of diarrhea, vomitus, and blood [13]. It is noteworthy that not all symptomatic cases are febrile; a study in Sierra Leone found that 18.0% of 61 confirmed cases did not present with fever, suggesting immune evasion and complicating early diagnosis [25].
Individuals who recover are minimally infectious during convalescence. Virus rapidly clears from the blood upon resolution of symptoms in survivors, although clearance is slower for immunologically protected sites, such as the kidney, gonads, and chambers of the eye [26]. Virus can persist in semen for months after it has cleared from the blood [27, 28]. Thus, some level of sexual transmission of EV likely occurs [29].
Asymptomatic Transmission
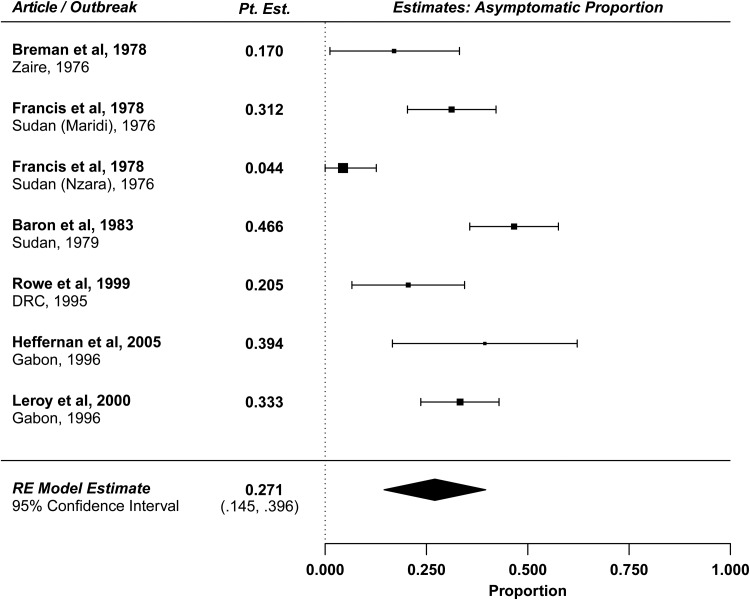
EV disease severity is known to vary, but the proportion of EV infections resulting in asymptomatic illness, otherwise known as the subclinical/asymptomatic proportion, or 1 minus the pathogenicity of the virus, is poorly understood [30]. Although no study has explicitly reported the asymptomatic proportion, we reviewed and analyzed the available literature to yield an estimate of this quantity. Presumed asymptomatic infections may be identified through serosurveys measuring Ebola-specific antibodies. We estimate the asymptomatic proportion from 7 serosurveys from previous outbreaks (Table 2) [9, 10, 12, 27, 31, 32]. To prepare these estimates (Figure 7), we adjust for the case-fatality rate in serosurveys conducted among survivors, or we adjust for the relevant SAR in serosurveys conducted among asymptomatic household contacts (methods described in the Supplementary Materials). Two of these studies have the highest-quality data because asymptomatic contacts are followed prospectively and are tested using enzyme-linked immunosorbent assay. The estimated asymptomatic proportions from these 2 studies are 20.5% (95% CI, 6.6%–34.4%) [27] and 33.3% (95% CI, 23.6%–42.9%) [30]. The limitations of the other studies are described in detail in the Supplementary Materials. The overall estimated asymptomatic proportion is 27.1% (95% CI, 14.5%–39.6%), with significant heterogeneity across the estimates (P < .001).
Table 2.
Description of Studies for Review of the Asymptomatic Proportion
| Outbreak | Study, Year | Description of Seroprevalence Testing |
|---|---|---|
| 1976, Zaire (sampling conducted 1976–1977) | Breman et al, 1978 [9] | Serosurvey of as many people as possible in Yamolembia I (village selected because of high attack rate). Used IFA at 1:64 dilution. |
| 1976, Sudan (Maridi) | Francis et al, 1978 [10] | Study of contacts of case patients. The majority of them were close family contacts, and several had helped to nurse sick relatives during their illnesses. Used IFA for detection of IgG at 1:8 dilution. |
| 1976, Sudan (Nzara) | Francis et al, 1978 [10] | Tested close family contacts of case patients. Used IFA for detection of IgG at 1:8 dilution. |
| 1979, Sudan | Baron et al, 1983 [12] | Tested asymptomatic relatives of suspected infected persons. As a comparator population, tested members of families in which none of the ill persons investigated were considered to have EVD. Used IFA at 1:16 dilution. |
| 1995, DRC | Rowe et al, 1999 [27] | Prospective study of convalescents and household contacts (person who resided in the same household or shared a cooking fire with an EVD convalescent at the time of enrollment). Monitored for up to 21 mo. Used ELISA for detection of IgM, IgG, and Ebola antigen. |
| 1996, Gabon (sampling conducted in 1997) | Heffernan et al, 2005 [31] | Population-based serosurvey >1 y after outbreak. Surveyed 8 villages, including site of February 1996 outbreak. Used ELISA for detection of IgG and IgM. |
| 1996, Gabon | Leroy et al, 2000 [30] | Study of asymptomatic close contacts of case patients. Sampled during 1 mo period after first exposure to patients. Used ELISA and Western blot for detection of IgG, IgM, Ebola antigen, cytokines, and chemokines. |
Abbreviations: DRC, Democratic Republic of the Congo; ELISA, enzyme-linked immunosorbent assay; EVD, Ebola virus disease; IFA, immunofluorescence assay; IgG, immunoglobulin G; IgM, immunoglobulin M.
Figure 7.
Forest plot: asymptomatic proportion. Abbreviations: DRC, Democratic Republic of the Congo; Pt. Est., point estimate; RE, random-effects.
DISCUSSION
We have summarized Ebola virus transmissibility by providing estimates of household SAR. The overall estimated household SAR based on 9 studies is 12.5% (95% CI, 8.6%–16.3%). Risk to household contacts is associated with direct physical contact, with little to no transmission observed otherwise. Risk is highest for contacts exposed to infectious body fluids through provision of nursing care, with an estimated SAR of 48.0% (95% CI, 25.5%–70.9%). Members of the immediate family are at greater risk, although data suggest children are at lower risk for infection even after controlling for direct physical contact. A significant decline in SAR over time (1976–2014) was observed for household members with direct physical contact, though this observation may be driven by the fact that earlier studies focused on highly infected households, whereas the most recent study included community (nonhousehold) contacts [18]. We consistently observe a decline in SAR over multiple generations of transmission within the same household, which could be due to changes in behavior as disease awareness increases, or could relate to the presence of asymptomatic individuals within the household with protective immunity. A similar phenomenon was observed in a study in Sierra Leone, with the population-level viral load of infected cases decreasing over the epidemic; however, the cause of this requires further investigation [33].
We estimate that 27.1% (95% CI, 14.5%–39.6%) of Ebola infections are asymptomatic. This estimate is based on limited data of variable quality, but it is our best estimate of a quantity that is otherwise unknown. Thus, we are underestimating secondary spread of the virus because a nonnegligible proportion of infections are subclinical. Although there is no evidence that these individuals are infectious [32], they are relevant if their subclinical infection confers protection against symptomatic disease.
The framework of Fraser et al suggests that the current epidemic could be controlled by standard surveillance and containment measures, if done aggressively [2]. The time scale of epidemic growth is reasonably slow, with an estimated serial interval (time between successive disease onsets) of 15.3 days observed in the current outbreak [34]. Transmission generally requires close physical contact with a symptomatic case patient, with peak infectivity not occurring until late infection. Furthermore, there is no evidence of asymptomatic transmission. Possible explanations for the large size of the West African outbreak include high rates of human migration in the region, poor health infrastructure, local burial practices, and fear of interventions such as isolation and hospitalization [35]. The recombinant vesicular stomatitis virus–based Ebola vaccine was evaluated in a phase 3 ring vaccination trial in Guinea and was found to have high efficacy, but the product is not yet licensed and was not available in previous outbreaks [36].
Estimating SAR based on historical data has important limitations. Data are often retrospective, and it is easier to track down infected contacts than healthy contacts [14]. In the future, collecting high-quality data on both infected and healthy contacts is essential for unbiased analysis. Studies may have different definitions of “household” and/or “contact.” A standardized definition of “contact” is critical for properly interpreting the data. Finally, without accounting for outside sources of infection, we always overestimate SAR [37].
Our estimates of the asymptomatic proportion should be interpreted with caution because they are approximated from limited data under simplifying assumptions. The studies have varying designs and lack a standardized case definition. The underlying test results may also be unreliable, especially for older data, as earlier approaches for measuring antibodies were prone to false positives [38], or may reflect exposure to inactivated virus or viral antigen (eg, bat saliva on fruit) [39].
Important questions remain about the household spread of Ebola, including the frequency of sexual transmission, the level of protection conferred by asymptomatic infection, and the ability of vaccines to interrupt household transmission. The results of well-designed household studies are important to help us select maximally effective control strategies and prevent future outbreaks from occurring.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers U54GM111274 and R37AI032042).
Potential conflicts of interest. M. E. H. has received institutional grant support through the National Institutes of Allergy and Infectious Diseases and National Institute of General Medical Sciences, consulting fees from Merck, and financial support for travel to meetings from Sanofi Pasteur. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization Clinical Response Team. Ebola situation report. 2016. Available at: http://apps.who.int/ebola/ebola-situation-reports Accessed 15 February 2016.
- 2.Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A 2004; 101:6146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellan SE, Pulliam JRCC, Dushoff J, Meyers LA. Ebola control: effect of asymptomatic infection and acquired immunity. Lancet 2015; 384:1499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brainard J, Hooper L, Pond K, Edmunds K, Hunter PR. Risk factors for transmission of Ebola or Marburg virus disease: a systematic review and meta-analysis. Int J Epidemiol 2015; doi:10.1093/ije/dyv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 6.R Core Team. R: a language and environment for statistical computing. R Core Team: Vienna, Austria: 2014. [Google Scholar]
- 7.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36:1–48. [Google Scholar]
- 8.Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 1978; 56:271–93. (Referred to as WHO 1978a.) [PMC free article] [PubMed] [Google Scholar]
- 9.Breman JG, Piot P, Johnson KM et al. . The epidemiology of Ebola haemorrhagic fever in Zaire, 1976. In: Pattyn S, ed Ebola virus haemorrhagic fever. Amsterdam: Elsevier/North Holland Biomedical Press, 1978:103–24. [Google Scholar]
- 10.Francis D, Smith D, Highton R. Ebola fever in the Sudan, 1976: epidemiological aspects of the disease. In: Pattyn S, ed Ebola virus haemorrhagic fever. Amsterdam: Elsevier/North Holland Biomedical Press, 1978:129–35. [Google Scholar]
- 11.Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ 1978; 56:247–70. (Referred to as WHO1978b.) [PMC free article] [PubMed] [Google Scholar]
- 12.Baron RC, Mccormick JB, Zubeir OA. Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ 1983; 61:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 13.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJP. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179(suppl):S87–91. [DOI] [PubMed] [Google Scholar]
- 14.Francesconi P, Yoti Z, Declich S et al. . Ebola hemorrhagic fever transmission and risk factors of contacts, Uganda. Emerg Infect Dis 2003; 9:1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okware SI, Omaswa FG, Zaramba S et al. . An outbreak of Ebola in Uganda. Trop Med Int Heal 2002; 7:1068–75. [DOI] [PubMed] [Google Scholar]
- 16.Borchert M, Mutyaba I, Van Kerkhove MD et al. . Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned. BMC Infect Dis 2011; 11:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nkoghe D, Kone ML, Yada A, Leroy E. A limited outbreak of Ebola haemorrhagic fever in Etoumbi, Republic of Congo, 2005. Trans R Soc Trop Med Hyg 2011; 105:466–72. [DOI] [PubMed] [Google Scholar]
- 18.Reaves EJ, Mabande LG, Thoroughman DA, Arwady MA, Montgomery JM. Control of Ebola virus disease—Firestone district, Liberia 2014. MMWR Morb Mortal Wkly Rep 2014; 63:959–65. [PMC free article] [PubMed] [Google Scholar]
- 19.Roels TH, Bloom AS, Buffington J et al. . Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis 1999; 179(suppl):S92–7. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Ebola Response Team. Ebola virus disease among children in West Africa. N Engl J Med 2015; 372:1274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowell SF. Ebola hemorrhagic fever: why were children spared? Pediatr Infect Dis J 1996; 15:189–91. [DOI] [PubMed] [Google Scholar]
- 22.Borio L, Schmaljohn AL, James M et al. . Hemorrhagic fever viruses as bioloigcal weapons: medical and public health management. JAMA 2002; 287:2391–405. [DOI] [PubMed] [Google Scholar]
- 23.Akerlund E, Prescott J, Tampellini L. Shedding of Ebola virus in an asymptomatic pregnant woman. N Engl J Med 2015; 372:2467–9. [DOI] [PubMed] [Google Scholar]
- 24.Towner JS, Rollin PE, Bausch DG et al. . Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 2004; 78:4330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin E, Bi J, Zhao M et al. . Clinical features of patients with Ebola virus disease in Sierra Leone. Clin Infect Dis 2015; 61:491–5. [DOI] [PubMed] [Google Scholar]
- 26.Varkey JB, Shantha JG, Crozier I et al. . Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 2015; 372:2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe AK, Bertolli J, Khan AS et al. . Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis 1999; 179(suppl):S28–35. [DOI] [PubMed] [Google Scholar]
- 28.Deen G, Knust N, Broutet F et al. . Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report [manuscript published online ahead of print 14 October 2015]. N Engl J Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mate S, Kugelman J, Nyenswah T et al. . Molecular evidence of sexual transmission of Ebola virus. N Engl J Med 2015; 373:2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leroy EM, Baize S, Debre P, Lansoud-Soukate J, Mavoungou E. Early immune responses accompanying human asymptomatic Ebola infections. Clin Exp Immunol 2001; 124:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffernan RT, Pambo B, Hatchett RJ, Leman PA, Swanepoel R, Ryder RW. Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooué-Ivindo region, northeastern Gabon, 1997. J Infect Dis 2005; 191:964–8. [DOI] [PubMed] [Google Scholar]
- 32.Leroy EM, Baize S, Volchkov VE et al. . Human asymptomatic Ebola infection and strong inflammatory response. Lancet 2000; 355:2210–5. [DOI] [PubMed] [Google Scholar]
- 33.de La Vega MA, Caleo G, Audet J et al. . Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J Clin Invest 2015; 125:4421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander KA, Sanderson CE, Marathe M et al. . What factors might have led to the emergence of Ebola in West Africa? PLoS Negl Trop Dis 2015; 9:e0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henao-Restrepo AM, Longini IM, Egger M et al. . Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857–66. [DOI] [PubMed] [Google Scholar]
- 37.Longini IM, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol 1982; 115:736–51. [DOI] [PubMed] [Google Scholar]
- 38.Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis 1999; 179(suppl):S192–8. [DOI] [PubMed] [Google Scholar]
- 39.Becquart P, Wauquier N, Mahlakõiv T et al. . High prevalence of both humoral and cellular immunity to Zaire ebolavirus among rural populations in Gabon. PLoS One 2010; 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.