Abstract
Background and Aims Apocynaceae and Orchidaceae are two angiosperm families with extreme flower synorganization. They are unrelated, the former in eudicots, the latter in monocots, but they converge in the formation of pollinia and pollinaria, which do not occur in any other angiosperm family, and for which extreme synorganization of floral organs is a precondition. In each family extensive studies on flower development and evolution have been performed; however, newer comparative studies focusing on flower synorganization and involving both families together are lacking.
Scope For this study an extensive search through the morphological literature has been conducted. Based on this and my own studies on flowers in various Apocynaceae and Orchidaceae and complex flowers in other angiosperms with scanning electron microscopy and with microtome section series, a review on convergent floral traits in flower development and architecture in the two families is presented.
Key Findings There is a tendency of protracted development of synorganized parts in Apocynaceae and Orchidaceae (development of synorganization of two or more organs begins earlier the more accentuated it is at anthesis). Synorganization (or complexity) also paves the way for novel structures. One of the most conspicuous such novel structures in Apocynaceae is the corona, which is not the product of synorganization of existing organs; however, it is probably enhanced by synorganization of other, existing, floral parts. In contrast to synorganized parts, the corona appears developmentally late.
Conclusions Synorganization of floral organs may lead to a large number of convergences in clades that are only very distantly related. The convergences that have been highlighted in this comparative study should be developmentally investigated directly in parallel in future studies.
Keywords: Apocynaceae, Orchidaceae, angiosperm flower development, flower evolution, flower symmetry, synorganization, congenital fusion, postgenital fusion, pollinium, pollinarium, species diversity.
INTRODUCTION
The integration of floral organs, resulting in functionally fitting positions of all floral organs and covariation of fitting parts, is an evolutionarily important trend in angiosperms (Armbruster et al., 2009, 2014). In contrast to ecological aspects, developmental aspects of integration have found much less attention (Wagner, 2014). A major developmental and evolutionary mechanism to increase such integration is synorganization of modules by highly symmetrical arrangement and tangential and radial congenital and postgenital fusion. Such synorganization is extreme in Apocynaceae (especially Asclepiadoideae) and Orchidaceae among angiosperms.
When we analyse a flower we commonly focus on the four organ categories sepals, petals, stamens and carpels. They are the organs that make up a flower. They are used in basic descriptions of flowers and are also the focus of molecular developmental studies on flowers. The classical ‘ABC model’ of flower development rests on them (Coen and Meyerowitz, 1991). In most flowers we encounter, these organs are easily seen and easy to distinguish from each other. However, looking at the flower of an orchid or an asclepiad, it is difficult to recognize the basic organs immediately, especially in androecium and gynoecium.
Harder and Johnson (2008) discussed the function and evolution of aggregated pollen in angiosperms, also addressing Apocynaceae and Orchidaceae. It was shown that pollen loss during pollination in plants with pollinia is considerably lower than in plants with granular pollen (Harder and Routley, 2006). However, the evolution of pollinia and pollinaria needed complex morphological preconditions by intimate synorganization of floral organs, and this was only achieved in Apocynaceae and Orchidaceae convergently as a prominent novelty. The present paper focuses on the morphology, development and evolution of the highly synorganized flowers of these two families. Without intimate knowledge of the developmental processes and diversity it is difficult to understand the structural evolution of these flowers. There is an extensive and exciting literature on the functional aspects of flowers of Apocynaceae and Orchidaceae. However, the literature on comparative floral development and morphology in the two families is much smaller (e.g. Vogel, 1959; Schick, 1980, 1982a, b, 1988, 1989; Kunze, 1981, 1990, 1991, 1994, 1995, 1996, 1997, 2005; Fallen, 1986; Kurzweil, 1987a, b, 1988, 1993, 1995, 1998; Kurzweil and Weber, 1992; Liede and Kunze, 1993; Endress, 1994, 2011; Liede, 1994; M. E. Endress, 2003; Kocyan and Endress, 2001; Kurzweil and Kocyan, 2002; Kunze and Wanntorp, 2008). As Apocynaceae and Orchidaceae are the only angiosperms that have pollinia and pollinaria, it is not surprising that initial comparisons between the two families were made long ago (Brown, 1833).
An important aspect of the convergent evolution of pollinia is economical use of available pollen and male fitness, which appears to be an important principle in floral biology (Barrett and Harder, 2006; Harder and Johnson, 2008). Various structural devices have evolved to this end (Erbar and Leins, 1995; Leins and Erbar, 2006, 2010), and the advent of pollinia is a particularly conspicuous trend in this respect.
Both families are species-rich, and the advent of pollinia and pollinaria may have been an important factor for this diversity. However, floral synorganization may also have led to other features driving speciation, such as devices forcing pollinators into precise positions on the flowers for pollinaria removal and deposition, or various ways of pollinator deception, especially in orchids. Apocynaceae comprises almost 5000 species. The subclade of Asclepiadoideae plus Secamonoideae, which is nested in Apocynaceae, has more species (3180) (Meve, 2002) than all other subclades of the family together (Periplocoideae, Apocynoideae and the basal grade of Rauvolfioideae), and it has 171 genera (M. E. Endress et al., 2014), whereas the rest of the family has 194 genera (M. E. Endress et al., 2014). Orchidaceae are one of the two most species-rich angiosperm families, with approx. 25 000 species and 735 genera (Chase et al., 2015). Apostasioideae have two genera and 14 species (Chase et al., 2015), Vanilloideae 14 genera and 245 species (Chase et al., 2015), Cypripedioideae five genera and 169 species (Chase et al., 2015), Orchidoideae 198 genera and 4575 species (Chase et al., 2015), Epidendroideae 516 genera (Chase et al., 2015) and 21 160 species (Freudenstein and Chase, 2015), and Cymbidieae and Vandeae of Epidendroideae together have 300 genera and 4528 species (Chase et al., 2015). Family stem ages were calculated as 52 Mya for Apocynaceae and 109 Mya for Orchidaceae (Magallón et al., 2015). However, when each of the salient morphological innovations first appeared within each family is largely unknown.
FLOWER SYNORGANIZATION IN GENERAL
The general evolutionary trend of synorganization in flowers
Angiosperm flowers are characterized by basically three kinds of organs, which serve the following basic functions: (1) protection and optical attraction (the perianth organs: tepals or sepals and petals), (2) male function (the androecial organs: stamens) and (3) female function (the gynoecial organs: carpels). These floral organs are modular structures, repetitive units of the same kind. Each kind of organ occurs in different numbers and arrangement in a flower, although they always have the same sequence from the periphery to the centre of the flower: perianth→androecium→gynoecium. There is a common evolutionary trend in angiosperms that organs of the same kind or also organs of different kinds become more intimately associated. Such association or integration of ancestrally independent organs into a complex structure is called synorganization. Thus, a common general evolutionary direction is from independent organs to a complex of organs. In the extreme, this complex of organs may become so synorganized that it behaves like a single complex unit or a single organ.
Preconditions for synorganization of floral organs
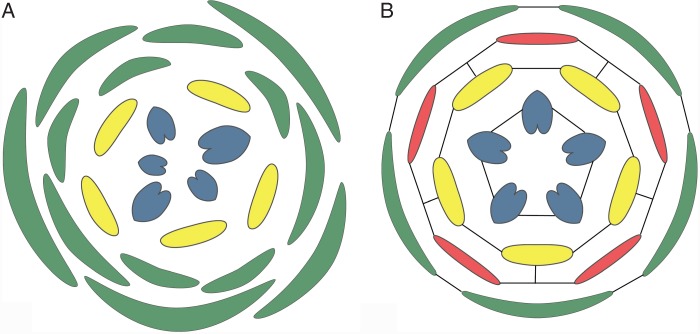
Precise localization of organs or organ parts is necessary for synorganization in development of the complex structure and at the same time for precise functioning of the flower [e.g. attachment of pollinaria to pollinators and deposition of pollinia on stigmas, such as in Catasetinae (Romero, 1990), see below]. An important precondition for such precise mutual position of the floral organs is whorled phyllotaxis, in contrast to spiral phyllotaxis (Endress, 1987, 1990, 2006). This allows (1) tangential, circumferential synorganization among the organs of a whorl (synsepaly, sympetaly, synstemony, syncarpy) and (2) sectorial synorganization (e.g. perianth organs and stamens in many monocots, and inner perianth organs and stamens in several basal eudicots) (Fig. 1). One of the most common tangential or circumferential synorganizations is syncarpy. It occurs in the majority of angiosperms (Endress, 1982). Also quite common is sympetaly, which is present in many eudicots, especially asterids, and syntepaly in several monocots. A combination of both tangential and radial synorganization also occurs, such as syntepaly plus fusion between tepals and stamens on the same radii in many monocots, and between petals and stamens in many asterids and some rosids (e.g. Galipeinae of Rutaceae, El Ottra et al., 2013), or between stamens and carpels in Orchidaceae and Apocynaceae (see below; Fig. 2).
Fig. 1.
Different potential for synorganization of floral organs in different phyllotaxis patterns. (A) Spiral phyllotaxis with limited potential. (B) Whorled floral phyllotaxis with high potential for radial and tangential synorganization. Black lines indicate preferred locations for synorganization.
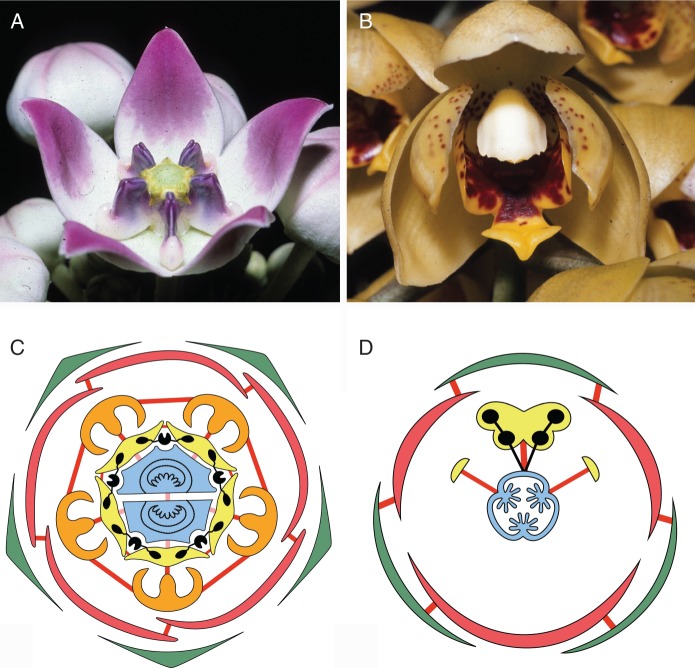
Fig. 2.
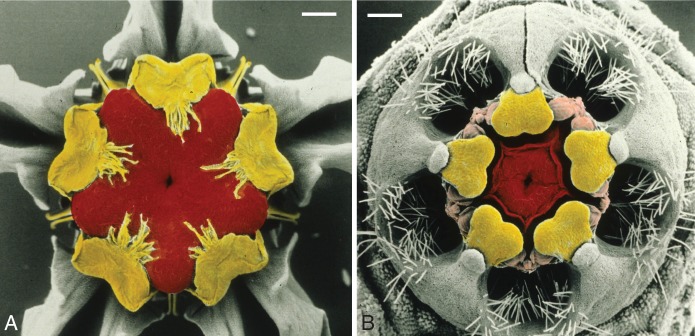
(A, B) Flowers with high degree of symmetry and firm consistency of floral organs with plastic-like appearance. (A) Calotropis procera (Apocynaceae-Asclepiadoideae). (B) Acineta densa (Orchidaceae-Epidendroideae). (C, D) Floral diagrams with synorganizations indicated. Green: outer whorl of perianth; red: inner whorl of perianth; orange: corona; yellow: androecium; blue: gynoecium; black: pollinarium. Thick red lines: congenital fusion; thick pink lines: postgenital fusion. (C) Asclepias (Apocynaceae). (D) Monandrous Orchidaceae, flower shown in resupinate position.
Developmental processes and results of synorganization; different kinds of fusion
Synorganization most commonly occurs by means of (1) postgenital fusion or (2) congenital fusion, and (3) rarely without fusion. In postgenital fusion adjacent free organs or parts of organs make secondary contact and the contiguous epidermises fuse. In congenital fusion the primary meristems of adjacent organs become confluent so that these organs develop as a unity (confluence of meristems or meristem fusion); thus, the epidermis is not involved in the fusion process. Postgenital and congenital fusion often co-occur in gynoecia and at specific locations. Postgenital fusion has also been called epidermal or ontogenetic fusion, and congenital fusion has also been called phylogenetic fusion (Cusick, 1966). For a discussion on meristem fusion, see Hagemann (1973), and for molecular aspects of fusion, see the review by Specht and Howarth (2015).
A general advantage of congenital fusion of organs in a flower is that differential elongation of the basal and upper part of all organs makes possible a broad potential of shapes with various proportions of free and fused parts. Without congenital fusion this is not easily achieved.
An interesting feature of postgenital fusion is the potential for easy reopening at anthesis after tight closure in bud (Endress, 2006). Petals are often (partially or completely) postgenitally united in bud (and partially also at anthesis) in many asterids, especially campanulids (Araliaceae, Asteraceae, Campanulaceae), in Gentianales (basal Apocynaceae sensu stricto (s.s.), Fallen, 1986; Rubiaceae, Robbrecht, 1988; basal lamiids: Icacinaceae, Endress and Rapini, 2014). Postgenital fusion is most common in gynoecia in intracarpellary and intercarpellary positions (e.g. Baum, 1948; Endress, 2015). It is also common in anthers, such as in many buzz-pollinated flowers (Endress, 1994, 2006) and in other complex flowers, such as in Balsaminaceae (e.g. Vogel and Cocucci, 1988). Rarely it occurs between stamens and carpels (balsaminoids, von Balthazar and Schönenberger, 2013).
A rare kind of intense synorganization without a particular fusion is known from Geranium robertianum (Endress, 2010). Here fusion only occurs in the gynoecium, which is syncarpous. All other synorganized parts (the sepals among themselves, and the sepals with petals, stamens and carpels) are free from each other but are held together by architectural modifications other than fusion.
General and conspicuous results of floral synorganization are an enhanced expression of the three-dimensional structure of the flowers and of internal morphological spaces. This may cause floral parts to become hidden. In addition, organs may become difficult to distinguish from their neighbours because their individuality becomes obliterated.
Also conspicuous is that synorganization leads to robustness or stability of the novel bauplan both in individual development and in evolution. Once the synorganized structures are established they are stable and are not easily lost again. This principle was nicely demonstrated by Simon (1962) with his watchmaker parable. At any new level of synorganization it is possible to experiment with variations at many single new points without harming the entirety of the bauplan and to attain new diversity.
EXTREME SYNORGANIZATION IN APOCYNACEAE AND ORCHIDACEAE
Apocynaceae (eudicots) and Orchidaceae (monocots) are the two families with the most extreme flower synorganizations among angiosperms. They are not phylogenetically related but have convergently evolved flowers with pollinia and pollinaria. A conspicuous difference is that the flowers of Apocynaceae are polysymmetric, those of Orchidaceae monosymmetric (Fig. 2).
Flowers of the most elaborate Apocynaceae (clade of Asclepiadoideae plus Secamonoideae)
It is easiest to begin with the most extremely complex flowers, thus flowers with the highest synorganization, and later show how the complexity develops during ontogeny and also show some of the evolutionary steps that led to the increasing synorganization. Floral organ numbers in the Asclepiadoideae plus Secamonoideae (this clade here called ‘ascleps’) are absolutely fixed: five sepals, five petals, five stamens and two carpels (as far as I know) in all 3100 species, without exception. Within some species, single aberrant flowers with four or six petals have been reported but are exceptional (Fuchs, 2013). In the basal grade of Apocynaceae it is similar, but there are a few taxa that have normally more than two (up to five) carpels (e.g. M. E. Endress et al., 1997), or rare mutants with surplus petals in species with normally 5-merous flowers (Wang et al., 2011). In ascleps, in addition, the floral organs of all floral whorls (except the sepals) are tangentially congenitally fused. Corona and stamens are also radially congenitally fused. Postgenital fusion occurs between the anthers and the style head and in the upper zone between the two carpels. In Apocynaceae in general, the corolla tube often has a postgenitally fused zone above the congenitally fused zone (e.g. Fallen, 1986; Kunze, 2005). This stability of the bauplan is necessary for synorganization. However, there is plasticity at other structural levels to attain the present diversity.
The complexity of the flowers in asclepiads is highlighted by three structures: (1) gynostegium, (2) pollinarium (five per flower) and (3) corona. These structures are not basic floral organs, but rather are already synorganized organs or new parts enabled by synorganization of other structures (see also Endress, 1994).
The uppermost part of the gynoecium, the style head, and the anthers are postgenitally fused. This organ complex is called gynostegium. On the surface of the gynostegium the functional units for pollen transport are formed. The style head itself develops by postgenital fusion of the two carpel tips. The corona is a novel structure between corolla and androecium; it is often highly subdivided into a number of parts, which together form a complex apparatus with several functions. In its simplest form there is a corona element behind each stamen and is fused with it, but other parts of the corona are often also present between the stamens. The pollinaria are formed by the synorganized androecium and gynoecium.
The pollinaria are the strangest parts of the flowers. Each pollinarium is a composite apparatus for pollen transfer. Pollen from each theca is united to form a compact pollinium, and is thus not dispersed as single grains. In addition, pollinia are not transported singly, but always two together, one each from two neighbouring anthers. They become connected by a translator. Each translator consists of a clip and two arms. Each pollinium is connected to an arm. The translator does not consist of tissue, but is composed of sculpted secretion. It is unusual for secreted materials to attain such a complex and precisely formed structure.
This raises several questions: Where and how is this translator formed? What are the mechanisms to take the translators out of a flower? What are the mechanisms to position the pollinia at the right site in the recipient flower? The answers to these questions are in the precise synorganization of the floral parts, especially the corona and the gynostegium.
The five translators are formed each on a corner of the expanded, pentagonal style head (Fig. 2A, C). Each corner has a longitudinally directed concavity, which functions as the mould for the secretion of a clip (Fig. 3A). The two flanks of the corner secrete the arms. Each arm contacts the adjacent pollinium, which is presented at this site from the opened anther and is attached to the arm. In this way the clip is presented exactly in the middle of the upper end of a guide rail (Figs 4 and 5A).
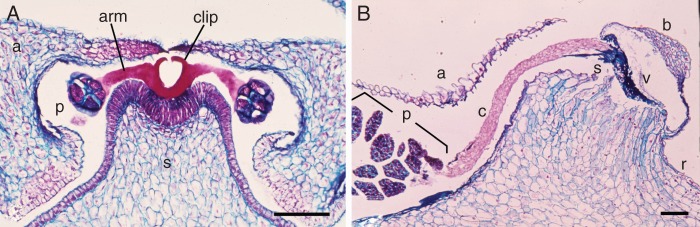
Fig. 3.
Pollinaria and their position in the flower. (A) Vincetoxicum nigrum (Apocynaceae-Asclepiadoideae), microtome transverse section of gynostegium, showing one corner of style head (s) with secreted translator consisting of clip and two arms, which have become attached to the pollinium (p) of the two adjacent anthers (a) and forming the pollinarium. See also overview of this section in Fig. 5A. (B) Ophrys fusca (Orchidaceae-Orchidoideae), microtome longitudinal section of gynostemium, showing part of the pollinarium, consisting of sectile pollinium (p) subdivided into massulae, caudicula (c) and viscidium (v) with scutellum (s), covered by bursicula (b); anther wall (a); rostellum (r). Scale bars: A = 100 µm, B = 200 µm.
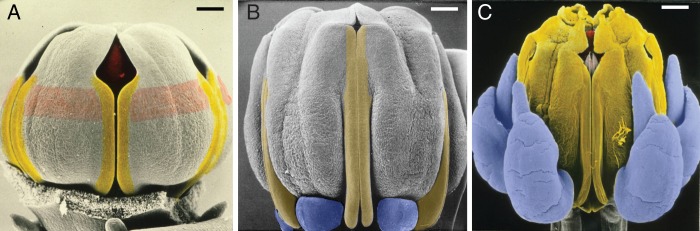
Fig. 4.
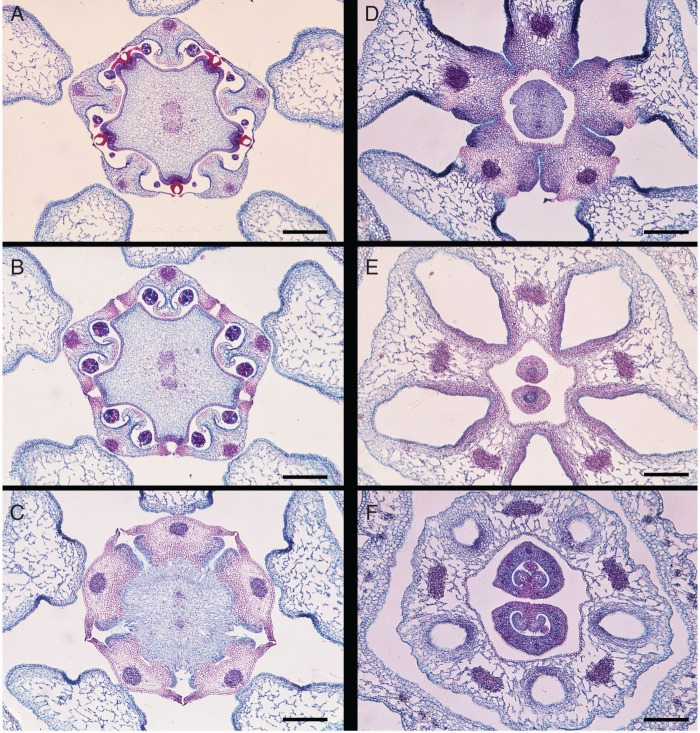
Young flower buds of Asclepias curassavica (Apocynaceae-Asclepiadoideae) showing developmental steps of synorganization, scanning electron micrographs, all from the side, with one guide rail in the centre. (A) The anther flanks that later form the guide rails are marked with yellow. The zone of postgenital fusion of the anther flanks with the style head marked with pink. The corona elements are not yet formed. (B) Later stage. The anther flanks that later form the guide rail are marked with yellow. The corona elements, marked with blue, beginning to be formed. (C) Still later stage. The entire anthers are marked with yellow; the lower entrance of the guide rail has become wider. Corona elements are now much longer. Clip of the pollinarium above the guide rail is marked with pink. Part of the style head above the clip is marked with red. Scale bars: A = 100 µm, B = 200 µm, C = 500 µm.
Fig. 5.
Centre of a flower with gynostegium of Vincetoxicum nigrum (Apocynaceae-Asclepiadoideae), microtome transverse section series at six levels, from top, downwards. (A) Level of the five translator glands at style head. The two carpels postgenitally fused and forming five edges, each with a translator gland, the two main carpellary vascular bundles still distinct. (B) Level of postgenital fusion of anther flanks with style head and histological reinforcement of anther flanks for guide rail function. (C) Level of the five stigmas at lower end of style head. (D) Level of the five nectaries in the five grooves below the guide rails. The five corona portions congenitally fused with the stamens. (E) Level of the five corona/stamen portions congenitally fused with each other, leaving five holes between them. The two carpels free. (F) Level of corona and stamens forming a ring around the gynoecium, at the base of the five holes. Carpels at the upper portion of the ovary. Scale bars: all = 500 µm.
In Asclepiadoideae, each guide rail is formed by the adjacent flanks of two neighbouring anthers. It guides pollinator body parts to the clip or to the stigma. Thus, there are five guide rails, five clips and five functional stigmatic units in each flower. The anther flanks that form the guide rails are the transformed (i.e. sterilized and histologically reinforced) dorsal pollen sacs of each theca. Thus, each anther produces only two pollinia (from the ventral pollen sacs). The anther flanks are postgenitally connected with the style head below the translator glands (Fig. 5B). The stigma is not on top of the gynoecium, but is located on the underside of the style head. It is subdivided into five functional units. Each unit is placed exactly in the radius of a guide rail. The stigma units are not visible from the outside because they are hidden in a concavity behind the guide rails on the underside of the style head (Fig. 5C). Below the stigma, at the base of the guide rails, there are five nectaries in niches between the (at this level) congenitally fused stamens (and stamens fused with the corona elements in the same radii) (Fig. 5D). The nectaries may also secrete additional substances, perhaps in conjunction with pollen tube growth (Christ and Schnepf, 1985; Vieira and Shepherd, 2002). Thus, in the five radii of the guide rails, exactly aligned from bottom to top, are the nectaries, the stigmas and the clips of the translators. The guide rails are broadest at the base and taper toward the top where the clip is located (Fig. 5A–D). Thus, body parts of visiting insects will easily get caught in the basal part of a guide rail and then be drawn upwards exactly into the clip when the insect moves about on the flower or leaves the flower. Below the guide rails the corona elements are, in addition, congenitally fused with each other (Fig. 5E, F).
Movements of insects on the flower are greatly encouraged by the compartmentalized location of the nectar. In Asclepias nectar is stored (and presented) in ten sectors of the flower by troughs formed by coronal elements, which are provided with nectar by capillary forces from the five nectaries (Galil and Zeroni, 1965). These flowers are thus intricately differentiated revolver flowers, i.e. flowers in which nectar is available for pollinators not from a single position, but in this case from five or ten different positions.
When a pollinarium is drawn out of a flower it is at first positioned perpendicular to the insect, but it soon (about 1 min) bends forward by a differential drying process of the translator. The translator is not homogeneous but is composed of lipophilic and hydrophilic components, which cause bending by the drying process (Schnepf et al., 1979). In this way it becomes optimally positioned for insertion into a guide rail of a recipient flower. If a pollinium comes into contact with the stigma, it sticks there and breaks off from the translator. The antestaminal elements of the corona also act as holding devices for insects and, because they protrude at the periphery of the floral centre, often the ends of the legs automatically come to lie into the guide rails, and most often the pollinaria become attached to the legs.
In most cases, an inserted pollinium provides only one of the two carpels with pollen tubes (Sage et al., 1990; survey in Vieira and Shepherd, 2002; Vieira et al., 2012). Thus, there is no functional compitum. Only rarely are both carpels served by the pollen tubes of a single pollinium (Kunze, 1991). There is a strange asymmetry because one carpel may be served from three stigmatic chambers, but the second carpel only from two. Often only one carpel develops into a fruit.
How can the style head of a dimerous gynoecium (Fig. 6A) produce five pollinaria? The mismatch between the pentamerous outer region of the flower and the dimerous gynoecium has been overcome by the early postgenital fusion of the carpels and by complete conformation of the gynoecium symmetry to that of the androecium. Thus, the upper part of the gynoecium becomes secondarily functionally pentamerous during development (Fig. 6B). This is nicely seen in cases in which the two carpels have a random, irregular position with respect to the outer floral whorls. This is an impressive example of an imprinted shape, a shape superimposed (moulded) by the five-angled shape of the immediate neighbourhood (Endress, 2008). The gynoecium remains clearly dimerous at the base, but looks pentamerous on top at anthesis. Only the two vascular bundles in the style head at anthesis give testimony to its dimerous origin (Fig. 5A–C). The disposition of a pentamerous androecium and dimerous gynoecium is most common in Gentianales (and in early branching Apocynaceae), and thus is most probably ancestral in the family and was present in the evolutionary history before the synorganization of androecium and gynoecium.
Fig. 6.
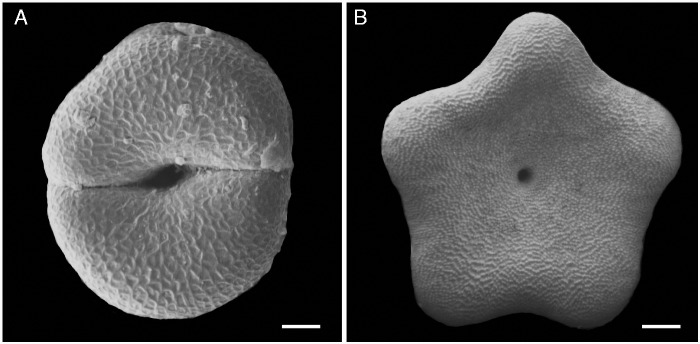
Style head moulding from disymmetry to pentasymmetry in two developmental stages, from above, in Gomphocarpus fruticosus (Apocynaceae-Asclepiadoideae), scanning electron micrographs (modified from Endress, 2006). (A) Very young stage, with the two carpels still distinct. (B) Older stage, with the two carpels postgenitally united and outline changed to five-angled. Scale bars: A = 50 µm, B = 500 µm.
Among asclepiads, diversity is expressed in particular in the shape of the corolla and corona. The corona is highly plastic with regard to nectar holder function (Kunze, 1997). Units of the corona may be simple in small flowers (e.g. Vincetoxicum) or complex in larger flowers (Fig. 7) (e.g. Asclepias, Galil and Zeroni, 1965, or Hoya, Kunze and Wanntorp, 2008), and convoluted in Calotropis (Puri and Shiam, 1966). Stamen shape in Asclepiadoideae is strongly influenced by the integration of all parts of the gynostegium (Liede, 1994; Kunze, 1996). The shape of the style head (Simões et al., 2007a) and the depth of the guide rails are evolutionarily plastic, correlated with the size of the pollinators (Fig. 8). Diversity in size of the entire flowers is addressed below. The synorganization of flowers in Asclepiadoideae underlying the diversity as described here is a constant feature through the subfamily, as several detailed morphological and developmental studies have shown (Corry, 1884; Demeter, 1922; Kunze, 1981, 1990, 1991, 1994, 1995, 2005; Demarco, 2014).
Fig. 7.
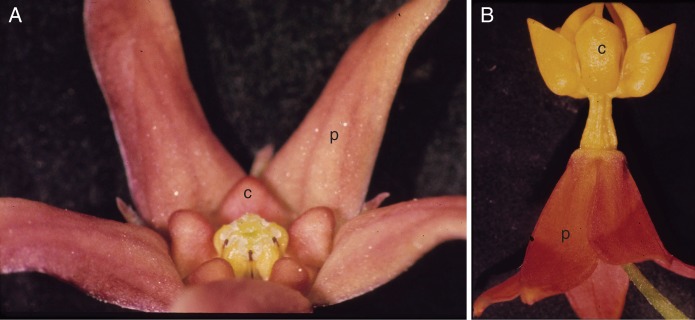
Flowers of Apocynaceae-Asclepiadoideae to show diversity in proportions of petals (p) and corona (c). (A) Vincetoxicum nigrum, with relatively small and simple corona. (B) Asclepias curassavica, with relatively large and complex corona.
Fig. 8.
Diversity in extension of the edges of the style head (marked with red), depth of the guide rails (marked with yellow, together with uppermost part of the stamens) and exposition of the pollinaria (marked with pink), correlated with pollinator size, in Apocynaceae-Asclepiadoideae. (A) Gomphocarpus fruticosus (style head edges not extended, guide rails deep, pollinaria hidden – for large pollinators). (B) Caralluma penicillata (style head edges long extended, guide rails shallow, pollinaria exposed – for small pollinators). Scale bars: both = 500 µm.
A pinnacle of complexity is exhibited by the flowers of Ceropegia, a genus of over 200 species (Huber, 1957; Vogel, 1961; The Plant List, http://www.theplantlist.org/; Figs 9 and 10). Here, not only are the corona, androecium and gynoecium intimately synorganized, but also the corolla is involved in the complexity in a unique way. The flowers are trap flowers with a long and slender tube formed by congenital fusion of the petals. The complex pollination apparatus is small and hidden in the base of the corolla tube. Thus, not only are parts of the androecium and gynoecium hidden, but the entire pollination apparatus. In the uppermost zone of the corolla the petals are free but are postgenitally fused in bud. At anthesis this postgenitally fused zone opens differentially. In the most complex species, such as C. distincta, at the base it opens to provide five separate entrances into the corolla tube, each with a wax-covered gliding zone. Then follows a zone where the petals remain fused and form a kind of central stalk. Above this, a second zone of opening presents a flag with the inner petal surfaces turned outward and acting as an osmophore to attract flies (Ollerton et al., 2009). The tips of the petals remain closed, forming a firm top of the flag. Some species have flickering hairs directed outwards at anthesis in the opening zones, which add to the attractivity for flies. Thus, the corolla exhibits five zones with regard to differential fusion, from base to top: (1) congenitally fused, (2) open, (3) postgenitally fused, (4) open and (5) postgenitally fused (Fig. 10).
Fig. 9.
Flowers with extremely complex corolla. (A) Ceropegia distincta (Apocynaceae-Asclepiadoideae). (B) Coryanthes macrantha (Orchidaceae-Epidendroideae).
Fig. 10.
Longitudinal differentiation of corolla and differential opening of zone of postgenital fusion of petals at anthesis in Ceropegia distincta (Apocynaceae-Asclepiadoideae). Left: floral bud. Corolla congenitally fused in the lower half, postgenitally fused in the upper half. Right: open flower. Corolla with five zones from base to top: (1) congenitally fused (floral tube), (2) open (five entrances with gliding zones for pollinators), (3) postgenitally fused (stalk of flag), (4) open (osmophoric flag), (5) postgenitally fused (upper end of flag).
Stepwise synorganization of the flowers in the phylogeny and evolution of Apocynaceae sensu lato (s.l.)
The phylogeny of Apocynaceae s.l. has been intensively studied in the past 20 years, and for some subclades aspects of floral evolution have been analysed (Nilsson et al., 1993; M. E. Endress et al., 1996, 2007a, b, 2014; Sennblad and Bremer, 1996, 2002; Civeyrel et al., 1998; Sennblad et al., 1998; M. E. Endress and Bruyns, 2000; M. E. Endress, 2003, 2004; M. E. Endress and Stevens, 2001; Rapini et al., 2003, 2006, 2007; Verhoeven et al., 2003; Simões et al., 2004, 2007b, 2010; Liede-Schumann et al., 2005; Ionta and Judd, 2007; Livshultz et al., 2007; Livshultz, 2010; Rapini, 2012; Nazar et al., 2013; M. E. Endress et al., 2014; Straub et al., 2014). In the basal subclades of Apocynaceae (grade of Rauvolfioideae), the degree of floral synorganization is relatively low. There are no pollinaria, no pollinia and no gynostegium. The very top of the gynoecium is commonly not secretory, but the secretory part somewhat lower down is without functional differentiation into receptive and non-receptive zones in some groups (e.g. Condylocarpon, Aspidosperma, M. E. Endress, pers. comm.). An incipient corona may be present as alternipetalous lobes on the corolla (corolline corona) as also in the sister family Gentianaceae; in some genera of Rauvolfioideae these corona lobes are conspicuous. The corolla is sympetalous, and the stamens are fused with the corolla and have two pollen sacs per theca. However, the flowers are already revolver flowers, as also in some other polysymmetric asterids.
There are trends in the evolutionary pathway of the pollinia and pollinaria and of style head differentiation. In Rauvolfioideae and Apocynoideae, pollen is normally dispersed as single grains, but in both subfamilies there are some taxa with tetrads. The proboscis of pollinating insects becomes sticky by touching the style head and takes up pollen, which may be deposited on the stigmas of recipient flowers. Schick (1982a) distinguished two types and Fallen (1986) found four levels of increasing complexity of the style head in Apocynaceae s.s.: (1) the entire style head is secretory and (probably) stigmatic in its entirety; (2) the stigma is restricted to the lowermost part of the style head below a downward directed collar, which collects incoming pollen, whereas the upper part of the style head secretes the adhesive for pollen transport, and an upper ring of hairs presents the pollen shed from the anthers; (3) in addition, the anthers are postgenitally fused with the style head, and thus the gynostegium emerged (occurs only in Apocynoideae); (4) the downward directed collar and the upper ring of hairs disappeared and, instead, hairs are present on the ventral side of the anthers, which scrape incoming pollen from the proboscis of insects. The most interesting innovation in Apocynoideae, found in Apocynum and Forsteronia, is a precursor of a translator. It consists of five small platelets of a gummy consistency secreted between the anthers on the style head, which take up pollen from the adjacent thecae of two anthers and are removed and transported by pollinators (Demeter, 1922; Nilsson et al., 1993; M. E. Endress, 2003); pollen still occurs as single grains (as tetrads in Apocynum), and the translator is without attachment device to pollinators, except for sticky secretion.
In Periplocoideae, the translator is spoon-shaped. Pollen is transported in the form of free tetrads or tetrads packed in soft pollinia (without pollinium wall) (Verhoeven and Venter, 2001), and is deposited in the sticky concave part of the spoon. The other end of the spoon, the ‘handle’, has a sticky pad underneath, which is attached to pollinators (Demeter, 1922; M. E. Endress, 2003). It has been assumed that pollinia evolved twice in Periplocoideae (Verhoeven and Venter, 2001) or at least three or four times (Ionta and Judd, 2007).
In Secamonoideae and Asclepiadoideae the pollinaria are attached to pollinators with a clip. In Secamonoideaae, the translator consists only of this clip, and pollen is in tetrads within the soft pollinia (without pollinium wall). In Asclepiadoideae the translator has, in addition, two arms, and the pollinia are hard, having a pollinium wall, and pollen is no longer in recognizable tetrads within the pollinium (Verhoeven and Venter, 2001). Within Asclepiadoideae, only Fockea still has tetrads in the pollinia, which are soft and lack a pollinium wall (Verhoeven and Venter, 2001).
Another important innovation in Asclepiadoideae is a reorganization of the anthers. The dorsal pollen sacs disappear, and instead the now sterile anther flanks form the rigid (lignified) guide rails that direct pollinator body parts precisely into the clip of the translators (Kunze, 1996). In addition to this enhanced precision, the diversity of the depth of the guide rails adds to enhanced pollinator specificity (Fig. 8). Another consequence is that each pollinarium here consists of only two pollinia (in contrast to the four pollinia from the ancestral two pollen sacs per theca in Secamonoideae; Safwat, 1962).
The corona is ancestrally corolline, and thus develops on the corolla in alternipetalous positions (Fishbein, 2001; Kunze, 2005). This is not only the case in Apocynaceae but also in other Gentianales (Gentianaceae). In addition, there is a staminal corona in some Periplocoideae and in Secamonoideae and Asclepiadoideae (Rudjiman, 1982; Kunze, 2005). This also develops in alternipetalous position but is closely associated morphologically and functionally with the androecium. A novelty here is that corona elements also develop in alternistaminal position. All elements together may form a complex nectar holder (and a holding device for nectar-seeking pollinators) of these revolver flowers (Liede and Kunze, 1993; Asclepias, Galil and Zeroni, 1965; Hoya, Kunze and Wanntorp, 2008). The consistency of the corona is then conspicuously firm. This is also true for the corolla in many Asclepiadoideae (Fig. 2A). Associated with such firm consistency is also a diversity of surface sculptures of corolla and corona (Ehler, 1975; Bruyns et al., 2005). If the staminal corona is large and the five units are bulging, as in Asclepias, each of these convex, smooth and slippery units leads a pollinator leg exactly towards a guide rail (Fig. 7B).
Flowers of the most elaborate Orchidaceae
As in Apocynaceae s.l., an impressive increase in synorganization can be observed following evolutionary trends through the family Orchidaceae. I will also begin with the most extremely synorganized clade, which constitutes the largest subfamily Epidendroideae (with 20 000 species; Chase et al., 2015) and then show aspects of the evolution of this complexity. As in the derived subfamilies of Apocynaceae, in Epidendroideae the number of floral organs is also completely fixed, always with 3 + 3 perianth organs, 1 stamen and 3 carpels. The same is true in Orchidoideae and Vanilloideae. Orchidaceae with a single stamen are often called monandrous orchids (but they do not form a clade). In the two smallest subfamilies stamen number is higher: 2–3 in Apostasioideae and 2 in Cypripedioideae. In many of the derived Orchidaceae (Orchidoideae and Epidendroideae) with a single stamen, two developmentally early formed lateral outgrowths of the gynostemium are interpreted as staminodia; they may canalize the movements of the pollinators (Burns-Balogh and Bernhardt, 1985; Kurzweil and Kocyan, 2002). However, lateral appendages in some Orchidoideae may not be remnants of staminodia but late elaborations of the fertile stamen (reviewed by Kurzweil and Kocyan, 2002). In contrast to the polysymmetric Apocynaceae, the flowers of Orchidaceae are monosymmetric. The single stamen is in the single symmetry plane (Fig. 2B, D). All Orchidaceae have an inferior ovary.
The organs (tepals) of the two perianth whorls are congenitally fused, at least at the base, and also between the whorls. The three carpels are congenitally fused up to the top. Also the single stamen is congenitally fused with the gynoecium up to the top, androecium and gynoecium together forming a gynostemium (also called column). Note the difference between Orchidaceae and Apocynaceae: gynostemium (congenitally fused) vs. gynostegium (postgenitally fused). The perianth organ on the opposite side of the flower to the stamen, the lip, is commonly more elaborate and larger than the other five. It is part of the inner perianth whorl. It mainly functions as a landing platform for pollinators. In all Orchidaceae early floral development is remarkably similar. The flowers are pronouncedly monosymmetric from the beginning, and all six tepals originate as a conspicuous medianly compressed ring wall, without distinction of the single organs, and thus they apear congenitally fused before their tips become visible (Bletia, Kurzweil, 1987a, fig. 2C; Malaxis, Kurzweil, 1987a, fig. 4A, B; Dactylorrhiza, Kurzweil, 1987b, fig. 1A–C; Prescottia, Kurzweil, 1988, fig. 4A, B; Listera, Kurzweil, 1988, fig. 6A; Phragmipedium, Kurzweil, 1993, fig. 4B, C; Oncidium, Endress, 1994, fig. 8.67.1–3; this study, Fig. 11; Satyrium, Kurzweil, 1996, fig. 3a; Pholidota, Kurzweil, 1998, fig. 6A; Hemipilia, Luo and Chen, 2000, fig. 1B, C; Amitostigma, Luo and Chen, 2000, fig. 3B, C; Gymnadenia, Luo and Chen, 2000, fig. 5B, C; Platanthera, Luo and Chen, 2000, fig. 5S, T; Telipogon, Pabón-Mora and González, 2008, figs 2D, 4D–F, 5B, E). Only in Apostasioideae is this early fusion less pronounced, but also present (Kocyan and Endress, 2001, figs 2B, C, H, I, N, O, 11B, G, L). Floral development of orchids has been reviewed by Kurzweil (1998) and Kurzweil and Kocyan (2002).
Fig. 11.
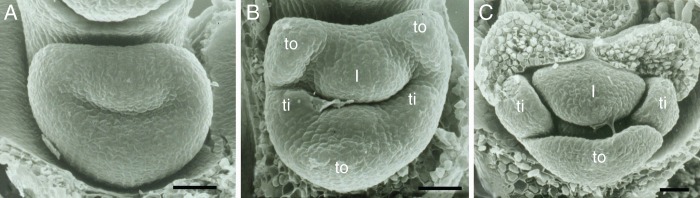
Congenital fusion of all organs in very young flowers of Oncidium ornithorhynchum (Orchidaceae-Epidendroideae), scanning electron micrographs, flowers not resupinated, and thus lip located in upper part (modified from Endress 1994). (A) All six perianth organs congenitally fused, forming a ring wall, individual organs not yet visible. (B) Individual perianth organs visible: outer tepals (to), inner tepals (ti), lip (l). (C) More advanced stage, with lateral outer tepals removed, gynostemium hidden by median outer tepal and lip; abbreviations as in (B). Scale bars: all = 50 µm.
The complete congenital fusion between the stamen and the gynoecium ensures the immediate proximity of the androecial and gynoecial parts that contribute to the formation of the pollinarium. Pollen of the anther is basically organized into four pollinia, one per pollen sac. In many groups the two pollen masses of the two pollen sacs in a theca form a single pollinium so that the pollinarium has only two pollinia (Rasmussen, 1986a), comparable to Asclepiadoideae (but where dimery results from transformation of the dorsal pollen sacs into sterile but mechanically reinforced parts). The stamen is in the symmetry plane of the flower and one of the three carpels is also in the symmetry plane and is adjacent to the stamen. The tip of this carpel is differentiated into a proximal stigmatic zone and a terminal secretory zone (viscidium) that comes into contact with the pollinia, and the secreted material acts as a glue to attach the pollinarium to a pollinator (Yeung, 1987a; Prutsch and Schill, 2000). The joint between the pollinia (coming from the androecium) and the viscidium (coming from the gynoecium) is a piece of disintegrated tissue from the anther (elastoviscin) with elastic properties (Dressler, 1986; Schill and Wolter, 1986; Wolter and Schill, 1986) in most Orchidaceae. However, in Cymbidieae and Vandeae of Epidendroideae, this joint is a piece of tissue from the short tip of the median carpel. This carpel tip is called a rostellum. Depending on the site of origin the joint is called a caudicle (caudicula) (if from the androecium) or a stipe (stipes) (if from the gynoecium); there are even additional terms used in the literature (Rasmussen, 1982, 1985, 1986a, b). Pollinaria with a stipe also have a short portion of a caudicle adjacent to the pollinia. Thus, there is a combination of caudicle and stipe, whereby the largest part of the joint is made up by the stipe. The joint functionally corresponds to the arms of the translator in Apocynaceae. Conspicuous diversity is also exhibited by the proportions and directions of curvature of the gynostemium, which aid in attaching pollinaria to different body parts of different pollinators (Vogel, 1959; Garay, 1972; Rasmussen, 1982, 1986a, b; Burns-Balogh and Bernhardt, 1985; Manning and Linder, 1992). Dressler (1981) mentioned 13 areas for pollinarium attachment in euglossine bees.
Because of their often firm structure, the perianth organs, especially the lip (labellum), are extremely plastic in shape and diverse in details of surface differentiations (holding structures, secretory structures, three-dimensional surface sculptures of optical significance) (e.g. Davies et al., 2002; Davies and Stipczynska, 2006; Bradshaw et al., 2010). The firm structure also allows the differentiation of a spur (another means of diversification is by spur diversity, e.g. Kurzweil and Weber, 1992; Micheneau et al., 2009). Rarely, two collateral spurs are present (Satyrium, Satyridium, Vogel, 1959; Kurzweil, 1996) or a complex spur in which the lip and neighbouring tepals participate (Vogel, 1969). Spurs on tepals of the outer whorl also occur but are less common than lip spurs (Vogel, 1959, 1969; Kurzweil, 1998). Optical, olfactorial and tactile devices on the lip are involved in different kinds of deception (food, brood site, sexual deceit, floral mimicry) of pollinators (e.g. Ackerman, 1986; Davies et al., 2002; Cozzolino and Widmer, 2005; Schiestl, 2005; Jersákova et al., 2006). Sexual deceit appears to be only known from orchids among angiosperms (Cozzolino and Widmer, 2005). The spur is involved in nectar production in many orchids. However, also here there is deception: many spurs do not secrete nectar. Nectar may also be produced from floral parts other than spurs. Loss and gain of nectar is plastic and may have occurred many times even within a genus (e.g. Disa, Hobbhahn et al., 2013). A number of orchids have flowers that produce oil, either exposed on the lip (Vogel, 1974) or in a spur (Steiner, 2010).
Because of its often highly three-dimensional structure and sculpture, the lip of the orchids has sometimes been interpreted as a complex organ, a tepal fused with staminodia, beginning with Brown (1833). At present the tendency is rather to assume it to simply represent a tepal (Endress, 1994; Rudall and Bateman, 2002, 2004; Rudall et al., 2013). However, a decisive answer is elusive because all outer floral organs are completely congenitally fused from the beginning (Fig. 11). In another, unrelated, clade of monocots, Zingiberaceae plus Costaceae, which also have a lip, the lip consists of two fused staminodia in the former, and of five fused staminodia in the latter (Kirchoff, 1988, 1997; Endress, 1995; 2–4 in Zingiberaceae, according to Specht et al., 2012). As there is such divergence of lip formation within a clade of two families in Zingiberales, the question of whether the lip is homologous in all Orchidaceae should also be explored. Although molecular developmental studies are being performed in orchids, the specific question of lip homology has been difficult to tackle because in monocots perianth determination is somewhat different from eudicots (to which Arabidopsis and Antirrhinum belong) and because in orchids androecium and gynoecium are completely congenitally united into a gynostemium (Mondragón-Palomino and Theissen, 2008, 2009, 2011; Pan et al., 2011; Mondragón-Palomino, 2013).
A speciality in some derived clades of Orchidaceae are extremely high ovule and seed numbers (689 000 ovules per ovary estimated for Coryanthes senghasiana; Nazarov and Gerlach, 1997). This is enabled by non-synchronous ovule development on large, convoluted surfaces. It is associated with an enormously high pollen number in each pollinium. In a study of eight species of eight genera, a range of 40 000–4000 000 pollen per pollinium was calculated (the maximum among them for Cochleanthus discolor) (Schill et al., 1992).
Coryanthes exhibits an extreme three-dimensional differentiation of the large, hanging flowers, in which the labellum takes part prominently; it is a genus of approx. 60 species (Gerlach and Schill, 1993; Fig. 9B). The lip is longitudinally differentiated into three conspicuous parts. The distal part has the shape of a hanging bucket. It tapers into a part bearing a helmet-shaped structure. The helmet is fastened with a horizontally directed stalk at the pendant floral base. The stalk is associated with two protrusions. The protrusions look like twin water taps (faucets), and secrete water drops, which at anthesis continuously drip into the bucket, filling it with water. The helmet is a gland, secreting a perfume, which is collected by male euglossine bees for attracting females (Dodson, 1965; Vogel, 1966). The bees flying around the helmet and collecting perfume will nolens volens touch the water drops hanging from the taps with their wings and, when these get wet, immediately fall into the water-filled bucket. From there they find only one narrow exit at the morphological tip of the labellum, which is more or less obstructed by the tip of the rostellum. In struggling on their way out they either become the pollinarium of this flower attached to their back or, if they already have one attached to their body from another flower, they pollinate the stigma with it (Gerlach, 2011).
Stepwise synorganization of the flowers in Orchidaceae
As in Apocynaceae, phylogeny across Orchidaceae and some aspects of flower evolution have been intensively studied in recent decades (for a phylogeny of the family or subfamilies: Burns-Balogh and Funk, 1986; Cameron et al., 1999; Cameron and Chase, 2000; Freudenstein et al., 2004; Kocyan et al., 2004; van den Berg et al., 2005; Cameron, 2006; Carlsward et al., 2006; Górniak et al., 2010; Chase et al., 2015; Freudenstein and Chase, 2015).
In the basal clade of Orchidaceae, Apostasioideae, there are no pollinaria, no pollinia, only an incipient gynostemium, floral monosymmetry is already present but not conspicuous, and flowers are only moderately complex. In some Apostasia species with superficially almost polysymmetric flowers, this feature appears to be secondary, as they are probably buzz-pollinated, and thus a special lip is not needed, whereas Neuwiedia has a lip and is not buzz-pollinated (Vogel, 1998; Kocyan and Endress, 2001). In addition, the androecium is monosymmetric in all Apostasioideae, also in early development; stamens are only formed on the developmentally abaxial side of the flower. In some cases stamen primordia also appear to be present on the adaxial side but do not develop into stamens (Kocyan and Endress, 2001).
In Apostasioideae the stamens (and staminode) are congenitally fused with the style for about half its length, and the style is shorter or longer than the stamens (Kocyan and Endress, 2001). In the other subfamilies (except for Cypripedioideae), only the median stamen is formed. It is developmentally abaxial but becomes secondarily adaxial in almost all orchids, either by torsion (resupination) of the flower or, in epiphytic groups, by the hanging position of the inflorescence. By complete congenital fusion of the remaining stamen with the gynoecium, and adjustment of the length of these two components, the position of the anther and the median carpel tip become closely associated. This is a precondition for the evolution of the pollinarium.
Pollen is present as separate grains (monads) surrounded by pollen kitt or elastoviscin in Apostasioideae and Cypripedioideae (Schill and Wolter, 1986; Pacini and Hesse, 2002; Pacini, 2009), rarely soft pollinia in Cypripedioideae (Johnson and Edwards, 2000). Vanilloideae have monads or tetrads, rarely pollinia. Orchidoideae and Epidendroideae predominantly have pollinia. In some Orchidoideae the pollinia are ‘sectile’ and portioned into numerous massulae, which are loosely connected by elastoviscin. In a flower visit, part of the massulae can be deposited on the stigma, and in this way a pollinium may be used for several pollinations (Freudenstein and Rasmussen, 1997; Johnson and Edwards, 2000; Pacini and Hesse, 2002; Pacini, 2009). Anthers with pollen not organized into pollinia or with pollinia with massulae occur in taxa with fewer ovules per flower.
The normal number of two or four pollinia per anther may be increased by septation of archesporial areas into eight, or this septation may be incomplete so that pollinia are incompletely divided (Hirmer, 1920; Freudenstein and Rasmussen, 1996). In Epidendroideae (and Vanilloideae), the rostellum with the stigma is bent down towards the lip and concomitantly the anther is also curved downwards (‘incumbent’) (Dressler, 1981; Freudenstein and Chase, 2015). In non-vandoids, curvature is at the base of the anther, whereas in vandoids it is in the sporogenous zone (Freudenstein et al., 2002). A consequence is that in vandoids the sporogenous tissue acquires a convolute shape. This increase of the surface area of the sporogenous tissue per volume may be advantageous for the synchronous process of meiosis of the large and pollen-rich microsporangia. However, because of the curvature it is not always easy to determine the number of microsporangia in an anther from serial microtome sections (Hirmer, 1920; Freudenstein and Rasmussen, 1996).
Viscidia are not present in the three small subfamilies, Apostasioideae, Cypripedioideae (Johnson and Edwards, 2000) and Vanilloideae (Cameron, 2003b), but are present throughout in Orchidoideae and Epidendroideae. Generally there is one viscidium, but in some Orchideae and some Vandeae there are two viscidia associated with two separate pollinaria, which are removed together or separately (Schill and Pfeiffer, 1977; Johnson and Edwards, 2000). In groups without viscidia, contact of the pollinator with secretion from the median stigmatic lobe may help in attaching pollen to the pollinator (Schick, 1989).
In some Orchidoideae, the viscidium is covered by a bursicula, a ‘small purse’, formed by the rostellum (Fig. 3B). The bursicula is pushed backwards by the pollinator, exposing the viscidium so that the pollinarium adheres to the pollinator. The viscidium is sometimes associated with a small plate (scutellum), which makes it more robust, and it is then called a retinaculum (Fig. 3B). This occurs especially in Orchidoideae and Epidendroideae. Elaborations of this region were studied in more detail by Schick (1988, 1989).
There is yet more complexity in the detailed structure of the pollinarium in Epidendroideae. In Cymbidieae and Vandeae, in particular, the joint between pollinia and viscidium consists not only of caudicles but, in addition and for its main part, of a stipe. In flowers with elaborate pollinarium application mechanisms, stipes can perform forceful movements by precise deformation (Catasetinae, Romero, 1990), which would perhaps not be possible for simple caudicles. This is an enhanced way of synorganization between androecium and gynoecium. It requires a developmentally early bending of the anther, in contrast to pollinarium development with simple caudicles (Kurzweil, 1987a; Freudenstein et al., 2002). This anther bending in flowers with stipes is congenital, i.e. the anther is incumbent from the beginning of development, whereas in other epidendroids bending occurs only late in development (Kurzweil, 1987a; Freudenstein et al., 2002; Freudenstein and Chase, 2015). Stipes and early anther incumbence probably evolved at least twice (Freudenstein and Chase, 2015). Additional synorganization in Epidendroideae also occurs between the lip and the gynostemium by extensive congenital fusion (especially in Epidendrum; with some 1400 species this is one of the largest genera of Orchidaceae).
A trilocular or almost trilocular ovary occurs in Apostasioideae, Vanilloideae and Cypripedioideae (Cameron, 2003a). However, it is unilocular and thus the carpels are more synorganized in Orchidoideae and Epidendroideae, as the presence of one unified locule allows more space than three separate locules for the development of an excessive number of ovules in ovaries of the same size.
Convergences in flowers of Apocynaceae and Orchidaceae: Fixed features
The advanced clades within Apocynaceae and Orchidaceae share many prominent features that are all connected with extreme synorganization, either as preconditions for or as results of synorganization or both:
stability of floral organ number,
highly regular floral symmetry (pentasymmetry in Asclepiadoideae, monosymmetry in Orchidaceae),
thick and firm consistency of floral organs (often plastic-like),
fusion of androecium and gynoecium (postgenital in Asclepiadoideae, congenital in Orchidaceae: gynostegium vs. gynostemium),
pollen aggregation into pollinia (Pacini and Hesse, 2002; Harder and Johnson, 2008),
two (or more) pollinia organized into pollinaria (with components from androecium and gynoecium),
pollinaria with a translator (at least partly secreted or of transformed tissue, i.e. elastoviscin) with an efficient apparatus to attach the pollinarium to a pollinator (glue or clip in Apocynaceae, glue in Orchidaceae),
attachment of pollinia to the translator with elastoviscin (Wolter and Schill, 1986; Dannenbaum and Schill, 1991; Pacini and Hesse, 2002),
number of pollinia per pollinarium two in all Asclepiadoideae and in many orchids (by a decrease of number of microsporangia per theca to one in Asclepiadoideae, and by synorganization of the two pollen sacs per theca into one in many Orchidaceae),
forward movement of pollinia by bending of the translator by desiccation after extraction from the flower, to reach optimal positioning of pollinia for application to a stigma, which is different from the position at extraction,
presence of floral guiding parts for exact positioning of pollinators (guide rails formed by anther flanks and shape of corona in Asclepiadoideae, floral monosymmetry and shape of lip and lateral outgrowths of gynostemium in Orchidaceae),
a consequence of the presence of pollinia is that the stigma becomes hidden in both families (in Asclepiadoideae the stigma is hidden in the guide rail, in some Orchidaceae it is a concavity below the rostellum; e.g. Dannenbaum et al., 1989),
strong sectorial differentiation of the flowers: in Apocynaceae, this has led to the functional differentiation of one flower into five meranthia; these flowers can be visited and pollinated from five different sides; an exceptional pattern also occurs in an orchid: Huttonaea pulchra has two meranthia, and thus the two-spurred flowers can be pollinated from two sides; each side has a separate pollinarium (Steiner, 2010).
Very prominent are novel floral parts that converge in both families: pollinia, pollinaria with translator, corona (corona only in Apocynaceae).
Convergences in flowers of Apocynaceae and Orchidaceae: new flexible features: sources of diversification
Orchids and ascleps with their highly synorganized flowers have attained a high diversity within the confinements of their bauplan. Although this bauplan is remarkably fixed with regard to the number, position and fusion of the conventional floral organs, flexibility has arisen superposed on it. Pollinaria are extremely diverse in size and proportions in both families, and in asclepiads also in restrictions of the site in the pollinium where pollen can germinate, which differs from species to species (Schill and Pfeiffer, 1977; Schill and Jäkel, 1978; Johnson and Edwards, 2000). The guiding organs for pollinators, guide rails in asclepiads and spurs in orchids, vary greatly in depth. The firm texture of the corolla and corona in asclepiads and of the lip in orchids allows a multitude of surface elaborations for diverse tactile, optical and scent properties (Ehler, 1975, 1976; Schiestl, 2005). Pollinia, although they are key innovations in both families, are by no means uniform but are diverse in some groups, especially in Orchidoideae, where they may become portioned (sectile) or lose coherence in other ways (Freudenstein and Rasmussen, 1997; Pacini, 2009). Pollinaria (translators) are also diverse in asclepiads (e.g. Schill and Jäkel, 1978; Cocucci et al., 2014) and orchids (Freudenstein and Chase, 2015).
Because of the fixed, precise position of the floral organs, progressive latitudinal and longitudinal floral differentiation with local separation of functions becomes possible. In basal Apocynaceae, there is no clear differentiation of the style head into a receptive area and an area with mere sticky secretion, but in more advanced clades there is spatial separation of stigma and translator secretion. Likewise in basal Orchidaceae, the receptive area and sticky part are identical and later in evolution there is separation of stigma and viscidium.
A highly specialized case of longitudinal elaboration in Apocynaceae is differential postgenital fusion in the corolla as described above for Ceropegia (Fig. 10), which has led to different functional zones of the petals and diversity within the genus. In Orchidaceae with longitudinal differentiation in shape and function, an impressive case is the lip of Coryanthes (Fig. 9B). In the two extreme cases, Ceropegia and Coryanthes, the perianth has become most diverse and inventive, after the formation of pollinaria was established and thus evolution proceeded to produce additional diversification at new levels.
In both families the novel floral organs or elaborated parts of old organs led to a confusing plethora of new terms (some of them redundant). In Apocynaceae this is especially the case for corona elements and parts of the pollinarium (e.g. Bhatnagar, 1975; Bookman, 1981), and in Orchidaceae also for parts of the pollinarium and for the rostellum (Rasmussen, 1986a). A source of confusion may also be that the term retinaculum is used in both families, but with different meanings. In Apocynaceae it has been used to refer to the postgenital attachment area of the stamens to the style head, whereas in Orchidaceae it means a viscidium that is associated with a scutellum.
Changes in floral size in Apocynaceae and Orchidaceae, compared with less synorganized angiosperms
A general means of diversification in angiosperms is evolutionary change in flower size. It is interesting to see how flowers become miniaturized. The pathways are different depending on the degree of synorganization. In clades with a lower degree of synorganization, miniaturization occurs by a decrease of floral organ number and decrease of organ size. In highly synorganized flowers a decrease of organ number is not possible but there is more potential for a decrease of organ size in flowers with strongly fused organs. Congenital fusion of organs leads to a reinforcement of the architecture of the entire flower so that less tissue is neccessary and may result in a more economical construction. An extreme case of each group, Asclepiadoideae and Orchidaceae, and an extreme case of an angiosperm clade with low degree of synorganization, Nymphaeales, may show this.
Among the smallest orchids are species of Oberonia with flowers 1 mm in diameter (Pridgeon et al., 2005). In Asclepiadoideae, flowers of some Tassadia species are also 1 mm in diameter (Medeiros et al., 2008). In both cases these flowers have the full set of floral organs (as described above for the two clades). The same set is also present in the largest flowers in the two families (Stapelia gigantea, up to 40 cm in diameter, Meve and Liede, 1994; and Phragmipedium caudatum, with inner tepals up to 75 cm long, Vogel, 1963). Also of interest is that in these cases what is increased are not the most synorganized parts (gynostemium or gynostegium) but the perianth organs, which are more independent and less functionally burdened.
In contrast, in Nymphaeales, although they exhibit about the same range of floral sizes, the extremes are reached differently. The smallest flowers are in Trithuria (Hydatellaceae), which are around 1 mm long (Rudall et al., 2007), and the largest are in Victoria (Nymphaeaceae), which are up to 50 cm in diameter (Schneider and Williamson, 1993). To reach the miniature size of flowers of Trithuria appears only possible by stripping the flowers of all organs but one. Trithuria species are wetland plants with unisexual flowers (for a discussion of evolution, see Endress and Doyle, 2009). Even within the genus Nymphaea, a change in flower size is conspicuoulsy linked to a decrease or increase in floral organ number. An example is Nymphaea micrantha, which produces two kinds of flowers. Flowers from dwarf plants, grown from bulbils, are much smaller and have many fewer organs than flowers from plants grown from seeds (Schmucker, 1932). The length of outer tepals is 72 mm in plants grown from seeds (21 mm in plants grown from bulbils). The difference in mean number of floral organs is as follows: outer (sepaloid) tepals 4 (4), inner (petaloid) tepals 18 (11), stamens 111 (21), carpels 23 (8) (Schmucker, 1932).
Differences between Apocynaceae and Orchidaceae
The main differences in the evolutionary behaviour of flowers of Apocynaceae and Orchidaceae are due to the different symmetry: polysymmetry (pentasymmetry) in the former, and monosymmetry in the latter. However, because Apocynaceae are so highly differentiated into five sectors they could also be viewed as consisting of five monosymmetric modules in some sense and representing five meranthia. In both families the pollinators, predominantly insects, are forced into fixed positions for the pollination process by the specific floral architecture. These positions are exactly in the symmetry planes, five in Apocynaceae and one in Orchidaceae. In the elaborate revolver flowers of the Apocynaceae the body parts are guided by the corona elements and anthers into the five guide rails; in Orchidaceae the body is guided by the shape of the lip and the specific position of the attractive site, be it nectar or oil, often in a spur, or a dummy in deceptive flowers, and perhaps also by the two lateral appendages of the gynostemium.
Regarding diversity, according to Johnson and Edwards (2000, p. 243), ‘The bilateral symmetry of orchids has allowed a greater degree of specialization in pollination systems and a much greater diversity in the morphology of pollinaria [than in ascleps].’ Although orchids are uniform in having an attachment mechanism with glue, whereas in Apocynaceae there was an evolutionary change from glue to a clip, there is great diversity in the differentiation of the sticky part in orchids (see above, Schick, 1988, 1989). However, it may be added that the diversity of the translator shape of the pollinaria is higher in Asclepiadoideae than in Orchidaceae in some respects (Schill and Jäkel, 1978; Wiemer et al., 2012; Cocucci et al., 2014). In contrast to Orchidaceae, there are no spurs in Apocynaceae. In the latter the access to the flowers for different-sized pollinators is mechanically regulated by the general flower size, the size and shape of the corona, and specifically the depth and robustness of the guide rails.
Early flower development is different in the two families. In the highly synorganized clades of Apocynaceae, the perianth appears as separate sepals and petals (although the petals are congenitally fused; late sympetaly, for term, see Erbar and Leins, 2011), the androecium as separate stamens, and the gynoecium as separate carpels (Endress, 1994). Synorganization begins largely only after all individual organs are present. In contrast, in the highly synorganized clades of Orchidaceae, synorganization appears at the beginning of flower development by early congenital fusion of the organs.
Aspects of synorganization take place early in development, but the corona as a new organ appears late
The evolution of floral synorganization in Apocynaceae and Orchidaceae took place in the crown group of each family. Aspects of synorganization also take place at different times during floral ontogeny. Major episodes of ontogenetic synorganization occur early in development. There appears to be a tendency that they occur earlier in more derived clades than in basal clades in both families. In contrast to the early expression of synorganization in floral development, the appearance of the corona as a new organ is late in ontogeny (Fig. 4).
In Asclepiadoideae, postgenital intercarpellary fusion takes place very early in flower development (Endress et al., 1983, figs 9–12), earlier than in other Apocynaceae with a lower degree of synorganization (Walker, 1975, figs 4–8; Gomes et al., 2008, figs 12–15). It is remarkable how the style head in Asclepiadoideae is transformed early in development from the genuine dimerous structure (the two carpels) to a pentangular structure, moulded by the neighbouring five stamens (Figs 2C and 6B). These five angles will be the five sectors where later the five translators are secreted. Thus, there is a change from two to five units in early ontogeny. Because of the very early postgenital fusion of the two carpels, this dimery is later no longer visible, except for the two main vascular bundles. Complexity obliterates the original morphological organ boundaries.
Particularly impressive is the early congenital fusion of all floral organs within whorls and between whorls in Orchidaceae (Figs 2D and 11A). In the basal Orchidaceae (Apostasioideae) fusion of the organs occurs later in the course of floral development (Kocyan and Endress, 2001). In some epidendroid orchids with particularly intimate synorganization of pollinaria by the addition of a stipe, the incumbence of the anthers is congenital (and not postgenital as in other orchids; Freudenstein and Chase, 2015). Thus, the connection between anther and rostellum is protracted in development.
Evolutionary sequence of synorganization and innovation in Apocynaceae and Orchidaceae
Several key innovations evolved sequentially from ancestral flowers to the highly synorganized flowers of orchids or asclepiads. These innovations did not necessarily appear in a straight line. It is to be expected that there was zig-zag evolution with gain and sometimes loss of features. Details are poorly known. However, there was a first time of evolutionary appearance for each of these innovations. The following are the putative sequences (of the first occurrence) of innovations.
Apocynaceae: fixation of pentamery → fixation of syncarpy (at least at the base of the gynoecium) → fixation of a single whorl in each organ category → sympetaly → postgenital fusion of carpel tips and of anthers with gynoecium → ontogenetic moulding of style head from two to five symmetry planes (→ translator number, nectary number, functional stigma number) → 4 or 2 pollinia per stamen → 5 pollinaria per flower (translators, including glue or clips, by secretion of 5 style head sectors).
Orchidaceae: fixation of trimery with two perianth whorls and two androecial whorls → congenital fusion of all tepals → lip differentiation → reduction of stamen number to 2 or 3 → complete sycarpy → reduction of stamen number to 1 → 4 or 2 pollinia per stamen → 1 or 2 pollinaria per flower (translator by tissue decay in the anther and – in some clades – by detachment of part of rostellum; viscidium by rostellum secretion).
Synorganization of organs by fusion also resulted in further stabilization of the position of organs and by the organs becoming more sturdy. In turn, it allowed further differentiation of the organs along their length (longitudinally) or along their width (latitudinally), and also differentiation of a simple basic structure into two substructures, which assume different functions (progressive separation of functions, division of labour). For instance, in both families the former stigma differentiates longitudinally into a subapical receptive part and an apical part that produces the translator (among orchids in Cymbidieae and Vandeae at least the viscidium of the translator; see, for example, Yeung, 1987b). In both families, the translator differentiates either longitudinally or latitudinally into a part for pollinator attachment (by glue or clip) and arms or joint connecting pollinia and part of pollinator attachment. In Apocynaceae (Asclepiadoideae), each theca with two fertile pollen sacs differentiates latitudinally into a fertile pollen sac and a sterile guide rail element. The corona differentiates longitudinally and latitudinally into a complex apparatus with several functions.
Orchidaceae with approx. 25 000 species are one of the two most diversified angiosperm families, and species and genus richness are especially concentrated in those subclades with the highest flower synorganization (Orchidoideae and Epidendroideae, approx. 21 500 species) (Chase et al., 2015). Apocynaceae have almost 5000 species. Also here, the clade with the most synorganized flowers (Asclepiadoideae and Secamonoideae) have more species (3180) than all other subclades of the family together (Meve, 2002). It may be expected that flower synorganization (in addition to other traits) played an important role in the high diversification in both families, although different ages and different habitats of the families make a detailed direct comparison difficult.
OUTLOOK
Comparison of the flowers of the unrelated families Apocynaceae and Orchidaceae, which share a conspicuous convergence by having pollinia and pollinaria (unique in angiosperms), highlights the developmental and evolutionary preconditions for these traits. The flowers of the two families also show a large number of other convergences in detail. These are largely a precondition for, or a result of, synorganization. Whereas the synorganization of androecium and gynoecium and the structure of pollinaria have been studied in many representatives of the two families, the structure and diversification of the corona in Apocynaceae has been explored less well in spite of its extreme diversity.
There are additional effects of the synorganization of the flowers in Apocynaceae and Orchidaceae: because of their firm consistency, the flowers lend themselves to three-dimensional studies using scanning electron microscopy or tomography for morphometrics or morphospace studies because these flowers are expected to undergo less distortion than flowers with more delicate organs (van der Niet et al., 2010; Gamisch et al., 2013; Chartier et al., 2014; Sedeek et al., 2014). Thus far, tomography has been applied to orchids but not yet to Apocynaceae. Molecular developmental genetics has provided results for orchids as mentioned in the section on Flowers of the most elaborate Orchidaceae. Given the prominence of synorganization in both families and the emergence of novel organs (corona) in Apocynaceae, molecular developmental research regarding organ fusion and boundary formation as studied in model organisms could be promising (e.g. Aida and Tasaka, 2006; Vandenbussche et al., 2009; Lampugnani et al., 2012; Žádníková and Simon, 2014; Zhong and Preston, 2015). There is great potential for interesting research topics.
ACKNOWLEDGEMENTS
I thank Günter Theissen and Rainer Melzer for the invitation to contribute to this special issue of the Annals of Botany. Rosemarie Siegrist is acknowledged for microtome sections, Urs Jauch for use of the scanning electron microscope, and Alex Bernhard for graphical work. I thank Mary Endress for suggestions and for reading the manuscript. Alessandro Rapini is thanked for information on flower size in ascleps. Two anonymous reviewers are acknowledged for helpful suggestions.
APPENDIX
Material used in this study is based on the following collections. The collection date is only mentioned if there is no collection number.
Acineta densa Lindl. (Orchidaceae-Epidendroideae), P.K. Endress s.n., 15.vii.1983 (not collected, only photographed), Botanic Garden, University of Zurich.
Asclepias curassavica L. (Apocynaceae-Asclepiadoideae), P.K. Endress 7368, Botanic Garden, University of Zurich.
Calotropis procera (Aiton) Dryand. (Apocynaceae-Asclepiadoideae), P.K. Endress s.n. (not collected, only photographed), Botanic Garden, University of Zurich.
Ceropegia distincta N.E.Br. (Apocynaceae-Asclepiadoideae), P.K. Endress 5210, Botanic Garden, University of Zurich.
Coryanthes macrantha (Hook.) Hook. (Orchidaceae-Epidendroideae), P.K. Endress s.n., 22.i.1996 (not collected, only photographed), Botanic Garden, University of Zurich.
Gomphocarpus fruticosus (L.) W.T.Aiton (Apocynaceae-Asclepiadoideae), P.K. Endress 7534, Botanic Garden, University of Zurich.
Oncidium ornithorhynchum Kunth (Orchidaceae-Epidendroideae), P.K. Endress 9759, Botanic Garden, University of Zurich.
Ophrys fusca Link (Orchidaceae-Orchidoideae), P. Voser s.n., 6.iii.1980, Corsica, France.
Vincetoxicum nigrum (L.) Moench (Apocynaceae-Asclepiadoideae), P.K. Endress 4690, Botanic Garden, University of Zurich.
Caralluma penicillata (Deflers) N.E.Br. (Apocynaceae-Asclepiadoideae), P.K. Endress 7516, Städtische Sukkulentensammlung, Zurich 81/1685.
Collected material was fixed and stored in 70 % ethanol. Material studied with a scanning electron microscope was critical point dried, sputter coated with gold and studied at 20 kV with a Hitachi S-4000 microscope. Material used for microtome section series was dehydrated and embedded in paraplast. Section series, 10 µm thick, were produced with a Leitz rotary microtome, stained with safranin and Astrablue, and embedded in Eukitt. Vouchers and permanent slides of the microtome sections are deposited at the Institute of Systematic Botany, University of Zurich (Z).
LITERATURE CITED
- Ackerman JD. 1986. Mechanisms and evolution of food-deceptive pollination systems in orchids. Lindleyana 1: 108–113. [Google Scholar]
- Aida M, Tasaka M. 2006. Genetic control of shoot organ boundaries. Current Opinion in Plant Biology 9: 72–77. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Pélabon C, Hansen TF, Bolstad GH. 2009. Macroevolutionary patterns of pollination accuracy: a comparison of three genera. New Phytologist 183: 600–617. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Pélabon C, Bolstad GH, Hansen TF. 2014. Integrated phenotypes: understanding trait covariation in plants and animals. Philosophical Transactions of the Royal Society B 369: 20130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. 2006. David G. Lloyd and the evolution of floral biology: from natural history to strategic analysis. In Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 1–21. [Google Scholar]
- Baum H. 1948. Postgenitale Verwachsung in und zwischen Karpell- und Staubblattkreisen. Sitzungsberichte der Österreichischen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Abteilung 1, 157: 17–38. [Google Scholar]
- Bhatnagar S. 1975. Floral polymorphism in sympatric populations of Calotropis procera (Ait.) R.Br. Acta Botanica Indica 3: 43–46. [Google Scholar]
- Bookman SS. 1981. The floral morphology of Asclepias speciosa (Asclepiadaceae) in relation to pollination and a clarification in terminology for the genus. American Journal of Botany 68: 675–679. [Google Scholar]
- Bradshaw E, Rudall PJ, Devey DS, Thomas MM, Glover BJ, Bateman RM. 2010. Comparative labellum micromorphology of the sexually deceptive temperate orchid genus Ophrys: diverse epidermal cell types and multiple origins of structural colour. Botanical Journal of the Linnean Society 162: 504–540. [Google Scholar]
- Brown R. 1833. On the organs and mode of fecundation in Orchideae and Asclepiadeae. Transactions of the Linnean Society 16: 685–745. [Google Scholar]
- Bruyns PV, Nowell TL, Hedderson TAJ. 2005. A revision and phylogenetic analysis of Stapeliopsis (Apocynaceae). Botanical Journal of the Linnean Society 148: 125–155. [Google Scholar]
- Burns-Balogh P, Bernhardt P. 1985. Evolutionary trends in the androecium of the Orchidaceae. Plant Systematics and Evolution 149: 119–134. [Google Scholar]
- Burns-Balogh P, Funk V. 1986. A phylogenetic analysis of the Orchidaceae. Smithsonian Contributions to Botany 61: 1–79. [Google Scholar]
- Cameron KM. 2003a. The structure and occurrence of trilocular ovaries within Orchidaceae. Botany 2003. http://www.2003.botanyconference.org/engine/search/detail.php?aid=71. [Google Scholar]
- Cameron KM. 2003b. Vanilloideae. In Pridgeon AM, Cribb PJ, Chase MW, eds. Genera Orchidacearum, Vol. 3 Oxford: Oxford University Press, 281–334. [Google Scholar]
- Cameron KM. 2006. A comparison and combination of plastid atpB and rbcL gene sequences for inferring phylogenetic relationships within Orchidaceae. In Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG, eds. Monocots: comparative biology and evolution (excluding Poales). Claremont: Rancho Santa Ana Botanic Garden, 447–464. [Google Scholar]
- Cameron KM, Chase MW. 2000. RDNA sequences of Orchidaceae confirm the subfamilial status and circumscription of Vanilloideae. In Wilson KL, Morrison DA, eds. Monocots: systematics and evolution. Collingwood: CSIRO, 457–464. [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, et al. 1999. A phylogenetic analysis of the Orchidaceae: evidence from rbcL nucleotide sequences. American Journal of Botany 86: 208–224. [PubMed] [Google Scholar]
- Carlsward BS, Whitten WM, Williams NH, Bytebier B. 2006. Molecular phylogenetics of Vandeae (Orchidaceae) and the evolution of leaflessness. American Journal of Botany 93: 770–786. [DOI] [PubMed] [Google Scholar]
- Chartier M, Jabbour F, Gerber S, et al. 2014. The floral morphospace – a modern comparative approach to study angiosperm evolution. New Phytologist 204: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MW, Cameron KM, Freudenstein JV, et al. 2015. An updated classification of Orchidaceae. Botanical Journal of the Linnean Society 177: 151–174. [Google Scholar]
- Christ P, Schnepf E. 1985. The nectaries of Cynanchum vincetoxicum (Asclepiadaceae). Israel Journal of Botany 34: 79–90. [Google Scholar]
- Civeyrel L, Le Thomas A, Ferguson K, Chase MW. 1998. Critical reexamination of palynological characters used to delimit Asclepiadaceae in comparison to the molecular phylogeny obtained from plastid matK sequences. Molecular Phylogenetics and Evolution 9: 517–527. [DOI] [PubMed] [Google Scholar]
- Cocucci AA, Marino S, Baranzelli M, Wiemer AP, Sérsic A. 2014. The buck in the milkweed: evidence of male-male interference among pollinaria on pollinators. New Phytologist 203: 280–286. [DOI] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM. 1991. The war of the whorls: genetic interactions controlling flower development. Nature 353: 31–37. [DOI] [PubMed] [Google Scholar]
- Corry TH. 1884. Structure and development of the gynostegium, and the mode of fertilization in Asclepias cornuti Decaisne (A. syriaca L.). Transactions of the Linnean Society of London, Series 2, 2: 173–207. [Google Scholar]
- Cozzolino S, Widmer A. 2005. Orchid diversity: an evolutionary consequence of deception? Trends in Ecology and Evolution 20: 487–494. [DOI] [PubMed] [Google Scholar]
- Cusick F. 1966. On phylogenetic and ontogenetic fusions. In Cutter EG, ed. Trends in plant morphogenesis. London: Longmans Green, 170–183. [Google Scholar]
- Dannenbaum C, Schill R. 1991. Die Entwicklung der Pollentetraden und Pollinien bei den Asclepiadaceae. Bibliotheca Botanica 141: 1–138. [Google Scholar]
- Dannenbaum C, Wolter M, Schill R. 1989. Stigma morphology of the orchids. Botanische Jahrbücher für Systematik 110: 441–460. [Google Scholar]
- Davies KL, Stipczynska M. 2006. Labellar micromorphology of Bifrenariinae Dressler (Orchidaceae). Annals of Botany 98: 1215–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Roberts DL, Turner MP. 2002. Pseudopollen and food-hair diversity in Polystachya Hook. (Orchidaceae). Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco D. 2014. Secretory tissues and the morphogenesis and histochemistry of pollinarium in flowers of Asclepiadeae (Apocynaceae). International Journal of Plant Sciences 175: 1042–1053. [Google Scholar]
- Demeter K. 1922. Vergleichende Asclepiadaceenstudien. Flora 15: 130–176. [Google Scholar]
- Dodson CH. 1965. Studies in orchid pollination: the genus Coryanthes. American Orchid Society Bulletin 34: 680–687. [Google Scholar]
- Dressler RL. 1981. The orchids: natural history and classification. Cambridge: Harvard University Press. [Google Scholar]
- Dressler RL. 1986. Features of pollinaria and orchid classification. Lindleyana 1: 125–130. [Google Scholar]
- Ehler N. 1975. Beitrag zur Kenntnis der Mikromorphologie der Coroll-Epidermen von Stapelieen und ihre taxonomische Verwendbarkeit. Tropische und Subtropische Pflanzenwelt 14: 83–139. [Google Scholar]
- Ehler N. 1976. Struktur und Funktion der Oberflächen von Orchideenblüten. In Senghas K, ed. Tagungsbericht der 8. Welt-Orchideen-Konferenz. Palmengarten, Frankfurt, 1975. Frankfurt am Main: Deutsche Orchideen-Gesellschaft, 456–462. [Google Scholar]
- El Ottra JHL, Pirani JR, Endress PK. 2013. Fusion within and between whorls of floral organs in Galipeinae (Rutaceae): structural features and evolutionary implications. Annals of Botany 111: 821–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress ME. 2003. Apocynaceae and Asclepiadaceae: united they stand. Haseltonia 8: 2–9. [Google Scholar]
- Endress ME. 2004. Apocynaceae: Brown and now. Telopea 10: 525–541. [Google Scholar]
- Endress ME, Bruyns PV. 2000. A revised classification of the Apocynaceae s.l. Botanical Review 66: 1–56. [Google Scholar]
- Endress ME, Stevens WD. 2001. The renaissance of the Apocynaceae s.l.: recent advances in systematics and evolution. Annals of the Missouri Botanical Garden 88: 517–522. [Google Scholar]
- Endress ME, Sennblad B, Nilsson S, et al. 1996. A phylogenetic analysis of Apocynaceae s.str. and some related taxa in Gentianales – a multidisciplinary approach. Opera Botanica Belgica 7: 59–102. [Google Scholar]
- Endress ME, Lorence DH, Endress PK. 1997. Structure and development of the gynoecium in Lepinia marquisensis and its systematic position in the Apocynaceae. Allertonia 7: 267–272. [Google Scholar]
- Endress ME, van der Ham RWJM, Nilsson S, et al. 2007a. A phylogenetic analysis of Alyxieae (Apocynaceae) based on rbcL, matK, trnL intron, trnL-F spacer sequences and morphological characters. Annals of the Missouri Botanical Garden 94: 1–35. [Google Scholar]
- Endress ME, Liede-Schumann S, Meve U. 2007b. Advances in Apocynaceae. The enlightenment, an introduction. Annals of the Missouri Botanical Garden 94: 259–267. [Google Scholar]
- Endress ME, Liede-Schumann S, Meve U. 2014. An updated classification for Apocynaceae. Phytotaxa 159: 175–194. [Google Scholar]
- Endress PK. 1982. Syncarpy and alternative modes of escaping disadvantages of apocarpy in primitive angiosperms. Taxon 31: 48–52. [Google Scholar]
- Endress PK. 1987. Floral phyllotaxis and floral evolution. Botanische Jahrbücher für Systematik 108: 417–438. [Google Scholar]
- Endress PK. 1990. Patterns of floral construction in ontogeny and phylogeny. Biological Journal of the Linnean Society 39: 153–175. [Google Scholar]
- Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- Endress PK. 1995. Major evolutionary traits of monocot flowers. In Rudall PJ, Cribb P, Cutler DF, Humphries CJ, eds. Monocotyledons: systematics and evolution. Kew: Royal Botanic Gardens, 43–79. [Google Scholar]
- Endress PK. 2006. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research 44: 1–61. [Google Scholar]
- Endress PK. 2008. The whole and the parts: relationships between floral architecture and floral organ shape, and their repercussions on the interpretation of fragmentary floral fossils. Annals of the Missouri Botanical Garden 95: 101–120. [Google Scholar]
- Endress PK. 2010. Synorganisation without organ fusion in the flowers of Geranium robertianum. Annals of Botany 106: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK. 2011. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany 98: 370–396. [DOI] [PubMed] [Google Scholar]
- Endress PK. 2015. Patterns of angiospermy development before carpel sealing across living angiosperms: diversity, morphological and systematic aspects. Botanical Journal of the Linnean Society 178: 556–591. [Google Scholar]
- Endress PK, Doyle JA. 2009. Reconstructing the ancestral flower and its initial specializations. American Journal of Botany 96: 22–66. [DOI] [PubMed] [Google Scholar]
- Endress PK, Rapini A. 2014. Floral structure of Emmotum (Icacinaceae sensu stricto or Emmotaceae), a phylogenetically isolated genus of lamiids with a unique pseudotrimerous gynoecium, bitegmic ovules and monosporangiate thecae. Annals of Botany 114: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK, Jenny M, Fallen ME. 1983. Convergent elaboration of apocarpous gynoecia in higher advanced dicotyledons (Sapindales, Malvales, Gentianales). Nordic Journal of Botany 3: 293–300. [Google Scholar]
- Erbar C, Leins P. 1995. Portioned pollen release and the syndrome of secondary pollen presentation in the Campanulales-Asterales-complex. Flora 190: 323–338. [Google Scholar]
- Erbar C, Leins P. 2011. Synopsis of some important non-DNA character states in the asterids with special reference to sympetaly. Plant Diversity and Evolution 129: 93–123. [Google Scholar]
- Fallen ME. 1986. Floral structure in the Apocynaceae: morphological, functional, and evolutionary aspects. Botanische Jahrbücher für Systematik 106: 245–286. [Google Scholar]
- Fishbein M. 2001. Evolutionary innovation and diversification in the flowers of Asclepiadaceae. Annals of the Missouri Botanical Garden 88: 603–623. [Google Scholar]
- Freudenstein JV, Chase MW. 2015. Phylogenetic relationships in Epidendroideae (Orchidaceae), one of the great flowering plant radiations: progressive specialization and diversification. Annals of Botany 115: 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenstein JV, Rasmussen FN. 1996. Pollinium development and number in the Orchidaceae. American Journal of Botany 83: 813–824. [Google Scholar]
- Freudenstein JV, Rasmussen FN. 1997. Sectile pollinia and relationships in the Orchidaceae. Plant Systematics and Evolution 205: 125–146. [Google Scholar]
- Freudenstein JV, Harris EM, Rasmussen FN. 2002. The evolution of anther morphology in orchids: incumbent anthers, superposed pollinia, and the vandoid complex. American Journal of Botany 89: 1747–1755. [DOI] [PubMed] [Google Scholar]
- Freudenstein JV, van den Berg C, Goldman DH, Kores PJ, Molvray M, Chase MW. 2004. An expanded plastid DNA phylogeny of Orchidaceae and analysis of jackknife branch support strategy. American Journal of Botany 91: 149–157. [DOI] [PubMed] [Google Scholar]
- Fuchs S. 2013. Die “Fünf” stimmt nicht immer. Abnorme Blütenbildung bei Asclepiadaceen. Kakteen und andere Sukkulenten 64: 301–305. [Google Scholar]
- Galil J, Zeroni M. 1965. Nectar system of Asclepias curassavica. Botanical Gazette (Crawfordsville) 126: 144–148. [Google Scholar]
- Gamisch A, Staedler YM, Schönenberger J, Fischer GA, Comes HP. 2013. Histological and micro-CT evidence of stigmatic rostellum receptivity promoting auto-pollination in the Madagascan orchid Bulbophyllum bicoloratum. PLoS ONE 8: e72688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay LA. 1972. On the origin of the Orchidaceae, II. Journal of the Arnold Arboretum 53: 202–215. [Google Scholar]
- Gerlach G. 2011. The genus Coryanthes: a paradigm in ecology. Lankesteriana 11: 253–264. [Google Scholar]
- Gerlach G, Schill R. 1993. Die Gattung Coryanthes Hook. (Orchidaceae). Eine monographische Bearbeitung unter besonderer Berücksichtigung der Blütenduftstoffe. Tropische und Subtropische Pflanzenwelt 83: 1–205. [Google Scholar]
- Gomes SM, Kinoshita LS, Castro MM. 2008. Hemisincarpia e nectário apendicular enfocadaos através de ontogênese floral em Mandevilla velame (A. St.-Hil.) Pichon, Apocynoideae. Revista Brasileira de Botânica 31: 81–93. [Google Scholar]
- Górniak M, Paun O, Chase MW. 2010. Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: congruence with organellar and nuclear ribosomal DNA results. Molecular Phylogenetics and Evolution 56: 784–795. [DOI] [PubMed] [Google Scholar]
- Hagemann W. 1973. The organization of shoot development. Revista de Biologia 9: 43–67. [Google Scholar]
- Harder LD, Johnson SD. 2008. Function and evolution of aggregated pollen in angiosperms. International Journal of Plant Sciences 169: 59–78. [Google Scholar]
- Harder LD, Routley MB. 2006. Pollen and ovule fates and reproductive performance by flowering plants. In Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 61–80. [Google Scholar]
- Hirmer M. 1920. Beiträge zur Organographie der Orchideenblüte. Flora 113: 213–310. [Google Scholar]
- Hobbhahn N, Johnson SD, Bytebier B, Yeung EC, Harder LD. 2013. The evolution of floral nectaries in Disa (Orchidaceae: Disinae): recapitulation or diversifying innovation? Annals of Botany 112: 1303–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H. 1957. Revision der Gattung Ceropegia. Memórias da Sociedade Broteriana 12: 1–203. [Google Scholar]
- Ionta GM, Judd WS. 2007. Phylogenetic relationships in Periplocoideae (Apocynaceae s.l.) and insights into the origin of pollinia. Annals of the Missouri Botanical Garden 94: 360–375. [Google Scholar]
- Jersákova J, Johnson SD, Kindlmann P. 2006. Mechanisms and evolution of deceptive pollination in orchids. Biological Review 81: 219–235. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Edwards TJ. 2000. The structure and function of orchid pollinaria. Plant Systematics and Evolution 222: 243–269. [Google Scholar]
- Kirchoff BK. 1988. Inflorescence and flower development in Costus scaber (Costaceae). Canadian Journal of Botany 66: 339–345. [Google Scholar]
- Kirchoff BK. 1997. Inflorescence and flower development in the Hedychieae (Zingiberaceae): Hedychium. Canadian Journal of Botany 75: 581–594. [Google Scholar]
- Kocyan A, Endress PK. 2001. Floral structure and development of Apostasia and Neuwiedia (Apostasioideae) and their relationships to other Orchidaceae. International Journal of Plant Sciences 162: 847–867. [Google Scholar]
- Kocyan A, Qiu Y-L, Endress PK, Conti E. 2004. A phylogenetic analysis of Apostasioideae (Orchidaceae) based on ITS, trnL-F and matK sequences. Plant Systematics and Evolution 247: 203–213. [Google Scholar]
- Kunze H. 1981. Morphogenese und Synorganisation des Bestäubungsapparates einiger Asclepiadaceen. Beiträge zur Biologie der Pflanzen 56: 133–170. [Google Scholar]
- Kunze H. 1990. Morphology and evolution of the corona in Asclepiadaceae and related families. Tropische und Subtropische Pflanzenwelt 76: 1–51. [Google Scholar]
- Kunze H. 1991. Structure and function in asclepiad pollination. Plant Systematics and Evolution 176: 227–253. [Google Scholar]
- Kunze H. 1994. Ontogeny of the translator in Asclepiadaceae s.str. Plant Systematics and Evolution 193: 223–242. [Google Scholar]
- Kunze H. 1995. Bau und Funktion der Asclepiadaceenblüte. Phyton 35: 1–24. [Google Scholar]
- Kunze H. 1996. Morphology of the stamen in the Asclepiadaceae and its systematic relevance. Botanische Jahrbücher für Systematik 118: 547–579. [Google Scholar]
- Kunze H. 1997. Corona and nectar system in Asclepiadinae (Asclepiadaceae). Corona and nectar system in Asclepiadinae. Flora 192: 175–183. [Google Scholar]
- Kunze H. 2005. Morphology and evolution of the corolla and corona in the Apocynaceae s.l. Botanische Jahrbücher für Systematik 126: 347–383. [Google Scholar]
- Kunze H, Wanntorp L. 2008. Corona and anther skirt in Hoya (Apocynaceae, Marsdenieae). Plant Systematics and Evolution 271: 9–17. [Google Scholar]
- Kurzweil H. 1987a. Developmental studies in orchid flowers I: Epidendroid and vandoid species. Nordic Journal of Botany 7: 427–442. [Google Scholar]
- Kurzweil H. 1987b. Developmental studies in orchid flowers II: Orchidoid species . Nordic Journal of Botany 7: 443–451. [Google Scholar]
- Kurzweil H. 1988. Developmental studies in orchid flowers III. Neottioid species. Nordic Journal of Botany 8: 271–282. [Google Scholar]
- Kurzweil H. 1993. Developmental studies in orchid flowers IV: Cypripedioid species. Nordic Journal of Botany 13: 423–430. [Google Scholar]
- Kurzweil H. 1995. Floral morphology and ontogeny in subtribe Satyriinae (fam. Orchidaceae). Flora 190: 1–20. [Google Scholar]
- Kurzweil H. 1998. Floral ontogeny of orchids: a review. Beiträge zur Biologie der Pflanzen 71: 45–100. [Google Scholar]
- Kurzweil H, Kocyan A. 2002. Ontogeny of orchid flowers. In Kull T, Arditti J, eds. Orchid biology: Reviews and perspectives. Dordrecht: Kluwer, 83–138. [Google Scholar]
- Kurzweil H, Weber A. 1992. Floral morphology of southern African Orchideae. II. Habenariinae. Nordic Journal of Botany 12: 39–61. [Google Scholar]
- Lampugnani ER, Kilinc A, Smyth DR. 2012. PETAL LOSS is a boundary gene tha inhibits growth between developing sepals in Arabidopsis thaliana. The Plant Journal 71: 724–735. [DOI] [PubMed] [Google Scholar]
- Leins P, Erbar C. 2006. Secondary pollen presentation syndromes of the Asterales – a phylogenetic perspective. Botanische Jahrbücher für Systematik 127: 83–103. [Google Scholar]
- Leins P, Erbar C. 2010. Flower and fruit. Stuttgart: Schweizerbart Science Publishers. [Google Scholar]
- Liede S. 1994. Anther differentiation in the Asclepiadaceae-Asclepiadeae: form and function. In D’Arcy WG, Keating RC, eds. The anther: form, function and phylogeny. Cambridge: Cambridge University Press, 221–235. [Google Scholar]
- Liede S, Kunze H. 1993. A descriptive system for corona analysis in Asclepiadaceae and Periplocaceae. Plant Systematics and Evolution 185: 275–284. [Google Scholar]
- Liede-Schumann S, Rapini A, Goyder DJ, Chase MW. 2005. Phylogenetics of the New World subtribes of Asclepiadeae (Apocynaceae-Asclepiadoideae): Metastelmatinae, Oxypetalinae, and Gonolobinae. Systematic Botany 30: 184–200. [Google Scholar]
- Livshultz T. 2010. The phylogenetic position of milkweeds (Apocynaceae subfamilies Secamonoideae and Asclepiadoideae): evidence from the nucleus and chloroplast. Taxon 59: 1016–1030. [Google Scholar]
- Livshultz T, Middleton D, Endress ME, Williams JK. 2007. Systematics of Apocynoideae (Apocynaceae s.str.) and the APSA clade. Annals of the Missouri Botanical Garden 94: 324–359. [Google Scholar]
- Luo Y-B, Chen S-C. 2000. The floral morphology and ontogeny of some Chinese representatives of orchid subtribe Orchidinae. Botanical Journal of the Linnean Society 134: 529–548. [Google Scholar]
- Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T. 2015. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist 207: 437–453. [DOI] [PubMed] [Google Scholar]
- Manning JC, Linder HP. 1992. Pollinators and evolution in Disperis (Orchidaceae), or why are there so many species? South African Journal of Science 88: 38–49. [Google Scholar]
- Medeiros JF, Rapini A, Barbosa UC, Py-Daniel V, Braga PIS. 2008. First record of Simuliidae (Diptera) with pollinaria of Asclepiadoideae (Apocynaceae) attached. Neotropical Entomology 37: 338–341. [DOI] [PubMed] [Google Scholar]
- Meve U. 2002. Species numbers and progress in asclepiad taxonomy. Kew Bulletin 57: 459–464. [Google Scholar]
- Meve U, Liede S. 1994. Floral biology and pollination in stapeliads – new results and a literature review. Plant Systematics and Evolution 192: 99–116. [Google Scholar]
- Micheneau C, Johnson SD, Fay MF. 2009. Orchid pollination: from Darwin to the present day. Botanical Journal of the Linnean Society 161: 1–19. [Google Scholar]
- Mondragón-Palomino M. 2013. Perspectives on MADS-box expression during orchid flower evolution and development. Frontiers in Plant Science 4: Article 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Theissen G. 2008. MADS about the evolution of orchid flowers. Trends in Plant Science 13: 51–59. [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Theissen G. 2009. Why are orchid flowers so diverse? Reduction of evolutionary constraints by paralogues of class B floral homeotic genes. Annals of Botany 104: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Theissen G. 2011. Conserved differential expression of paralogous DEFICIENS- and GLOBOSA-like MADS-box genes in the flowers of Orchidaceae: refining the ‘orchid code’. Plant Journal 66: 1008–1019. [DOI] [PubMed] [Google Scholar]
- Nazar N, Goyder DJ, Clarkson JJ, Mahmood T, Chase MW. 2013. The taxonomy and systematics of Apocynaceae: where we stand in 2012. Botanical Journal of the Linnean Society 171: 482–490. [Google Scholar]
- Nazarov VV, Gerlach G. 1997. The potential seed productivity of orchid flowers and peculiarities of their pollination systems. Lindleyana 12: 188–204. [Google Scholar]
- Nilsson S, Endress ME, Grafström E. 1993. On the relationship of the Apocynaceae and Periplocaceae. Grana, Suppl. 2: 3–20. [Google Scholar]
- Ollerton J, Masinde M, Meve U, Picker M, Whittington A. 2009. Fly pollination in Ceropegia (Apocynaceae: Asclepiadaceae): biogeographic and phylogenetic perspectives. Annals of Botany 103: 1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabón-Mora N, González F. 2008. Floral ontogeny of Telipogon spp. (Orchidaceae) and insights on the perianth symmetry in the family. International Journal of Plant Sciences 169: 1159–1173. [Google Scholar]
- Pacini E. 2009. Orchids pollen dispersal units and reproductive consequences. In Kull T, Arditti J, Wong SM, eds. Orchid biology: reviews and perspectives. X. Berlin: Springer Science + Business Media B.V., 185–218. [Google Scholar]
- Pacini E, Hesse M. 2002. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Annals of Botany 89: 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z-J, Cheng C-C, Tsai W-C, et al. 2011. The duplicated B-class MADS-box gene display dualistic characters in orchid floral identity and growth. Plant Cell Physiology 52: 1515–1531. [DOI] [PubMed] [Google Scholar]
- Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, eds. 2005. Genera Orchidacearum, Vol. 4 Epidendroideae (Part one). Oxford: Oxford University Press. [Google Scholar]
- Prutsch J, Schill R. 2000. Die Ontogenese der Narbe bei den Orchideen. Bibliotheca Botanica 151: 1–82. [Google Scholar]
- Puri V, Shiam R. 1966. Studies in floral anatomy. VIII. Vascular anatomy of the flower of certain species of the Asclepiadaceae with special reference to corona. Agra University Journal of Research (Science) 15: 189–216. [Google Scholar]
- Rapini A. 2012. Taxonomy “under construction”: advances in the sytematics of Apocynaceae, with emphasis on the Brazilian Asclepiadoideae. Rodriguésia 63: 75–88. [Google Scholar]
- Rapini A, Chase MW, Goyder DJ, Griffiths J. 2003. Asclepiadoideae classification: evaluating the phylogenetic relationships of New World Asclepiadoideae (Apocynaceae). Taxon 52: 33–50. [Google Scholar]
- Rapini A, Chase MW, Konno TUP. 2006. Phylogenetics of South American Asclepiadeae (Apocynaceae). Taxon 55: 119–124. [Google Scholar]
- Rapini A, van den Berg C, Liede-Schumann S. 2007. Diversification of Asclepiadoideae (Apocynaceae) in the New World. Annals of the Missouri Botanical Garden 94: 407–422. [Google Scholar]
- Rasmussen FN. 1982. The gynostemium of the neottioid orchids. Opera Botanica 65: 1–96. [Google Scholar]
- Rasmussen FN. 1985. Orchids. In Dahlgren RMT, Clifford HAT, Yeo PF, eds. The families of the monocotyledons. Berlin: Springer, 249–274. [Google Scholar]
- Rasmussen FN. 1986a. On the various contrivances by which pollinia are attached to viscidia. Lindleyana 1: 21–32. [Google Scholar]
- Rasmussen FN. 1986b. Ontogeny and phylogeny in Orchidaceae. Lindleyana 1: 114–124. [Google Scholar]
- Robbrecht E. 1988. Tropical woody Rubiaceae. Opera Botanica Belgica 1: 1–271. [Google Scholar]
- Romero GA. 1990. Phylogenetic relationships in subtribe Catasetinae (Orchidaceae, Cymbidieae). Lindleyana 5: 160–181. [Google Scholar]
- Rudall PJ, Bateman RM. 2002. Role of synorganisation, zygomorphy and heterotopy in floral evolution: the gynoecium and labellum of orchids and other lilioid monocots. Biological Review of the Cambridge Philosophical Society 77: 403–441. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. 2004. Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytologist 162: 25–44. [Google Scholar]
- Rudall PJ, Sokoloff DD, Remizowa MV, et al. 2007. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. American Journal of Botany 94: 1073–1092. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Perl CD, Bateman RM. 2013. Organ homologies in orchid flowers re-interpreted using the musk orchid as a model. PeerJ 1: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudjiman RR. 1982. A revision of Vallaris Burm.f. (Apocynaceae). Mededelingen Landbouwhogeschool Wageningen 82–11: 1–17. [Google Scholar]
- Safwat FM. 1962. Floral morphology of Secamone and evolution of the pollinating apparatus in Asclepiadaceae. Annals of the Missouri Botanical Garden 49: 95–129. [Google Scholar]
- Sage TL, Broyles SB, Wyatt R. 1990. The relationship between the five stigmatic chambers and two ovaries of milkweed (Asclepias amplexicaulis Sm.) flowers: a three-dimensional assessment. Israel Journal of Botany 39: 187–196. [Google Scholar]
- Schick B. 1980. Untersuchungen über die Biotechnik der Apocynaceenblüte I. Morphologie und Funktion des Narbenkopfes. Flora 170: 394–432. [Google Scholar]
- Schick B. 1982a. Untersuchungen über die Biotechnik der Apocynaceenblüte II. Bau und Funktion des Bestäubungsapparates. Flora 172: 347–371. [Google Scholar]
- Schick B. 1982b. Zur Morphologie, Entwicklung, Feinstruktur und Funktion des Translators von Periploca L. (Asclepiadaceae). Tropische und Subtropische Pflanzenwelt 40: 1–45. [Google Scholar]
- Schick B. 1988. Zur Anatomie und Biotechnik des Bestäubungsapparates der Orchideen. I. Dactylorhiza majalis (Rchb.) Hunt & Summerh., Disa uniflora Bergius und Oncidium hastatum Lindl. Botanische Jahrbücher für Systematik 110: 215–262. [Google Scholar]
- Schick B. 1989. Zur Anatomie und Biotechnik des Bestäubungsapparates der Orchideen. Epipactis palustris (L.) Crantz und Listera ovata (L.) R. Br. Botanische Jahrbücher für Systematik 110: 289–323. [Google Scholar]
- Schiestl FP. 2005. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften 92: 255–264. [DOI] [PubMed] [Google Scholar]
- Schill R, Jäkel U. 1978. Beitrag zur Kenntnis der Asclepiadaceen-Pollinarien. Tropische und Subtropische Pflanzenwelt 22: 1–122. [Google Scholar]
- Schill R, Pfeiffer W. 1977. Untersuchungen an Orchideenpollinien unter besonderer Berücksichtigung ihrer Feinskulpturen. Pollen et Spores 19: 5–118. [Google Scholar]
- Schill R, Wolter M. 1986. On the presence of elastoviscin in all subfamilies of the Orchidaceae and the homology to pollenkitt. Nordic Journal of Botany 6: 321–324. [Google Scholar]
- Schill R, Dannenbaum C, Neyer P. 1992. Quantitative Untersuchungen an Orchideen-Pollinien. Botanische Jahrbücher für Systematik 114: 153–171. [Google Scholar]
- Schmucker T. 1932. Physiologische und ökologische Untersuchungen an Blüten tropischer Nymphaea-Arten. Planta 16: 376–412. [Google Scholar]
- Schneider EL, Williamson PS. 1993. Nymphaeaceae. In Kubitzki K, Rohwer JG, Bittrich V, eds. The families and genera of vascular plants, Vol. 3 Berlin: Springer, 486–493. [Google Scholar]
- Schnepf E, Witzig F, Schill R. 1979. Über Bildung und Feinstruktur des Translators der Pollinarien von Asclepias curassavica und Gomphocarpus fruticosus (Asclepiadaceae). Tropische und Subtropische Pflanzenwelt 25: 1–39. [Google Scholar]
- Sedeek KEM, Scopece G, Staedler YM, et al. 2014. Genic rather than genome-wide differences between sexually deceptive Ophrys orchids with different pollinators. Molecular Ecology 23: 6192–6205. [DOI] [PubMed] [Google Scholar]
- Sennblad B, Bremer B. 1996. The familial and subfamilial relationships of Apocynaceae and Asclepiadaceae evaluated with rbcL data. Plant Systematics and Evolution 202: 153–175. [Google Scholar]
- Sennblad B, Bremer B. 2002. Classification of Apocynaceae s.l. according to a new approach combining Linnean and phylogenetic taxonomy. Systematic Biology 51: 1–21. [DOI] [PubMed] [Google Scholar]
- Sennblad B, Endress ME, Bremer B. 1998. Morphology and molecular data in phylogenetic fraternity: The tribe Wrightieae (Apocynaceae) revisited. American Journal of Botany 85: 1143–1158. [PubMed] [Google Scholar]
- Simões AO, Endress ME, van der Niet T, Kinoshita LS, Conti E. 2004. Tribal and intergenetic relationships of Mesechiteae (Apocynoideae, Apocynaceae): evidence from five noncoding plastid DNA regions and morphology. American Journal of Botany 91: 1409–1418. [DOI] [PubMed] [Google Scholar]
- Simões AO, do Rio MCS, Castro MM, Kinoshita LS. 2007a. Gynostegium morphology of Mesechiteae Miers (Apocynaceae, Apocynoideae) as it pertains to the classification of the tribe. International Journal of Plant Sciences 168: 999–1012. [Google Scholar]
- Simões AO, Livshultz T, Conti E, Endress ME. 2007b. Phylogeny and systematics of the Rauvolfioideae (Apocynaceae) based on molecular and morphological evidence. Annals of the Missouri Botanical Garden 94: 268–297. [Google Scholar]
- Simões AO, Endress ME, Conti E. 2010. Systematics and character evolution of Tabernaemontaneae (Apocynaceae, Rauvolfioideae) based on molecular and morphological evidence. Taxon 59: 772–790. [Google Scholar]
- Simon HA. 1962. The architecture of complexity. Proceedings of the American Philosophical Society 106: 467–482. [Google Scholar]
- Specht CD, Howarth DG. 2015. Adaptation in flower form: a comparative evodevo approach. New Phytologist 206: 74–90. [DOI] [PubMed] [Google Scholar]
- Specht CD, Yockteng R, Almeida AM, Kirchoff BK, Kress WJ. 2012. Homoplasy, pollination, and emerging complexity during the evolution of floral development in the tropical gingers (Zingiberales). Botanical Review 78: 440–462. [Google Scholar]
- Steiner KE. 2010. Twin oil sacs facilitate the evolution of a novel type of pollination unit (meranthium) in a South African orchid. American Journal of Botany 97: 311–323. [DOI] [PubMed] [Google Scholar]
- Straub SCK, Moore MJ, Soltis PS, Soltis DE, Liston A, Livshultz T. 2014. Phylogenetic signal detection from an ancient rapid radiation: Effects of noise reduction, long-branch attraction, and model selection in crown clade Apocynaceae. Molecular Phylogenetics and Evolution 80: 169–185. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Goldman DH, Freudenstein JV, Pridgeon AM, Cameron KM, Chase MW. 2005. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae). American Journal of Botany 92: 613–624. [DOI] [PubMed] [Google Scholar]
- van der Niet T, Zollikofer CPE, Ponce de León MS, Johnson SD, Linder HP. 2010. Three-dimensional geometric morphometrics for studying floral shape variation. Trends in Plant Science 15: 423–426. [DOI] [PubMed] [Google Scholar]
- Vandenbussche M, Horstman A, Zethof J, Koes R, Rijpkema AS, Gerats T. 2009. Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. The Plant Cell 21: 2269–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven RL, Venter HJT. 2001. Pollen morphology of the Periplocoideae, Secamonoideae, and Asclepiadoideae (Apocynaceae). Annals of the Missouri Botanical Garden 88: 569–582. [Google Scholar]
- Verhoeven RL, Liede S, Endress ME. 2003. The tribal position of Fockea and Cibirhiza (Apocynaceae: Asclepiadoideae). Grana 42: 70–81. [Google Scholar]
- Vieira MF, Shepherd GJ. 2002. Oxypetalum banksii subsp. banksii: a taxon of Asclepiadaceae with an extragynoecial compitum. Plant Systematics and Evolution 233: 199–206. [Google Scholar]
- Vieira MF, Fonseca RS, Shepherd GJ. 2012. Morfologia floral e mecanismos de polinização em espécies de Oxypetalum R.Br. (Apocynaceae, Asclepiadoideae). Revista Brasileira de Biociências 10: 314–321. [Google Scholar]
- Vogel S. 1959. Organographie der Blüten kapländischer Ophrydeen mit Bemerkungen zum Koaptations-Problem I/II. Abhandlungen der Mathematisch-Naturwissenschaftlichne Klasse, Akademie der Wissenschaften und der Literatur in Mainz 1959: 265–532. [Google Scholar]
- Vogel S. 1961. Die Bestäubung der Kesselfallen-Blüten von Ceropegia. Beiträge zur Biologie der Pflanzen 36: 159–237. [Google Scholar]
- Vogel S. 1963. Duftdrüsen im Dienste der Bestäubung. Über Bau und Funktion der Osmophoren. Abhandlungen der Mathematisch-Naturwissenschaftlichen Klasse, Akademie der Wissenschaften und der Literatur in Mainz 1962: 599–763. [Google Scholar]
- Vogel S. 1966. Parfümsammelnde Bienen als Bestäuber von Orchideen und Gloxinia. Österreichische Botanische Zeitschrift 113: 302–361. [Google Scholar]
- Vogel S. 1969. Über synorganisierte Blütensporne bei einigen Orchideen. Österreichische Botanische Zeitschrift 116: 244–262. [Google Scholar]
- Vogel S. 1974. Ölblumen und ölsammelnde Bienen. Tropische und Subtropische Pflanzenwelt 7: 1–267. [Google Scholar]
- Vogel S. 1998. Floral biology [in monocots]. In Kubitzki K, ed. The families and genera of vascular plants, Vol. 3 Berlin: Springer, 34–48. [Google Scholar]
- Vogel S, Cocucci A. 1988. Pollen threads in Impatiens: their nature and function. Beiträge zur Biologie der Pflanzen 63: 271–287. [Google Scholar]
- von Balthazar M, Schönenberger J. 2013. Comparative floral structure and systematics in the balsaminoid clade including Balsaminaceae, Marcgraviaceae and Tetrameristaceae (Ericales). Botanical Journal of the Linnean Society 173: 325–386. [Google Scholar]
- Wagner GP. 2014. Homology, genes, and evolutionary innovations. Princeton: Princeton University Press. [Google Scholar]
- Walker DB. 1975. Postgenital carpel fusion in Catharanthus roseus (Apocynaceae): I. Light and scanning electron microscopic study of gynoecial ontogeny. American Journal of Botany 62: 457–467. [Google Scholar]
- Wang Y-Q, Melzer R, Theissen G. 2011. A double-flowered variety of lesser periwinkle (Vinca minor fl. pl.) that has persisted in the wild for more than 160 years. Annals of Botany 107: 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer AP, Sérsic AN, Marino S, Simões AO, Cocucci AA. 2012. Functional morphology and wasp pollination of two South American asclepiads (Asclepiadoideae-Apocynaceae). Annals of Botany 109: 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter M, Schill R. 1986. Ontogenie von Pollen, Massulae und Pollinien bei den Orchideen. Tropische und Subtropische Pflanzenwelt 56: 1–93. [Google Scholar]
- Yeung EC. 1987a. Development of pollen and accessory structures in orchids. In Arditti J, ed. 1987. Orchid biology. Reviews and perspectives. VI. Ithaca, NY: Comstock Publ., 193–226. [Google Scholar]
- Yeung EC. 1987b. The development and structure of the viscidium in Epidendrum ibaguense H.B.K. (Orchidaceae). Botanical Gazette (Crawfordsville) 148: 149–155. [Google Scholar]
- Žádníková P, Simon R. 2014. How boundaries control plant development. Current Opinion in Plant Biology 17: 116–125. [DOI] [PubMed] [Google Scholar]
- Zhong J, Preston JC. 2015. Bridging the gaps: evolution and development of perianth fusion. New Phytologist. doi:10.1111/nph.13517. [DOI] [PubMed] [Google Scholar]