Abstract
Background and Aims Obdiplostemony has long been a controversial condition as it diverges from diplostemony found among most core eudicot orders by the more external insertion of the alternisepalous stamens. In this paper we review the definition and occurrence of obdiplostemony, and analyse how the condition has impacted on floral diversification and species evolution.
Key Results Obdiplostemony represents an amalgamation of at least five different floral developmental pathways, all of them leading to the external positioning of the alternisepalous stamen whorl within a two-whorled androecium. In secondary obdiplostemony the antesepalous stamens arise before the alternisepalous stamens. The position of alternisepalous stamens at maturity is more external due to subtle shifts of stamens linked to a weakening of the alternisepalous sector including stamen and petal (type I), alternisepalous stamens arising de facto externally of antesepalous stamens (type II) or alternisepalous stamens shifting outside due to the sterilization of antesepalous stamens (type III: Sapotaceae). In primary obdiplostemony the alternisepalous stamens arise before the antesepalous stamens and are more externally from initiation. The antesepalous stamen whorl is staminodial and shows a tendency for loss (type I), or the petals are missing and the alternisepalous stamens effectively occupy their space (type II). Although obdiplostemony is often related to an isomerous gynoecium, this is not essential. Phylogenetically, both secondary and primary obdiplostemony can be seen as transitional stages from diplostemony to either haplostemony or obhaplostemony. Obdiplostemony is the consequence of shifts in the balance between the two stamen whorls, affecting either the alternisepalous stamens together with the petals, or the antesepalous stamens.
Conclusions We advocate a broad definition of obdiplostemony, to include androecia with incomplete whorls, staminodial whorls, anisomerous gynoecia and an absence of petals. As such, the taxonomic significance of obdiplostemony is transient, although it is a clear illustration of how developmental flexibility is responsible for highly different floral morphs.
Keywords: asterids, common petal–stamen primordia, diplostemony, floral bauplan, haplostemony, isomery, obdiplostemony, obhaplostemony, petal loss, rosids, staminodes
GLOSSARY OF TERMS
Alternisepalous stamens: stamens between the sepals; this corresponds to antepetalous stamens in the presence of petals.
Antesepalous stamens: stamens opposite the sepals.
Obdiplostemony: an androecial configuration with two stamen whorls, with the outer whorl alternating with the sepals (opposite the petals when these are present), and the inner whorl opposite the sepals. One of the stamen whorls may be staminodial or consist of paired (multiple) stamens.
Primary obdiplostemony (cf. Ronse De Craene and Smets, 1995): alternisepalous stamens arise before and are more external to the antesepalous stamens from the start, and do not shift position. This condition may be associated with sterilization of the antesepalous stamens (type I) or loss of petals (type II).
Secondary obdiplostemony (cf. Ronse De Craene and Smets, 1995): antesepalous stamens arise before and generally more externally to the alternisepalous stamens and shift position; this condition may be associated with sterilization of the alternisepalous (type I) or antesepalous stamens (type III). In rare cases antesepalous stamens arise before but more internally to the alternisepalous stamens and do not shift position (type II). Type II corresponds to centrifugal obdiplostemony of Ronse De Craene and Smets (1995).
INTRODUCTION
Obdiplostemony is usually associated with flowers with a biseriate perianth (sepals and petals). It represents the unusual condition in the two-whorled androecium where the order of stamen whorls is inversed compared with a diplostemonous flower, so that the outer stamen whorl is opposite the petals and the inner stamen whorl opposite the sepals. Obdiplostemony has been a major topic of morphological research and debate since Chatin’s 1855 description of a ‘type obdiplostémone’ (e.g. Čelakovský, 1875, 1894; Eichler, 1878; Jordan, 1883; Stroebl, 1925; Mattfeld, 1938; Corner, 1946; Eckardt, 1963; Leins, 1964; Eckert, 1966; Gelius, 1967; Rohweder, 1967, 1970; Mayr, 1969; Klopfer, 1973; Ronse De Craene and Smets, 1995; Endress, 2010). Obdiplostemony was considered to be a puzzling and anomalous condition, because it implied that Hofmeister’s (1868) rule of alternating whorls has been violated. Different interpretations for the origin of obdiplostemony have been presented in the past and are reviewed by Eckert (1966) and Ronse De Craene and Smets (1995). Androecial configurations have generally been described as obdiplostemonous based on mature flowers, without knowledge of the ontogeny of the stamens (Ronse De Craene and Smets, 1995). Nonetheless, the interpretation of obdiplostemony is wholly dependent on developmental processes. The significance of obdiplostemony has been questioned since several developmental studies (especially in the 1960s) demonstrated the less fundamental nature of the condition in flowers and the difficulty of establishing an unequivocal distinction from diplostemony (e.g. Leins, 1964; Eckert, 1966; Rohweder, 1967, 1970; Gelius, 1967). The main consensus is that obdiplostemony is a secondary phenomenon arising during floral development as the result of a delay in development of the alternisepalous sector (including petals) and a strengthening of the antesepalous sector. This transition can be read as a gradual shift from diplostemony to a condition resembling obdiplostemony (where alternisepalous stamens appear visually externally to the antesepalous stamens, although their insertion is more internal), and further to cases with externally inserted alternisepalous stamens, as can be illustrated in several families (e.g. Saxifragaceae, Rutaceae, Ericaceae). Klopfer (1973) described this condition as secondary obdiplostemony. On the basis of a broad study of angiosperms, Ronse De Craene and Smets (1995) reviewed the occurrence of obdiplostemony within a premolecular context and distinguished between three forms of obdiplostemony on the basis of developmental evidence. In an ontogenetic context, the relative position (inner vs. outer) and relative timing of initiation (before vs. after) of stamen whorls is key in determining among different types of obdiplostemony. In primary obdiplostemony the alternisepalous stamens arise before the antesepalous stamens and are external to them from initiation. In secondary obdiplostemony alternisepalous stamens are initiated after the antesepalous stamens and shifted outside during ontogeny. Ronse De Craene and Smets (1995) distinguished a third condition (centrifugal obdiplostemony), where alternisepalous stamens arise after the antesepalous stamens but are external to them from initiation. Endress (2010) reviewed the developmental basis of obdiplostemony, based on the example of Geranium robertianum, and concluded that there is no organizational difference between obdiplostemony and diplostemony in this particular case, corresponding to the secondary obdiplostemony of previous authors. Indeed, obdiplostemony results from a reduction of the attachment area of alternisepalous stamens and petals, and a difference in orientation of both stamen whorls. According to Endress (2010), a clear indication for the occurrence of obdiplostemony is the alternisepalous position of the carpels when the gynoecium is isomerous, with a maximal space occupation in the petal sectors. Additionally, Endress precludes a centrifugal androecium initiation in flowers with two fully developed stamen whorls, implying that alternisepalous stamens may not arise before antesepalous stamens. The interpretation of Endress (2010) is more restrictive than that of Ronse De Craene and Smets (1995), who advocated three different cases of obdiplostemony.
Obdiplostemony represents a complicated concept. One can either ignore the condition (as being simply a derivative of diplostemony) or consider it as an important transitional character state in floral evolution. The difficulty in the circumscription of obdiplostemony was also acknowledged by Endress (2010), because of variable parameters during development. In an earlier paper, Ronse De Craene and Smets (1998a) considered that obdiplostemony plays a more fundamental role in floral evolution than suggested by those advocating obdiplostemony to be just a late developmental modification in the flower; they implied that obdiplostemony could be transitional in the evolution of diplostemony to either haplostemony (a single stamen whorl opposite the sepals) or obhaplostemony (a single stamen whorl opposite the petals or alternating with the sepals). In line with the controversial interpretations of obdiplostemony, the purpose of this opinion paper is to clarify the significance of obdiplostemony from a developmental and phylogenetic point of view. Changes in developmental parameters are important drivers of speciation. As such, obdiplostemony represents evolutionary change in progress, where floral developmental evidence shows specific pathways linking different androecial configurations. Obdiplostemony could thus represent an escape from the developmental robustness of diplostemonous flowers, but at the same time be an indication of a possible shift towards a more stable configuration with a single stamen whorl.
IS OBDIPLOSTEMONY A CHARACTER OF ANY SIGNIFICANCE?
Previous discussions, as presented by Eckert (1966), demonstrate that theories about obdiplostemony are often built on rigid concepts that lack developmental evidence, in implying that floral whorls are fixed and cannot change in position. Several flower configurations have been described as obdiplostemony, although evidence for this is often lacking. Different arguments have been used to explain the existence of obdiplostemony, such as the floral vasculature, the presence of nectaries in flowers or changes in the floral development (e.g. Venkata Rao, 1949, 1952; Al-Nowaihi and Khalifa, 1973; Narayana and Rao, 1977a, b). A more external position of alternisepalous stamen bundles will often accompany obdiplostemonous flowers (e.g. Ronse De Craene and Smets, 1995), but can be conflicting when developmental evidence shows the contrary. The vascular connections develop merely as a consequence of the position of organs in the flower (see discussion in Carlquist, 1969; Ronse De Craene and Smets, 1995). Venkata Rao and Ramalakshmi (1968) argued that the large antesepalous nectaries are responsible for the obdiplostemonous arrangement in Euphorbiaceae. However, this explanation for obdiplostemony needs to be used with caution, as nectaries are initiated late in floral development and nectary position may fluctuate strongly in structurally closely similar obdiplostemonous taxa. For example, comparable obdiplostemonous flowers of genera such as Oxalis and Geranium differ in the former having glands in alternisepalous position and the latter in antesepalous position (Endress, 2010).
Čelakovský (1894) and Corner (1946) argued that obdiplostemony could be derived from a polyandrous androecium with centrifugal initiation (described as centrifugal obdiplostemony by Corner, 1946). The examples used by Corner are androecia with 15 stamens (as in Visnea, Monsonia and Peganum). Ronse De Craene and Smets (1995, 1996) described centrifugal obdiplostemony in relation to a doubling of alternisepalous stamen positions based on the example of Peganum, in which an external alternisepalous stamen pair was seen to arise after the antesepalous stamens (Ronse De Craene et al., 1996). This observation, based partially on anatomical data, was shown to be erroneous and was rectified by Bachelier et al. (2011) as the androecium consists of antesepalous triplets, similar to Nitraria, the sister genus of Peganum. This observation puts other cases with alternisepalous stamen pairs in outer position as doubtfully obdiplostemonous (e.g. Crinodendron in Elaeocarpaceae; Monsonia or Hypseocharis in Geraniaceae: Matthews and Endress, 2002; Endress, 2010). A detailed developmental investigation of cases with paired alternisepalous stamens, as mentioned by Ronse De Craene and Smets (1996), may show that the pairs are parts of antesepalous triplets (haplostemony) without any evidence of obdiplostemony. Wei et al. (2015) considered the multistaminate androecia of Aurantioidae (Rutaceae) as derived from precursors with obdiplostemony by doubling of the antepetalous stamen positions. However, clear observations by Lord and Eckard (1985) and Moncur (1988) on Citrus demonstrate that the many stamens develop laterally of antesepalous precursors. This demonstrates that the definition of centrifugal obdiplostemony has been overstretched and has been object of major confusion in the past, with no connection to obdiplostemony.
Another doubtful case of obdiplostemony has been presented by Tucker and Bernhardt (2000), who described the unusual centrifugal androecium of Adrastea salicifolia (Dilleniaceae) as obdiplostemonous. However, the androecium in Adrastea is dimorphic with alternisepalous stamens arising externally of the antesepalous stamen whorl on a ring primordium. This might indicate that this specific two-whorled androecium is derived from a more complex polyandrous androecium, as is common in the genus Hibbertia. The not exactly alternisepalous position and occasional presence of some extra outer stamens is additional support for this interpretation. Androecia with a centrifugal stamen development restricted to two stamen whorls probably also belong to this category, which has nothing to do with obdiplostemony although it has been described as such in the past (e.g. Sericolea in Elaeocarpaceae: van Heel, 1966, see Table 1; Sesuvium in Aizoaceae, Glinus in Molluginaceae, Phytolacca dodecandra in Phytolaccaceae: see Brockington et al., 2013; Ronse De Craene, 2013).
Table 1.
Occurrence of obdiplostemony in the Core Eudicots
| Orders, families and genera investigated * | Presence of petals | Petals retarded in growth | Primary obdiplostemony | Secondary obdiplostemony | Size of antesepalous vs. alternisepalous stamens | Alternisepalous staminodes | Antesepalous staminodes | Presence of diplostemony in family | Presence of obhaplostemony in family | Presence of haplostemony in family | Carpel number and position if isomerous | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalidales | ||||||||||||
| Brunelliaceae Brunellia | – | n/a | type II? | – | > | – | – | + | – | – | (4–)5 alternisepalous | Matthews and Endress (2002) † |
| Cephalotaceae Cephalotus | – | n/a | type II | – | > | – | – | – | – | – | 6 alternisepalous | Baillon (1865), Matthews and Endress (2002) †; Fig. 2D–F |
| Connaraceae Connarus, Cnestis, etc. | + | Yes | – | type I | >/= | + (Connarus, etc.) | – | ? | – | + | 5 antepetalous | Breteler (1989), Matthews & Endress (2002), Fig. 2A |
| Cunoniaceae Acsmithia, Davidsonia, Geissois, etc. | +/– | Yes | type II?‡ | type I | > | – | – | + | – | – | 2–3(4) antepetalous | Dickison (1975), Moody and Hufford (2000), Matthews et al. (2001), Schönenberger et al. (2001), Matthews and Endress (2002), Fig. 2C |
| Oxalidaceae Oxalis, Biophytum, Averrhoea | + | yes | – | type I | > | + (Averrhoea) | – | – | – | – | 5 antepetalous | Matthews and Endress (2002), Fig. 1A–C |
| Elaeocarpaceae§ Platytheca, Sericolea, Elaeocarpus | + | no | type I? | type I | > | – | – | – | – | + (pairs or triplets) | 2–3 | van Heel (1966), Ronse De Craene and Smets (1996), Matthews and Endress (2002) |
| Saxifragales | ||||||||||||
| Crassulaceae Monanthes, Semperivum, Kalanchoe etc. | + | yes | – | type I | > | + (Sempervivum) | – | – | – | + | 4–5 antepetalous | Eichler (1878), Gelius (1967), Ronse De Craene (2016) |
| Haloragaceae Haloragis | +/– | yes | – | type I | = | – | – | + | – | + | 2–4 antepetalous | Eichler (1878), L. Ronse De Craene, unpubl. data |
| Iteaceae Pterostemon | + | ? | – | type I? | > | + | – | – | – | – | 5 antepetalous? | Engler (1930) |
| Aphanopetalaceae Aphanopetalum | +/– | + | type II? | type I? | > | – | – | – | – | – | 4 alternisepalous | Dickison (1975), Dickison et al. (1994) |
| Penthoraceae Penthorum | – | n/a | type II? | – | < | – | – | – | – | – | 5–6 alternisepalous | Baillon (1865), L. Ronse De Craene, unpubl.data |
| Tetracarpaeaceae Tetracarpaea | + | yes | – | type I | > | – | – | – | – | + | 4–5 antepetalous | Hils et al. (1988) |
| Saxifragaceae Rodgersia, Chrysosplenium | +/– | yes | type II? | type I | > | – | – | – | + | + | 2 | Engler (1930), Gelius (1967), Klopfer (1968, 1970, 1973), Ronse De Craene et al. (1998c) |
| Malpighiales | ||||||||||||
| Euphorbiaceae Crozophora, Capuronia, Jatropha | – (+) | yes | type I? | – | >/< | – | – | + | + | + (Crozophora) | 2–3 | Eichler (1878), Michaelis (1924), Venkata Rao and Ramalakshmi (1968) |
| Linaceae Durandea, Hugonia | + | yes | – | type I | >/< | + | – | + | – | + | 3–5 antepetalous | Narayana (1964), Schewe et al. (2011), Matthews and Endress (2011) |
| Malpighiaceae Malpighia, Galphimia, etc. | + | ? | – | type I | > | – | – | – | – | + | 2–3–(–5) antepetalous | Eichler (1878), Ronse De Craene (2010) |
| Ochnaceae Ouratea, Sauvagesia | + | no | – | type I | > | + (Sauvagesia) | – | + (Ouratea) | – | + | 3–5 antepetalous | Farrar and Ronse De Craene (2013), Fig. 1G–I |
| Rhizophoraceae Anopyxis, Bruguieria, Ceriops, Crossostylis | + (–) | yes | ? | type I | >, =,< | – | – | + | – | – | 2–5(–20) ¶ antepetalous/antesepalous | Baillon (1862), Juncosa and Tomlinson (1987), Juncosa (1988), Setoguchi et al. (1996), Matthews and Endress (2011) |
| Fabales | ||||||||||||
| Surianiaceae Suriania | + | yes | – | type I | > | + (occ. incomplete) | – | – | – | 5 antepetalous | Tschunko and Nickerson (1976), Bello et al. (2007) | |
| Ericales | ||||||||||||
| Clethraceae Clethra | + | no | - | type I | > | – | – | + | – | – | 3–4 | Leins (1964), Caris (2013) |
| Diapensiaceae Galax, Pyxidanthera, etc. | + | yes | - | type I | > | + | – | – | – | + (Pyxidanthera) | 3 | Caris (2013) |
| Ericaceae Erica, Monotropa, etc. | + | yes/no | – | type I | = | – | – | + | – | + | 4–5 antepetalous | Payer (1857), Leins (1964), Caris (2013) |
| Sapotaceae Palaquium, Sideroxylon, etc. | + | no | – | type III | < | – | + | + | + | – | 4–5–∞ antesepalous/antepetalous | Caris (2013), Kümpers et al. (2016), Fig. 3D–F |
| Styracaceae Styrax, Pterostyrax | + | no | – | type I | >/= | – | – | + | – | + (Pamphilia) | 2–5 antepetalous | Dickison (1993), Caris (2013) |
| Geraniales | ||||||||||||
| Geraniaceae Geranium, Erodium, etc. | + | yes | – | type I | > | + (Erodium) | – | – | – | + | 5 antepetalous | Endress (2010), Ronse De Craene et al. (1993) |
| Melanthiaceae Francoa, Greyia | + | yes | – | type I | > | – | – | – | – | + | 4–5 antepetalous | Ronse De Craene and Smets (1999) |
| Vivianaceae Viviania, Rhynchotheca | + (–) (Rhynchotheca) | yes? | – | type I | > | – | – | – | – | + | (2–)3–5 antepetalous | Weigend (2005), L. Ronse De Craene, pers. observ. |
| Zygophyllales | ||||||||||||
| Zygophyllaceae Tribulus, Balanites, etc | + | yes | – | type I | > | – | – | – | – | ? | 4–5 antepetalous | Payer (1857), Ronse De Craene and Smets (1995) |
| Malvales | ||||||||||||
| Malvaceae -Byttnerioideae Melochia, Byttneria, Theobroma, Lasiopetalum | + | yes | type I | – | < | – | + | – | + (Hermannia, Melochia, Lasiopetalum) | – | 5 antepetalous, antesepalous (when obhaplostemony) | van Heel (1966), Payer (1857), Ronse De Craene and Smets (1995), Fig. 3A–C |
| Malvaceae - Grewioideae Triumfetta annua, T. bartriania | + | yes | – | type I? | > | – | – | – | – | + | 5 antepetalous | van Heel (1966) |
| Caryophyllales | ||||||||||||
| Asteropeiaceae Asteropeia | + | + | – | type I? | > | – | – | – | – | – | 3 | Schatz et al. (1999), L. Ronse De Craene, unpubl. data |
| Caryophyllaceae Gypsophila, Dianthus, etc. | +/– | yes | – | type I | > | + | – | + | + (Colobanthus) | + | 2–3–5 antepetalous | Eckert (1966), Rohweder (1967, 1970), Ronse De Craene et al. (1998b), Ronse De Craene (2013) |
| Nepenthaceae Nepenthes | – | n/a | type II | – | > | – | – | – | + | – | 4 alternisepalous | L. Ronse De Craene, unpubl. data |
| Tamaricaceae Myricaria | + | yes | – | type 1 | > | – | – | –? | – | + | 3–5 antepetalous | Payer (1857), L. Ronse De Craene, unpubl. data |
| Myrtales | ||||||||||||
| Combretaceae Terminalia, Thiloa, Combretum | +/– | yes | – | type II | </> | + (Thiloa) | +/– | ? | – | + | (2–3)5, antepetalous | Payer (1857), Eichler (1878), Ronse De Craene (2010), L. Ronse De Craene, unpubl. data |
| Lythraceae Decodon, etc. | +/– | yes | – | type I | > | – | – | + | + | + | 2–6 antepetalous/antesepalous (Lagerstroemia) | Mayr (1969), Tobe et al. (1998) |
| Onagraceae Ludwigia, Epilobium | + (–) | yes | – | type I | > | – | + (Clarkia) | + | – | + (Ludwigia) | 2–4(5) antepetalous | Eichler (1878), Mayr (1969) |
| Myrtaceae Heteropyxis | + | no | – | type I? | ? | – | – | + (Psiloxylon) | + | + | 2–3 (–5) antepetalous | Eichler (1878), Schmid (1980) |
| Sapindales | ||||||||||||
| Meliaceae Cedrella, Melia | + | no | – | type I | > | – | – | + | – | ? | 5 antepetalous | Payer (1857), Eichler (1878) |
| Rutaceae Ruta, Dictamnus, etc. | + | no | – | type I | > | +(Agathosma, Flindersia) | – | + | – | + | 2–3(5) antepetalous | Payer (1857), Eckert (1966), Lal and Narayana (1994), Wei et al. (2011) |
| Simaroubaceae Ailanthus, Quassia, etc. | + | no | – | type I | > | + | – | + | – | + | 2–3(5) antepetalous | Payer (1857), Eichler (1878), Eckert (1966) |
| Cornales | ||||||||||||
| Loasaceae Schismocarpus | + | no | – | type I | > | + | – | – | – | + | 2–3–5 antepetalous | Hufford (1989), Moody and Hufford (2000), Ronse De Craene (2010) |
| Crossosomatales | ||||||||||||
| Geissolomataceae Geissoloma** | – | n/a | type II? | – | > | – | – | – | – | – | 4 alternisepalous | Matthews & Endress (2005a) |
| Strasburgeriaceae (Strasburgeria) | + | no | – | type I | > | – | – | – | – | – | 5 antepetalous | Matthews & Endress (2005a) |
| Cucurbitales | ||||||||||||
| Anisophylleaceae | + | ? | – | + | > | – | – | ? | – | – | 3–4 antepetalous | Matthews et al. (2001), Matthews and Endress (2004) |
*The following families were excluded although they were reported to be obdiplostemonous [see also Ronse De Craene and Smets (1995): Anacardiaceae, Eichler (1878), Bachelier and Endress (2009), Burseraceae, Bachelier and Endress (2009)], Humiriaceae [with increased stamen number: Narayana and Rao (1977b) – although the condition is marginal with either antesepalous or antepetalous carpels: Kubitzki (2014), Celastraceae [with antepetalous staminodes in Parnassia and possibly Brexia (Eichler, 1878; Matthews and Endress, 2005b)].
†Described as diplostemony by Matthews and Endress (2002).
‡No observations of early stamen initiation are young enough to give undisputed evidence of early obdiplostemony type II [e.g. Moody and Hufford (2000), contrary to Cephalotus (Fig. 2D–F).
§van Heel (1966) describes a condition resembling obdiplostemony in Sericolea. However, the number of antepetalous stamens is variable, with stamens occurring singly or in pairs. Other Elaeocarpaceae have antesepalous triplets or a multistaminate androecium with stamen groups in antepetalous sectors. Our unpublished data on Elaeocarpus cyaneus show a simultaneous initiation of the antepetalous stamen fascicles with the antesepalous stamens. This is correlated with a star-shaped floral apex (cf. Fig. 3A–C).
¶Antepetalous in Macarisia according to Baillon (1862), but antesepalous in Crossostylis according to Setoguchi et al. (1996).
**Matthews and Endress (2005a) described the flower as diplostemonous but the alternisepalous stamens are in outer position similar to other obdiplostemonous apetalous flowers.
How should obdiplostemony be defined? Is the definition strictly linked to developmental processes (as suggested by many authors), or is it a systematically diagnostic feature in mature flowers with different origins? Is it possible to consider a staminodial whorl as part of the definition of obdiplostemony? Can apetalous flowers with external alternisepalous stamens and inner antesepalous stamens be treated as examples of obdiplostemony, or is there any other developmental pathway responsible for this floral arrangement? Are flowers with fewer carpels or with isomerous carpels that are not in antepetalous position obdiplostemonous? Table 1 summarizes reports of the distribution of obdiplostemony in the core eudicots, including different concepts of the character. This list is not exhaustive and is also restricted by the meaning different authors attach to obdiplostemony.
Based on the early initiation of the respective stamen whorls in flowers we consider two developmental conditions, namely primary and secondary obdiplostemony, corresponding to the definition in Ronse De Craene and Smets (1995) with some modifications (see below), and argue that each represents a major disruption of the common floral bauplan (developmental robustness) leading to highly divergent floral morphs (see below).
Secondary obdiplostemony
Secondary obdiplostemony is characterized by following parameters:
the earlier initiation of antesepalous stamens, mostly in outer position and with an outward shift of alternisepalous stamens and inward shift of antesepalous stamens;
a stronger development of the antesepalous stamens than the alternisepalous stamens and petals, which may be delayed or arise as common primordia.
Eckardt (1963) argued that there is a progressive shift in the expression of obdiplostemony leading to two distinct forms. In other words, obdiplostemony may be weakly or more strongly expressed. The weakest condition is comparable to diplostemonous flowers but the orientation of anthers and filaments (and not their insertion) gives a vague impression of obdiplostemony and has sometimes been described as such (e.g. Limnanthes in Limnanthaceae: Eichler, 1878, but see Hofmann and Ludewig, 1985; Hamamelis in Hamamelidaceae: Eichler, 1878, but see Mione and Bogle, 1990; Hydrangeaceae: Eicher, 1878, but see Hufford, 2001; Humiriaceae: Narayana and Rao, 1977b). In some families (e.g. Rutaceae, Simaroubaceae, Onagraceae, Lythraceae) there is a whole gradation between conditions where the alternisepalous stamens are forcibly orientated towards the periphery by the larger size of the antesepalous stamens, although their insertion can be more internal, to flowers with a more external alternisepalous stamen insertion (e.g. Eichler, 1878; Eckert, 1966; Mayr, 1969). These families generally show well-developed petals with little interaction with the antepetalous stamens (e.g. Simaroubaceae: Eckert, 1966). Secondary obdiplostemony becomes more strongly established by cohesion between petal and its facing stamen. As illustrated by several authors (e.g. Leins, 1964; Eckert, 1966; Gelius, 1967; Klopfer, 1973; Ronse De Craene and Smets, 1995; Endress, 2010), secondary obdiplostemony is strongly linked to a reinforcement of antesepalous stamens and a weakening of alternisepalous stamens and petals. The antesepalous stamens are initiated before (or at the same time as) the alternisepalous stamens and are initially inserted more externally to the alternisepalous whorl. The development of flowers of Oxalidaceae, Caryophyllaceae and Geraniaceae is similar in the way broad antesepalous stamen primordia develop, extending both abaxially and adaxially beyond the insertion of the alternisepalous stamen whorl (Fig. 1A–F; Ronse De Craene et al., 1993, 1998b; Endress, 2010; Bull-Hereñu et al., 2016). By differential growth and a different orientation, antesepalous stamens may eventually shift inside the outer boundaries of the alternisepalous stamens, which together with the petals are usually delayed and smaller from inception, occupying a reduced primordial surface area (cf. Figs 1A–F and 2A, B). With a smaller-sized petal, the insertion area of the alternisepalous stamens is effectively reduced below the antesepalous stamen and this is stressed in later development by the more external placement of the anthers (Figs 1C, F and 2C). A strong cohesion between petal primordium and alternisepalous stamen may result in the development of common petal–stamen primordia by a process of heterochrony (e.g. Oxalidaceae, Zygophyllaceae, Geraniaceae, Caryophyllaceae; Fig. 1A). The importance of common primordia has perhaps been overemphasized in the past (e.g. Mattfeld, 1938; Ronse De Craene et al., 1993), but it represents a clear developmental indicator of petal reduction. A further delay in petal initiation leads to apetaly, allowing the alternipetalous stamen to expand fully on the otherwise former common primordium. It is no wonder that families or orders where obdiplostemony occurs in combination with weakly developed, delayed petals often contain apetalous genera (see below; e.g. Saxifragaceae: Chrysosplenium, Rodgersia, Gelius, 1967; Cunoniaceae: Davidsonia, Geissois, Dickison, 1975; Matthews et al., 2001; Cephalotus: Fig. 2E, F).
Fig. 1.
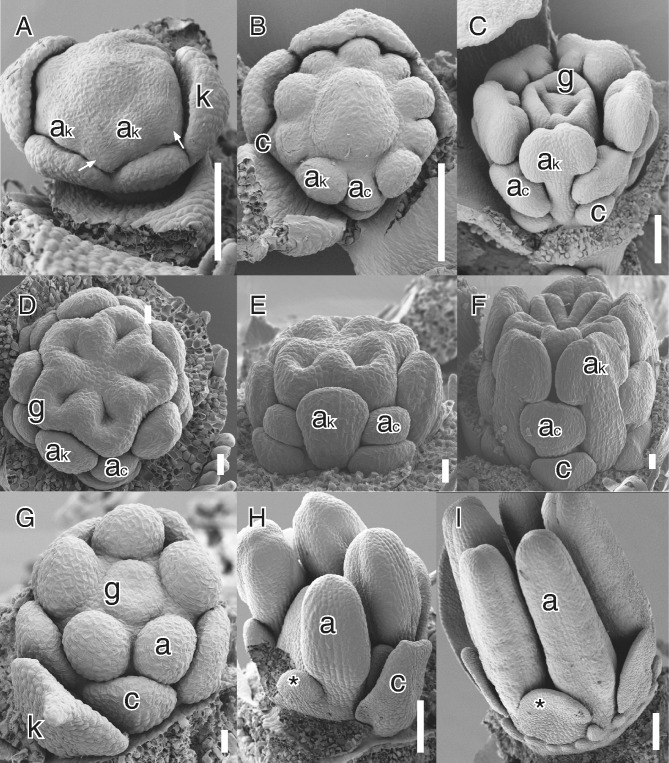
Different developmental modes of secondary obdiplostemony type I. (A–C) Silene pusilla Waldst. & Kit. (Caryophyllaceae); (D–F) Biophytum dendroides DC. (Oxalidaceae); (G–I) Sauvagesia erecta L. (Ochnaceae). (A) Lateral view with early initiation of androecium; antesepalous stamen primordia and common petal–stamen primordia (white arrows). (B) View of flower after the division of the common primordia; note the different direction of growth of antesepalous and alternisepalous stamens. (C) Lateral view of preanthetic flower; note the larger antesepalous stamens and small petals. (D) Apical view of flower after septum initiation; note the distinct size of the two stamen whorls. (E) Lateral view of the same. Note the lower insertion of the antesepalous stamen. (F) Lateral view of older bud; note the smaller alternisepalous stamen and petal. (G) Initiation of antesepalous stamens and gynoecium; there is no trace of the alternisepalous stamens. (H) Late stage of the initiation of the staminodes (asterisk) at the same level as the stamens, most petals and one stamen removed. (I) Nearly mature bud with external petaloid staminode (asterisk) and ring of pseudostaminodes emerging externally. Abbreviations: ac, alternisepalous stamen; ak, antesepalous stamen; c, petal; k, sepal; g, carpel. Scale bars: D–F, G = 20 μm; A–C, H I = 100 μm.
Fig. .2.
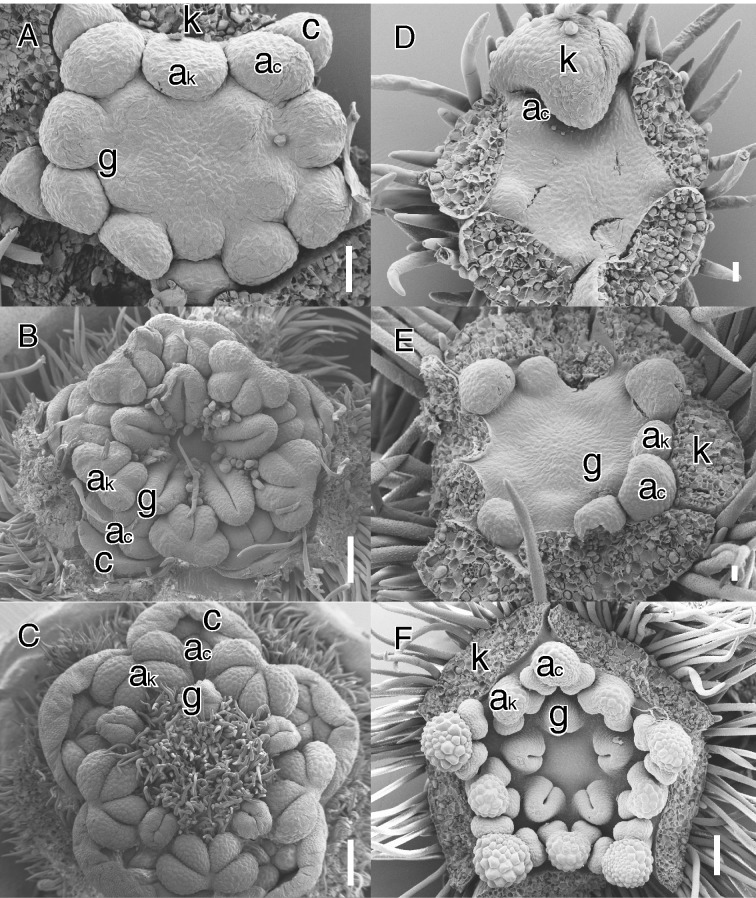
Illustrations of secondary obdiplostemony type I (A–C, Cnestis ferruginea DC., Connaraceae) and primary obdiplostemony type II (D–F, Cephalotus follicularis Labill., Cephalotaceae). (A) Apical view of young obdiplostemonous flower at initiation of the carpels. (B) Older stage with fully developed carpels. Note the extent of peripheral carpel development in alternisepalous position. (C) Nearly mature stage showing the strongly displaced stigmas pressed against the antepetalous stamens. (D) Early initiation of alternisepalous stamens; four sepals removed. (E) Initiation of second stamen whorl and carpel primordia. (F) Apical view of older pentamerous flower. Note the larger alternisepalous anthers and large central area pushing the carpels away from the centre in E and F. Abbreviations: ac, antepetalous or alternisepalous stamen; ak, antesepalous stamen; c, petal; k, sepal; g, carpel or style. Scale bars: C = 200 μm; D, E = 20 μm; A, B, F = 100 μm.
The fate of alternisepalous stamens in larger families with secondary obdiplostemony is variable. These stamens are generally shorter than antesepalous stamens (Fig. 1C, F), although some species can usually be found with sterile alternisepalous stamens while others may be even haplostemonous by complete loss of the sterile whorl (see Table 1). For example, in Linum alternisepalous staminodes arise very late and their position is strongly influenced by the already large antesepalous stamens (e.g. Schewe et al., 2011). As a result several Linaceae are haplostemonous, although clearly derived from a condition similar to obdiplostemony (present in Durandea: Narayana, 1964). Other Linaceae have either two stamen whorls of different length (e.g. Indorouchera: Narayana and Rao, 1978) or a strongly reduced antepetalous whorl (e.g. Catharolinum, Hesperolinon: Narayana and Rao, 1976, 1977a). In Sauvagesia (Ochnaceae) petaloid staminodes arise very late and are shifted outside the stamen whorl by the growth of an androgynophore (Fig. 1G–I; Farrar and Ronse De Craene, 2013). By contrast, the condition where flowers are strictly diplostemonous but with a sterile antesepalous whorl is rare (e.g. Moringa in Moringaceae: Ronse De Craene et al., 1998a; Corynocarpus in Corynocarpaceae: Matthews and Endress, 2004).
In Caryophyllaceae the nature of the petals is controversial, as they could represent either organs in a state of reduction or staminodial structures derived by the division of complex alternisepalous stamens (see Ronse De Craene et al., 1998b; Ronse De Craene, 2013). However, the fact that petals always arise by the division of common primordia in petaliferous Caryophyllaceae, and the frequent occurrence of sterile alternisepalous structures in apetalous Caryophyllaceae, indicates that there is a strong pressure for the reduction of the alternisepalous sector of the flower, linked to secondary obdiplostemony.
It has been emphasized that obdiplostemony is linked to an isomerous gynoecium pressing the alternisepalous stamens further to the periphery (e.g. Chatin, 1855; Eckert, 1966; Mayr, 1969; Endress, 2010; Figs 1E, F and 2B, C). Ronse De Craene (2013, 2016) demonstrated that carpel position has great influence on the stamen whorl that happens to be in a more central position of the flower, even before the morphological appearance of the carpels. Obdiplostemony is stressed by a large floral meristem pushing carpels towards the periphery. We observed this in several cases, such as Cnestis (Connaraceae: Fig. 2A–C) and Cephalotus (Cephalotaceae: Fig. 2F). Endress (2010), Bachelier and Endress (2009), and Matthews and Endress (2002, 2011) indicated that the presence of an isomerous alternisepalous carpel whorl is the best indication for obdiplostemony. We believe that carpel position can be a good indication for obdiplostemony, but is not binding in all cases for the following reasons:
When carpel number is reduced to three or two as is common in core eudicots, this does not influence the relative position of both anther whorls and the androecium should still be described as obdiplostemonous (e.g. Malpighiaceae, Saxifragaceae, Cunoniaceae, Caryophyllaceae; Table 1; see Klopfer, 1973; Fig. 1C).
An alternisepalous carpel position is not always reflected by the existence of a more external alternisepalous stamen whorl. Several diplostemonous flowers have carpels opposite the petals (e.g. Ebenaceae: Caris, 2013; Burseraceae: Bachelier and Endress, 2009; Lythraceae: Tobe et al., 1998; Melastomataceae: Eichler, 1878; Anacardiaceae: Wannan and Quinn, 1991), effectively disturbing Hofmeister’s rule. In some families (e.g. Caryophyllaceae, Rhizophoraceae, Humiriaceae, Hydrangeaceae) the position of isomerous carpels is variable (see Table 1; Engler, 1930; Matthews and Endress, 2011; Ronse De Craene, 2013; Kubitzki, 2014). The flexibility in carpel position can be illustrated in some Malvaceae: in species with an antesepalous staminodial whorl, carpels are in alternisepalous position (e.g. Melochia pyramidata L.), while in the absence of staminodes, carpels are in antesepalous position (e.g. Melochia umbellata (Houtt.) Stapf, Hermannia candicans Ait.: see van Heel, 1966).
Secondary obdiplostemony is not always linked to a postgenital shift of the alternisepalous stamens to an external position. In several studied cases, including Suriana (Surianaceae; Bello et al., 2007), Combretum indicum (Combretaceae; Payer, 1857) and possibly Bruguiera (Rhizophoraceae; Juncosa and Tomlinson, 1987), antesepalous stamens arise before alternisepalous stamens or staminodes and have a more central insertion from the start; the alternisepalous stamens or staminodes emerge later and more to the outside. In apetalous Caryophyllaceae alternisepalous stamens or staminodes (e.g. Corrigiola, Paronychia, Scleranthus) also arise outside the antesepalous stamen whorl (Ronse De Craene et al., 1998b). In Combretum, as in other Myrtales with a strongly developed hypanthium, receptacular growth is probably responsible for the more external initiation of alternisepalous stamens. In Suriana carpels arise at a considerable distance from the central floral receptacle, leaving little space for the alternisepalous stamens (Bello et al., 2007: fig. 9i). This developmental pattern was described as centrifugal obdiplostemony by Ronse De Craene and Smets (1995), but is clearly linked to secondary obdiplostemony due to the later initiation of the alternisepalous stamen (but without a shift of stamens). For this reason we tentatively prefer to distinguish two types, secondary obdiplostemony type I, corresponding to a shift of stamens, and secondary obdiplostemony type II, corresponding to the external initiation of alternisepalous stamens without a shift of stamens. We acknowledge that more evidence of early development is necessary in support of the latter case.
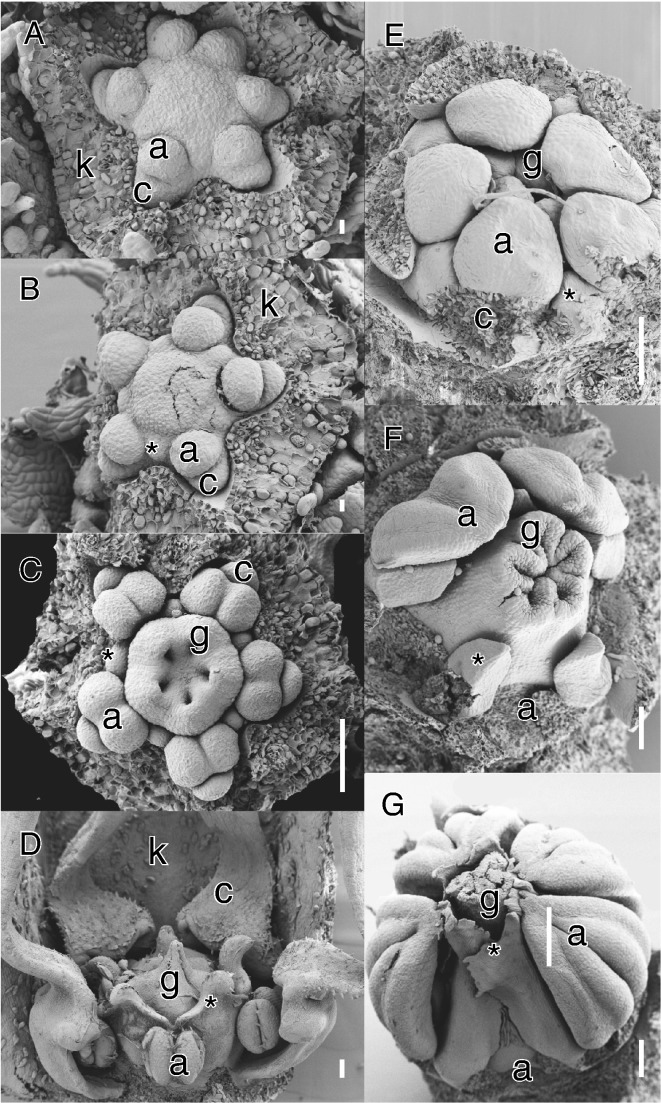
Another unusual derivation from diplostemony is found in Sapotaceae. The majority of species in the family are characterized by an androecium with the antesepalous whorl transformed into staminodes, or by obhaplostemony (Fig. 3F, G; Caris, 2013; Kümpers et al., 2016). However, in all cases studied so far the early stamen initiation is similar to diplostemonous flowers, with the antesepalous staminodes arising first outside the alternisepalous stamen whorl and opposite the carpels (Fig. 3E). Antesepalous stamens are arrested in growth relative to the alternisepalous stamens and become shifted inwards as staminodes. In some species both whorls are fertile and obdiplostemony is linked to a progressive shift of the larger alternisepalous stamens as the external whorl (e.g. Palaquium: Kümpers et al., 2016). This demonstrates that in some Ericales secondary obdiplostemony is the result of alternipetalous stamens shifting outside, but shows a reversed trend of secondary obdiplostemony type I, i.e. in favouring the alternisepalous sector of the flower by a progressive reduction of the antesepalous sector. This development bears resemblance to Sauvagesia (Ochnaceae), where initials of staminodes are formed at the same level as the antesepalous stamens, but their outward shift is linked to hypanthial growth (Fig. 1G–I; Farrar and Ronse De Craene, 2013). This form of secondary obdiplostemony in Sapotaceae does not affect the petals and alternipetalous stamens (no common primordia are formed) and is best described as secondary obdiplostemony type III.
Fig. 3.
Examples of primary obdiplostemony type I (A–D) and secondary obdiplostemony type III (E-G). (A–D) Byttneria aculeata (Jacq.) Jacq. (Malvaceae). (E–G) Sideroxylon inerme L. (Sapotaceae). (A) Apical view of young flower at the initiation of antesepalous staminodes; sepals removed. Note the abnormal hexamerous flower with two petals closer together on top of the image. (B) Apical view at gynoecium initiation; staminode marked with asterisk, sepals removed. (C) Apical view at septum differentiation. Note the well-developed alternisepalous stamens and small inner staminodes (asterisk). (D) Lateral view of nearly mature flower. Stamens are covered by spoon-shaped petals (frontal removed); staminodes (asterisk) form collar around ovary. (E) Early development of androecium, petals and sepals removed. Note the position of staminodes (asterisk) outside the alternisepalous stamens. (F) Outward shift of alternisepalous stamens (three removed to show the staminodes, asterisk). (G) Lateral view of nearly mature bud, one stamen removed. The staminodes form an inner collar around the ovary. Abbreviations: a, antepetalous or alternisepalous stamen; c, petal; k, sepal; g, carpel. Scale bars: A, B = 10 μm; C–F = 100 μm; G = 200 μm.
Primary obdiplostemony
The main differences between primary obdiplostemony and secondary obdiplostemony are:
the earlier initiation of alternisepalous stamens in outer position,
a stronger development of the alternisepalous stamens than the antesepalous stamens, and
the absence of an outward shift of alternisepalous stamens and inward shift of antesepalous stamens.
We argue that in some cases the normal order of initiation of the androecium is reversed, because the alternisepalous stamens arise before the antesepalous stamens (Figs 2D, E and 3A–C). The loss of petals (as in Cephalotus) or the delayed initiation of staminodes (as in Malvaceae) leads to a de facto condition resembling obdiplostemony with alternisepalous stamens in an external position. In primary obdiplostemony type I, alternisepalous stamens are generally outside the antesepalous stamen whorl and may be connected with the petals as common primordia. This condition is restricted to Malvaceae subfamily Byttnerioideae and is always linked to a weakening of the antesepalous stamen whorl, which can be delayed in its development and is generally staminodial (Fig. 3A–D). There are no known cases of fully fertile antesepalous stamens, indicating that primary obdiplostemony is a derived, transitional condition. Within Malvaceae–Byttnerioideae, species with primary obdiplostemony type I are often accompanied by purely obhaplostemonous species without any trace of a staminodial whorl (e.g. Hermannia, Melochia: van Heel, 1966). The origin of the diversity in androecial configurations in Malvaceae is complex (see van Heel, 1966; von Balthazar et al., 2006; Ronse De Craene, 2010). However, a common feature is a triplet arrangement consisting of an outer alternisepalous stamen pair and a single inner antesepalous stamen. This may indicate that the observed obdiplostemony is fictitious and ultimately derived through the expansion of antesepalous triplets linked to the sterilization of the upper stamen and the shift of the outer pair in alternisepalous sectors of the flower (see below).
In contrast to the condition in Malvaceae, where sterilization of the antesepalous stamens leads to obdiplostemony, a complete loss of petals may lead to the earlier initiation of alternisepalous stamens. We define the latter condition as primary obdiplostemony type II. Type II is more widespread than type I and is closely linked to secondary obdiplostemony. As mentioned before, secondary obdiplostemony is often associated with a weakening or loss of petals and this shift should be included in the definition of obdiplostemony. We argue for not describing the condition with apetalous flowers as diplostemony because (1) the arrangement of the stamens corresponds to the definition of obdiplostemony we adhere to, and (2) it makes it possible to distinguish these from apetalous flowers with the antesepalous stamens inserted more externally. Saxifragaceae include several apetalous genera (e.g. Chrysosplenium, Rodgersia). Floral developmental data show that in Chrysosplenium antesepalous stamens arise before alternisepalous stamens; the outward shift of alternisepalous stamens and the obdiplostemonous appearance is stressed by the development of a broad nectary and absence of petals (Ronse De Craene et al., 1998c). However, a developmental shift from this pattern may lead to a condition where alternisepalous stamens take over the position of the petals from the start, arising before the antesepalous stamens. The alternisepalous stamens differentiate on the whole primordium (possibly an ancestral petal–stamen primordium), thus gaining an earlier time of initiation and a larger size compared with the antesepalous stamens (Fig. 2D–F). This process is linked to apetalous flowers where pollinator attraction is taken over by the sepals, a hypanthium or occasionally the androecium. If space is provided the alternisepalous stamens arise before and more externally to the antesepalous stamens in a situation similar to primary obdiplostemony (e.g. Cephalotus: Baillon, 1862; Fig. 2D, E). Primary obdiplostemony type II appears to be widespread, although observations of critical stages are often missing (e.g. Davidsonia, Geissois and Acsmithia in Cunoniaceae: Matthews et al., 2001; Shepherdia in Elaeagnaceae: Eichler, 1878; Geissolomataceae, Saxifragaceae, Brunelliaceae or Penthoraceae: Table 1). Polygonaceae with paired stamens inserted higher on the hypanthium than the antesepalous stamens can also be described as primary obdiplostemony type II, even if the inner whorl is reduced in number due to the pressure of the gynoecium [see also Ronse De Craene (2010) for an interpretation of the paired stamens in the family]. In these potential representatives for primary obdiplostemony type II, alternisepalous stamens can be longer and more strongly developed than antesepalous stamens, indicating that they arise before the latter (e.g. Cunoniaceae, Elaeagnaceae, Polygonaceae). In Cunoniaceae with petals, antesepalous stamens are longer and broader, in contrast to the apetalous Davidsonia (Matthews et al., 2001), although stamen sizes may fluctuate during development (see Endress, 2010). In Cephalotus, antesepalous stamens are the longest at maturity (Matthews and Endress, 2002), despite their later initiation (Fig. 2D). A logical derivation of primary obdiplostemony type II is the loss of the antesepalous stamens, leading to apetalous flowers with a single whorl of stamens in alternisepalous position, effectively reversing the trend initiated by secondary obdiplostemony type I and leading to obhaplostemony.
Phylogenetic importance
Figure 4 shows the relationship between different forms of obdiplostemony. Diplostemony probably represents the basal androecial configuration in the core eudicots, as it is both widespread and considered to be plesiomorphic in most major clades (Ronse De Craene, 2010; L. Ronse De Craene, unpubl. data). Obdiplostemony is markedly absent from large orders, such as Brassicales, Rosales, Fagales and Cucurbitales (except Anisophylleaceae: Matthews et al., 2001), and Fabales (except Surianiaceae: Bello et al., 2007).
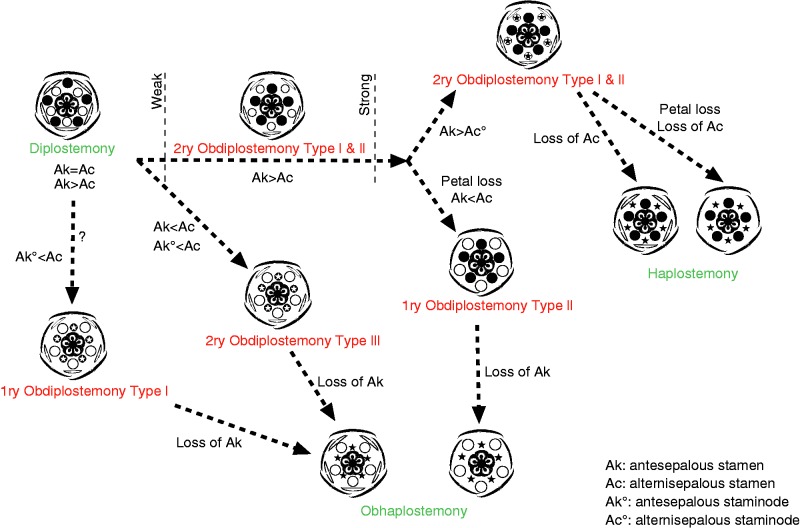
Fig. 4.
Diagram showing possible main evolutionary relationships in the androecium of core eudicots (see text for explanations). In the floral diagrams sepals and antesepalous stamens are in black; petals and alternisepalous stamens are in white; encircled stars show staminodial structures; stars without circles represent lost stamens.
An important factor influencing the occurrence of obdiplostemony is a strong coherence of whorls linked to the synorganization of the flower, as illustrated by Endress (2010) for Geranium robertianum. Different species within rosids sharing secondary obdiplostemony type I show a remarkable similarity in early development of the flower. A comparison between Biophytum (Fig. 1D–F) or Oxalis (Bull-Hereñu et al., 2016) and illustrations of Geranium robertianum in Endress (2010) demonstrates a similar initiation sequence, arrangement of organ whorls and smaller sizes for the alternisepalous stamen primordia.
Secondary obdiplostemony type I is common in rosid families belonging to the Zygophyllales, Sapindales, Saxifragales, Oxalidales, Geraniales, Malpighiales and Myrtales, as well as early-diverging asterids (Ericales) and Caryophyllales (Table 1). Obdiplostemony is often weakly expressed and occasionally difficult to distinguish from diplostemony (see Eckert, 1966; Gelius, 1967; Mayr, 1969) with occasional reports of both conditions in the same family (e.g. Onagraceae, Rutaceae, Lythraceae). Genera or species with secondary obdiplostemony are often closely related to species affected by weakening of the alternisepalous sector: these species have alternisepalous stamens and petals that are strongly delayed in development, often arising as common primordia; petals become lost; alternisepalous stamens become staminodial or vanish altogether. When isomerous, carpels occupy the best available space, which is alternisepalous.
Isomerous haplostemonous flowers mostly have carpels opposite the petals (i.e. facing the absent androecial whorl), which lends support to the interpretation of secondary obdiplostemony type I as a break-away from diplostemony and as a transitional state to haplostemony in cases where the two conditions are present in the same family (e.g. Ericaceae, Geraniaceae, Oxalidaceae, Caryophyllaceae). However, the significance of this correlation is often ignored by the fact that gynoecia in most rosids and asterids have a tendency to become reduced to three or two carpels by space restrictions (cf. Ronse De Craene and Smets, 1998b). The presence of an alternisepalous staminodial whorl is also an indication of the transitional nature of secondary obdiplostemony reflecting a shift to haplostemony (e.g. Averrhoa in Oxalidaceae, Erodium in Geraniaceae, Flindersia in Rutaceae, Parnassia in Celastraceae). This indicates that diplostemony and secondary obdiplostemony type I can be linked as stages of one gradual change, eventually leading to the loss of the alternisepalous stamens and the establishment of haplostemony (Fig. 4). It is not certain whether obdiplostemony and a sterile alternisepalous stamen whorl are necessary conditions for the origin of haplostemony, although both conditions are frequently associated (Table 1; Connaraceae, Linaceae, Rutaceae, etc.). Clearly, haplostemony can arise directly from diplostemony by loss of the alternisepalous whorl, and isomerous carpels become accommodated in the available space according to Hofmeister’s rule. Smaller flowers would be the origin of this shift, although there is little evidence to suggest that one of the two stamen whorls can easily vanish, as the establishment of diplostemony appears to be robust and well conserved in core eudicots (see Ronse De Craene, 2010). This also implies that petals are not affected by the alternisepalous stamen reduction. However, even without evidence for the presence of secondary obdiplostemony in a clade, the condition may have occurred in ancestral flowers preceding the occurrence of haplostemony.
Haplostemony is the most widespread stamen configuration in core eudicots, and is found predominantly in asterids (Ronse De Craene, 2010). The presence of obdiplostemony in some early-diverging asterids (Ericales: Ericaceae and related families), linked to an occasional tendency for developing alternisepalous staminodes (e.g. Diapensiaceae) and carpels in alternisepalous position, is probably precursor to the condition in the bulk of asterids. Although it is not the sole pathway to haplostemony, secondary obdiplostemony may function as a main disruptor of the ‘developmental robustness’ of diplostemony in rosids, leading to an increased transition to haplostemony common in asterids. The majority of Celastrales is haplostemonous, although antepetalous staminodes in an outer position are found in Parnassia and possibly Brexia (Matthews and Endress, 2005b). Haplostemony is also directly linked to the widespread occurrence of common petal–stamen tubes and a strong synorganization, which is more difficult to obtain with diplostemonous flowers, because of the two stamen whorls. The result is a mega-diversification in the haplostemonous asterids, which occurs to a far lesser extent in rosids.
Interestingly, apetalous flowers with an obdiplostemonous stamen arrangement are often nested in clades with secondary obdiplostemony type I (Oxalidales, Caryophyllales, Saxifragales: Table 1), suggesting a simple evolutionary shift between these two bauplans. This may indicate that the typical weakening of the alternisepalous stamens in late obdiplostemonous flowers may be halted by loss of the petals and occupation of the available space by the alternisepalous stamen, effectively replacing the petal in time of initiation and space occupied (Fig. 2D–F). Common petal–stamen primordia are often associated with secondary obdiplostemony and may represent a trigger leading the androecium away from a robust diplostemonous configuration. Primary obdiplostemony type II may eventually evolve into obhaplostemonous apetalous flowers, with a single stamen whorl alternating with the sepals (e.g. Rumex in Polygonaceae: Fig. 4). Indeed, the Polygonacreae have a peculiar two-whorled androecium, which is linked to the loss of petals and the development of a hypanthium (Ronse De Craene, 2010). The outer (alternisepalous) stamen whorl is rarely affected by stamen loss, while the inner antesepalous whorl is reduced to two or three stamens alternating with the carpels (e.g. Rheum, Persicaria, Coccoloba). In Rumex the inner stamens vanish altogether. This process effectively breaks away from a trend towards haplostemony, which is widespread in Caryophyllales. Another example of a shift towards obhaplostemony with loss of petals is found in Myrtales. Penaeaceae and some Crypteroniaceae have effectively lost the petals linked to an alternisepalous stamen position (Schönenberger and Conti, 2003). In other related related obhaplostemonous families (e.g. Alzateaceae, Rhynchocalycaceae, Oliniaceae) petals may still be present, or flowers have two stamen whorls and petals (e.g. Axinandra in Crypteroniaceae).
Primary obdiplostemony type I is far less common among core eudicots, as it is mainly concentrated in Malvaceae (and possibly in Euphorbiaceae), where antesepalous stamens become typically reduced and lost, and alternisepalous stamens are duplicated or proliferate (e.g. von Balthazar et al., 2006). The androecium in Malvaceae represents a unique obdiplostemonous bauplan (see also Venkata Rao, 1949, 1952; van Heel, 1966), and von Balthazar et al. (2006) consider this to be plesiomorphic for Malvaceae. In all investigated cases alternisepalous stamens emerge externally to the antesepalous stamens. However, as pointed out above, this obdiplostemonous configuration may be the result of a shift from an original antesepalous triplet arrangement, linked to a reinforcement of alternisepalous stamens and a reduction of the antesepalous stamens, making a plesiomorphic condition doubtful. Secondary obdiplostemony type III and associated obhaplostemony found in Sapotaceae probably represents an apomorphic derivation from the diplostemonous bauplan arising through a comparable reversal in the normal shift of secondary obdiplostemony type I towards haplostemony.
CONCLUSIONS
As stated by Endress (2010), the circumscription of obdiplostemony is far from simple. The original definition reflects a rigid concept of flower morphology, emphasizing the absence of alternation between the petal whorl and stamen whorl. However, this definition is too limited and does not reflect the diversity observed in apetalous flowers or flowers with a sterile stamen whorl. Developmental evidence shows the existence of several independent changes from diplostemony through subtle shifts in the robustness of whorls, leading to different conditions that are often defined as obdiplostemony (Fig. 4).
Flowers with the alternisepalous stamens appearing to be inserted outside the antesepalous stamens are generally described as obdiplostemonous, even if their insertion area is more central. Such flowers are not much different from diplostemonous flowers, but the observation, if consistent, may be useful for diagnostic purposes. By contrast, alternisepalous stamens may arise de facto as an outer whorl before the antesepalous stamens, in the presence or absence of petals. The particular case of the apetalous flowers of Cephalotus (and possibly other non-investigated apetalous flowers) is considered by Endress (2010) and Matthews and Endress (2002) to be a case of diplostemony. However, the link between Cephalotus and other obdiplostemonous Oxalidales implies that petals have been lost and that the remaining alternisepalous stamens have hijacked the position and earlier initiation of the petals. In other Oxalidales, such as Cunoniaceae, petals are variously developed or absent (Matthews and Endress, 2002; Ronse De Craene, 2010). As such the flower of Cephalotus is obdiplostemonous and not contradictory to the definition of obdiplostemony.
The restriction of the definition of obdiplostemony to flowers with petals and antepetalous stamens is contrary to our approach, in ignoring more complicated cases where petals are lost, stamen whorls are sterile, incomplete or in double positions, and carpels are not isomerous. These derived cases should be described within the context of the definition of obdiplostemony. Our approach to obdiplostemony includes those cases with altered morphologies (loss of petals, sterile stamens), taking in consideration that certain conditions may change during evolution, leading to strongly diverging morphologies. A delay in petal development, and a more external shift of carpels, undoubtedly emphasizes the perception of obdiplostemony as space becomes available for a more extensive development of alternisepalous stamens towards the periphery of the flower.
Obdiplostemony is representative of at least three major shifts in the floral bauplan occurring mainly in the rosids, and leading to a profound change in flower morphology (Fig. 4). Secondary obdiplostemony type I reflects a widespread and major trend from diplostemonous flowers to haplostemonous flowers, accompanied by a reduction and loss of alternisepalous stamens (Fig. 4). The loss or reinforcement of petals may be crucial for the direction of evolution of flowers and happens at an early stage. Well-developed, rapidly growing petals will be responsible for the maintenance and widespread occurrence of haplostemony, as in several rosids and most asterids. Heterochrony in the development of petal primordia as observed in several rosids is linked to a progressive delay in petal initiation, which may lead to petal loss, and their replacement by outer alternisepalous stamens resembling a homeotic transformation, as in Cephalotaceae (Fig. 2D–F) and possibly several other families (primary obdiplostemony type II). By contrast, alternisepalous sectors may become secondarily reinforced, accompanied by the reduction of the antesepalous stamens (as in Sapotaceae or Primuloid families) and shift towards obhaplostemony (secondary obdiplostemony type III). Primary obdiplostemony type I is rare compared with secondary obdiplostemony type I, and is linked to a shift to obhaplostemony in Malvaceae. Primary obdiplostemony type I is an anomalous condition that may have an origin separate from diplostemony but it shares the presence of common petal–stamen primordia with secondary obdiplostemony type I. Primary obdiplostemony type I and type II, as well as secondary obdiplostemony type III effectively break away from the transition between diplostemony and haplostemony, shifting the balance between the two stamen whorls in favour of the alternisepalous stamen whorl (Fig. 4).
Both primary and secondary obdiplostemony reflect a similar phenomenon, namely the weakening of one of the two stamen whorls. The delay in petal development is an additional but not a necessary factor, as flowers with rapidly growing petals may also be obdiplostemonous. The phenomenon of obdiplostemony thus represents a secondarily induced condition in floral evolution, where a progressive developmental shift causes major changes in floral morphology. The establishment of secondary obdiplostemony is linked to the best occupation of space and can be seen as a key step, leading to a shift between two robust androecial configurations in core eudicot flowers. Eckert (1966: p. 598) concludes that obdiplostemony may describe a condition in mature flowers, without disclosing the way in which it has arisen [Der Begriff der Obdiplostemonie kann daher nur den Zustand in der fertigen Blüte beschreiben, ohne etwas über dessen Zustandekommen aussagen zu können]. Obdiplostemony tells us that changes in floral structure are not random but follow clearly set routes regulated by constraints of floral development. However, the set-up of these routes is a random process, and any changes in developmental regulation can lead to totally different pathways. The perception of obdiplostemony is the consequence of the establishment of these pathways, with major phylogenetic significance.
ACKNOWLEDGEMENTS
We thank Rainer Melzer and Günther Theissen for their kind invitation to contribute to the present volume. We acknowledge the Royal Botanic Garden Edinburgh, especially Frieda Christie, for technical assistance with SEM and Britta Kümpers for photographing Sideroxylon. We also acknowledge the information kindly given by Botanic Gardens Conservation International (BCGI) regarding the location of particular plant specimens in European botanical gardens. We thank two reviewers, Pieter Caris and Peter Endress, for providing constructive criticism on different versions of this paper. We are very grateful to the institutions that kindly provided plant material: Botanical Gardens of the University of Mainz (Biophytum), the University of Zürich (Cephalotus) and the University of Bonn (Cnestis). We are also very grateful for the collecting assistance given by Eileen Dworaczek, Peter Endress and Wolfram Lobin. This work was partly funded by Fondecyt post-doc project 3130417 to K.B.
LITERATURE CITED
- Al-Nowaihi AS, Khalifa SF. 1973. Studies on some taxa of the Geraniales II. Floral morphology of certain Linaceae, Rutaceae and Geraniaceae with a reference to the consistency of some characters. Journal of the Indian Botanical Society 52: 198–206. [Google Scholar]
- Bachelier JB, Endress PK. 2009. Comparative floral morphology and anatomy of Anacardiaceae and Burseraceae (Sapindales), with a special focus on gynoecium structure and evolution. Botanical Journal of the Linnean Society 159: 499–571. [Google Scholar]
- Bachelier JB, Endress PK, Ronse De Craene LP. 2011. Comparative floral structure and development of Nitrariaceae (Sapindales) and systematic implications In: Wanntorp L, Ronse De Craene LP, eds. Flowers on the tree of life. Cambridge: Cambridge University Press, 181–217. [Google Scholar]
- Baillon H. 1862. Observations sur les affinités du Macarisia et sur l’organisation de quelques Rhizophorées. Adansonia 3: 15–41. [Google Scholar]
- Baillon H. 1865. Observations sur les Saxifragées. Adansonia 6: 1–15. [Google Scholar]
- Bello MA, Hawkins JA, Rudall PJ. 2007. Floral morphology and development in Quillajaceae and Surianaceae (Fabales), the species-poor relatives of Leguminosae and Polygalaceae. Annals of Botany 100: 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull-Hereñu K, Ronse De Craene LP, Pérez F. 2016. Flower meristematic size correlates with heterostylous morphs in two Chilean Oxalis L. species. Flora, doi:10.1016/j.flora.2016.02.009. [Google Scholar]
- Breteler FJ. (ed). 1989. The Connaraceae. A taxonomic study with emphasis on Africa. Agricultural University Wageningen Papers 89–6. Wageningen: Veenman. [Google Scholar]
- Brockington SF, Dos Santos P, Glover B, Ronse De Craene LP. 2013. Evolution of the androecium in Caryophyllales: insights from a paraphyletic Molluginaceae. American Journal of Botany 100: 1757–1778. [DOI] [PubMed] [Google Scholar]
- Caris P. 2013. Bloemontogenetische patronen in the Ericales sensu lato. Doctoral thesis, Katholieke Universiteit Leuven, Belgium. [Google Scholar]
- Carlquist S. 1969. Toward acceptable evolutionary interpretations of floral anatomy. Phytomorphology 19: 332–362. [Google Scholar]
- Čelakovský LJ. 1875. Über den ‘eingeschalteten’ epipetalen Staubgefäβkreis. Flora 58: 481. [Google Scholar]
- Čelakovský LJ. 1894. Das Reduktionsgesetz der Blüthen, das Dédoublement und die Obdiplostemonie. Sitzungsberichte der königlichen Böhmischen Gesellschaft der Mathematisch-naturwissenschaftlichen Classe 1894 3: 1–142. [Google Scholar]
- Chatin A. 1855. Sur les types obdiplostémone et diplostémone direct, ou de l’existence et des caractères de deux types symétriques distincts chez les fleurs diplostémones. Bulletin de la Société Botanique de France 2: 615–625. [Google Scholar]
- Corner EJH. 1946. Centrifugal stamens. Journal of the Arnold Arboretum 27: 423–437. [Google Scholar]
- Dickison WC. 1975. Studies on the floral anatomy of the Cunoniaceae. American Journal of Botany 62: 433–447. [Google Scholar]
- Dickison WC. 1993. Floral anatomy of the Styracaceae, including observations on intra-ovarian trichomes. Botanical Journal of the Linnean Society 112: 223–255. [Google Scholar]
- Dickison WC, Hils MH, Lucansky T, Stern WL. 1994. Comparative anatomy and systematics of woody Saxifragaceae. Aphanopetalum Endl. Botanical Journal of the Linnean Society 114: 167–182. [Google Scholar]
- Eckardt T. 1963. Zum Blütenbau der Angiospermen in Zusammenhang mit ihrer Systematik 6: Entwicklungsgeschichtliche und blütenanatomische Untersuchungen zum Problem der Obdiplostemonie. Berichte der Deutschen Botanische Gesellschaft 76: 43–45. [Google Scholar]
- Eckert G. 1966. Entwicklungsgeschichtliche und blütenanatomische Untersuchungen zum Problem der Obdiplostemonie. Botanische Jahrbücher für Systematik 85: 523–604. [Google Scholar]
- Eichler AW. 1878. Blüthendiagramme 2. Leipzig: W. Engelmann.
- Endress PK. 2010. Synorganisation without organ fusion in the flowers of Geranium robertianum (Geraniaceae) and its not so trivial obdiplostemony. Annals of Botany 106: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. 1930. Saxifragaceae In: A Engler, K Prantl, eds. Die natürlichen Pflanzenfamilien 18a. Leipzig: W. Engelmann, 74–226. [Google Scholar]
- Farrar J, Ronse De Craene LP. 2013. To be or not to be a staminode: the floral development of Sauvagesia (Ochnaceae) reveals different origins of presumed staminodes In: T Berntsen, K Alsvik, eds. Flowers, morphology, evolutionary diversification and implications for the environment. New York: Nova Science Publishers, 89–103. [Google Scholar]
- Gelius L. 1967. Studien zur Entwicklungsgeschichte an Blüten der Saxifragales sensu lato mit besonderer Berücksichtigung des Androeceums. Botanische Jahrbücher für Systematik 87: 253–303. [Google Scholar]
- Hils MH, Dickison WC, Lucansky TW, Stern WL. 1988. Comparative anatomy and systematics of woody Saxifragaceae: Tetracarpaea. American Journal of Botany 75: 1687–1700. [Google Scholar]
- Hofmann U, Ludewig J. 1985. Morphologie und systematische Stellung von Limnanthes douglasii R.Br., einem repräsentativen Vertreter der Limnanthaceae. Botanische Jahrbücher für Systematik 105: 401–431. [Google Scholar]
- Hofmeister W. 1868. Allgemeine Morphologie. Leipzig: W. Engelmann.
- Hufford LD. 1989. The structure and potential loasaceous affinities of Schismocarpus. Nordic Journal of Botany 9: 217–227. [Google Scholar]
- Hufford LD. 2001. Ontogeny and morphology of the fertile flowers of Hydrangea and allied genera of tribe Hydrangeeae (Hydrangeaceae). Botanical Journal of the Linnean Society 137: 139–187. [Google Scholar]
- Jordan KF. 1883. Ueber Abortus, Verwachsung, Dédoublement und Obdiplostemonie in der Blüthe. Österreichische Botanische Zeitschrift 33: 250–255, 287–291. [Google Scholar]
- Juncosa AM. 1988. Floral development and character evolution in Rhizophoraceae In: P Leins, SC Tucker, PK Endress, eds. Aspects of floral development. Vaduz: Cramer, 83–101. [Google Scholar]
- Juncosa AM, Tomlinson PB. 1987. Floral development in mangrove Rhizophoraceae. American Journal of Botany 74: 1263–1279. [Google Scholar]
- Klopfer K. 1968. Beiträge zur floralen Morphogenese und Histogenese der Saxifragaceae 2. Die Blütenentwicklung von Tellima grandiflora. Flora 158: 1–21. [Google Scholar]
- Klopfer K. 1970. Beiträge zur floralen Morphogenese und Histogenese der Saxifragaceae 4. Die Blütenentwicklung einiger Saxifraga-Arten. Flora 159: 347–365. [Google Scholar]
- Klopfer K. 1973. Florale Morphogenese und Taxonomie der Saxifragaceae sensu lato. Feddes Repertorium 84: 475–516. [Google Scholar]
- Kubitzki K. 2014. Humiriaceae In: K Kubitzki, ed. The familes and genera of vascular plants XI. Berlin: Springer, 223–228. [Google Scholar]
- Kümpers BMC, Richardson JE, Anderberg AA, Wilkie P, Ronse De Craene LP. 2016. The significance of meristic changes in the flowers of Sapotaceae Juss. Botanical Journal of the Linnean Society 180: 161–192. [Google Scholar]
- Lal S, Narayana LL. 1994. Floral anatomy and systematic position of Flindersia R.Br. Feddes Repertorium 105: 31–36. [Google Scholar]
- Leins P. 1964. Entwicklungsgeschichtliche Studien an Ericales-Blüten. Botanische Jahrbücher für Systematik 83: 57–88. [Google Scholar]
- Lord EM, Eckard KJ. 1985. Shoot development in Citrus sinensis L. (Washington navel orange) I. Floral and inflorescence ontogeny. Botanical Gazette 146: 320–326. [Google Scholar]
- Mattfeld J. 1938. Das morphologische Wesen und die phylogenetische Bedeutung der Blumenblätter. Berichte der Deutschen Botanisches Gesellschafts 56: 86–116. [Google Scholar]
- Matthews ML, Endress PK. 2002. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). Botanical Journal of the Linnean Society 140: 321–381. [Google Scholar]
- Matthews ML, Endress PK. 2004. Comparative floral structure and systematics in Cucurbitales (Corynocarpaceae, Coriariaceae, Tetramelaceae, Datiscaceae, Begoniaceae, Cucurbitaceae, Anisophylleaceae). Botanical Journal of the Linnean Society 145: 129–185. [Google Scholar]
- Matthews ML, Endress PK. 2005a. Comparative floral structure and systematics in Crossosomatales (Crossosomataceae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae). Botanical Journal of the Linnean Society 147: 1–46. [Google Scholar]
- Matthews ML, Endress PK. 2005b. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae). Botanical Journal of the Linnean Society 149: 129–194. [Google Scholar]
- Matthews ML, Endress PK. 2011. Comparative floral structure and systematics in Rhizophoraceae, Erythroxylaceae and the potentially related Ctenolophonaceae, Linaceae, Irvingiaceae and Caryocaraceae. Botanical Journal of the Linnean Society 166: 331–416. [Google Scholar]
- Matthews ML, Endress PK, Schönenberger J, Friis EM. 2001. A comparison of floral structures of Anisophylleaceae and Cunoniaceae and the problem of their systematic position. Annals of Botany 88: 439–455. [Google Scholar]
- Mayr B. 1969. Ontogenetische Studien an Myrtales-Blüten. Botanische Jahrbücher für Systematik 89: 210–271. [Google Scholar]
- Mione TM, Bogle AL. 1990. Comparative ontogeny of the inflorescence and flower of Hamamelis virginiana and Loropetalum chinense (Hamamelidaceae). American Journal of Botany 77: 77–91. [Google Scholar]
- Michaelis P. 1924. Blütenmorphologische Untersuchungen an den Euphorbiaceen, unter besonderer Berücksichtigung der Phylogenie der Angiospermenblüte. Goebel Botanische Abhandlungen 3: 1–150. [Google Scholar]
- Moncur MW. 1988. Floral development of tropical and subtropical fruit and nut species. An atlas of scanning electron micrographs. Melbourne: Natural Resources Series 8, CSIRO. [Google Scholar]
- Moody M, Hufford L. 2000. Floral development and structure of Davidsonia (Cunoniaceae). Canadian Journal of Botany 78: 1034–1043 [Google Scholar]
- Narayana LL. 1964. A contribution to the floral anatomy and embryology of Linaceae. Journal of the Indian Botanical Society 43: 344–357. [Google Scholar]
- Narayana LL, Rao D. 1976. Contributions to the floral anatomy of Linaceae 7. Journal of Japanese Botany 51: 349–352. [Google Scholar]
- Narayana LL, Rao D. 1977a. Contributions to the floral anatomy of Linaceae 8. Journal of Japanese Botany 52: 56–59. [Google Scholar]
- Narayana LL, Rao D. 1977b. Contributions to the floral anatomy of Humiriaceae 6. Journal of Japanese Botany 52: 145–153. [Google Scholar]
- Narayana LL, Rao D. 1978. Contributions to the floral anatomy of Linaceae 11. Journal of Japanese Botany 53: 12–14. [Google Scholar]
- Payer JB. 1857. Traité d’organogénie comparée de la fleur. Paris: Victor Masson. [Google Scholar]
- Rohweder O. 1967. Centrospermen-Studien 3: Blütenentwicklung und Blütenbau bei Silenoideen (Caryophyllaceae). Botanische Jahrbücher für Systematik 86: 130–185. [Google Scholar]
- Rohweder O. 1970. Centrospermen-Studien 4: Morphology und Anatomie der Blüten, Früchte und Samen bei Alsinoideen und Paronychioideen s.lat. (Caryophyllaceae). Botanische Jahrbücher für Systematik 90: 201–271, [Google Scholar]
- Ronse De Craene LP. 2010. Floral diagrams. An aid to understanding flower morphology and evolution. Cambridge: Cambridge University Press. [Google Scholar]
- Ronse De Craene LP. 2013. Reevaluation of the perianth and androecium in Caryophyllales: implications for flower evolution. Plant Systematics and Evolution 299: 1599–1636. [Google Scholar]
- Ronse De Craene LP. 2016. Meristic changes in flowering plants: how flowers play with numbers. Flora, doi:10.1016/j.flora.2015.08.005. [Google Scholar]
- Ronse De Craene LP, Smets E. 1995. The distribution and systematic relevance of the androecial character oligomery. Botanical Journal of the Linnean Society 118: 193–247. [Google Scholar]
- Ronse De Craene LP, Smets E. 1996. The morphological variation and systematic value of stamen pairs in the Magnoliatae. Feddes Repertorium 107: 1–17. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1998a. Notes on the evolution of androecial organization in the Magnoliophytina (Angiosperms). Botanica Acta 111: 77–86. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1998b. Meristic changes in gynoecium morphology, exemplified by floral ontogeny and anatomy In: SJ Owens, PJ Rudall, eds. Reproductive biology in systematics, conservation and economic botany. Kew: Royal Botanic Gardens, 85–112. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1999. Similarities in floral ontogeny and anatomy between the genera Francoa (Francoaceae) and Greyia (Greyiaceae). International Journal of Plant Science 160: 377–393. [Google Scholar]
- Ronse De Craene LP, Clinckemaillie D, Smets E. 1993. Stamen–petal complexes in Magnoliatae. Bulletin du Jardin Botanique National de Belgique 62: 97–112. [Google Scholar]
- Ronse De Craene LP, De Laet J, Smets EF. 1996. Morphological studies in Zygophyllaceae. II. The floral development and vascular anatomy of Peganum harmala. American Journal of Botany 83: 201–215. [Google Scholar]
- Ronse De Craene LP, De Laet J, Smets EF. 1998a. Floral development and anatomy of Moringa oleifera (Moringaceae): what is the evidence for a capparalean or sapindalean affinity? Annals of Botany 82: 273–284. [Google Scholar]
- Ronse De Craene LP, Smets EF, Vanvinckenroye P. 1998b. Pseudodiplostemony, and its implications for the evolution of the androecium in the Caryophyllaceae. Journal of Plant Research 111: 25–43. [Google Scholar]
- Ronse De Craene LP, Roels P, Smets EF, Backlund A. 1998c. The floral development and floral anatomy of Chrysosplenium alternifolium, an unusual member of the Saxifragaceae. Journal of Plant Research 111: 573–580. [Google Scholar]
- Schatz GE, Lowry PP, II, Wolf A-E. 1999. Endemic families of Madagascar. IV. A synoptic revision of Asteropeia (Asteropeiaceae). Adansonia Ser. 3, 21: 255–268. [Google Scholar]
- Schewe LC, Sawhney VK, Davis AR. 2011. Ontogeny of floral organs in flax (Linum usitatissimum; Linaceae). American Journal of Botany 98: 1077–1085. [DOI] [PubMed] [Google Scholar]
- Schmid R. 1980. Comparative anatomy and morphology of Psiloxylon and Heteropyxis, and the subfamilial and tribal classification of Myrtaceae. Taxon 29: 559–595. [Google Scholar]
- Schönenberger J, Conti E. 2003. Molecular phylogeny and floral evolution of Penaeaceae, Oliniaceae, Rhynchocalycaceae, and Alzateaceae (Myrtales). American Journal of Botany 90: 293–309. [DOI] [PubMed] [Google Scholar]
- Setoguchi H, Ohba H, Tobe H. 1996. Floral morphology and phylogenetic analysis in Crossostylis (Rhizophoraceae). Journal of Plant Research 109: 7–19. [Google Scholar]
- Stroebl F. 1925. Die Obdiplostemonie in den Blüten. Botanisches Archiv 9: 210–224. [Google Scholar]
- Tobe H, Graham SA, Raven PH. 1998. Floral morphology and evolution in Lythraceae sensu lato In: SJ Owens, PJ Rudall, eds. Reproductive biology in systematics, conservation and economic botany. Kew: Royal Botanic Gardens, 329–344. [Google Scholar]
- Tschunko AH, Nickerson NH. 1976. The androecium of Suriana maritima. Rhodora 78: 162–164. [Google Scholar]
- Tucker SC, Bernhardt P. 2000. Floral ontogeny, pattern formation, and evolution in Hibbertia and Adrastea (Dilleniaceae). American Journal of Botany 87: 1915–1936. [PubMed] [Google Scholar]
- van Heel WA. 1966. Morphology of the androecium in Malvales. Blumea 13: 177–394. [Google Scholar]
- Venkata Rao C. 1949. Floral anatomy of some Sterculiaceae with special reference to the position of the stamens. Journal of the Indian Botanical Society 28: 237–245. [Google Scholar]
- Venkata Rao C. 1952. Floral anatomy of some Malvales and its bearing on the affinities of families included in the order. Journal of the Indian Botanical Society 31: 171–203. [Google Scholar]
- Venkata Rao C, Ramalakshmi T. 1968. Floral anatomy of Euphorbiaceae I. Some non-cyathium taxa. Journal of the Indian Botanical Society 47: 278–298. [Google Scholar]
- von Balthazar M, Schönenberger J, Alverson WS, Janka H, Bayer C, Baum DA. 2006. Structure and evolution of the androecium in the Malvatheca clade (Malvaceae s.l.) and implications for Malvaceae and Malvales. Plant Systematics and Evolution 260: 171–197. [Google Scholar]
- Wannan BC, Quinn CJ. 1991. Floral structure and evolution in the Anacardiaceae. Botanical Journal of the Linnean Society 107: 349–385. [Google Scholar]
- Wei L, Wang Y-Z, Li Z-Y. 2011. Floral ontogeny of Ruteae (Rutaceae) and its systematic implications. Plant Biology 14: 190–197. [DOI] [PubMed] [Google Scholar]
- Wei L, Xiang X-G, Wang Y-Z, Li Z-Y. 2015. Phylogenetic relationships and evolution of the androecia in Ruteae (Rutaceae). PLOS ONE 10: e0137190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigend M. 2005. Notes on the floral morphology in Vivianiaceae (Geraniales). Plant Systematics and Evolution 253: 125–131. [Google Scholar]