Abstract
To evaluate the relationship between tooth loss and metabolic syndrome (MS) in South Korean adults.
Subjects and Methods: A total of 3589 adults (1511 men and 2078 women aged over 40 years) from the 2012 Korean National Health and Nutrition Examination Survey were included and divided into 3 groups according to the number of remaining teeth (0–19, 20–27, and 28). We recorded the number of remaining teeth and measured MS components such as waist circumference, systolic and diastolic blood pressure, fasting blood glucose, serum high-density lipoprotein-cholesterol, and triglyceride concentration. We also calculated the number of subjects who met the inclusion criteria of MS in each group. Multiple logistic regression analysis was performed to estimate the prevalence of MS components according to the number of remaining teeth after adjusting for covariates.
Women without MS had significantly more teeth than those with MS (24.5 ± 0.2 vs 21.0 ± 0.3). In men, the prevalence of high blood pressure and high fasting blood glucose levels were significantly different among the 3 groups (P = 0.003 and P < 0.001, respectively); however, the prevalence of MS and all MS components were significantly different in women (P < 0.001 for all comparisons). Men with 0 to 19 remaining teeth were most likely to have high blood pressure and high fasting blood glucose, while women with 0 to 19 remaining teeth had the highest prevalence of MS and each MS component. Multiple logistic regression analysis revealed that women with fewer remaining teeth had a higher prevalence of MS and MS components after adjusting for covariates.
Having only a few remaining teeth was associated with MS in women in South Korea.
INTRODUCTION
Tooth loss is defined as the exfoliation of at least 1 tooth from the alveolar bone of the jaw,1 and was considered to be a part of the aging process until the middle of the 20th century. However, as dental care procedures progressed and developed, teeth could be better preserved with the aid of restorative and prosthodontic treatment.2–4 Nevertheless, tooth loss remains a problem, especially in the elderly. According to the National Health and Nutrition Examination Survey, 91% of adults aged 20 to 64 years had dental caries, and only 34% of adults aged 40 to 64 years had all of their teeth.5 Tooth loss gives rise to nutritional deficiencies and general weakness that is involved in several medical problems such as subclinical atherosclerosis,6 physical disability, and mental impairment.7–9 Tooth loss is caused by many conditions such as periodontal problems, dental caries, and trauma,10,11 and some recent studies found that metabolic syndrome (MS) was also associated with tooth loss.12–14
MS is defined as a cluster of metabolic abnormalities including abdominal obesity, high blood pressure (BP), high fasting blood glucose (FBG), elevated serum triglycerides (TG), and low high-density lipoprotein cholesterol (HDL-C) levels.15–18 The main mechanism of MS is insulin resistance, which results in type 2 diabetes mellitus and subsequent cardiovascular diseases.19–21 The prevalence of MS has been increasing and is now an important social problem in both Western and Asian countries. The prevalence of MS is about 20% to 25% in the adults of western countries22–25 and 5% to 20% in Asian adults.26–28 The risk factors for MS include aging, stress, a sugar-rich diet, limited physical activity, alcohol abuse, irregular sleep patterns,29–33 and a postmenopausal state.34–36 MS is also associated with dental problems such as poor oral health behavior37,38 and periodontal diseases.39–41
For reducing these metabolic risk factors, routine monitoring of several cholesterol levels, FBG, BP, body weight, and waist circumference (WC) is needed. The general treatments of MS include remedies to alleviate metabolic risk factors by low caloric diet, enhancing physical activity, use of medication such as antihypertensive, antidyslipidemic, and antihyperglycemic agents.17 In some obese subjects with MS, weight-loss drugs or bariatric surgery is also recommended.42 Another study reported that some people with MS were more likely to use complementary and alternative medicine such as herbal supplements, aromatherapy, or massage therapy compared to people without MS.43
Although associations between tooth loss and MS have been previously reported,12–14 none of these studies involved a Korean population. Therefore, we examined the relationship between tooth loss and MS in a representative South Korean population. We hypothesized that tooth loss is associated with MS.
SUBJECTS AND METHODS
Survey Overview
Data from the 2012 Korean National Health and Nutrition Examination Survey were used in this study. This survey consisted of 3 main categories, which were a nutrition survey, health examination, and health interview. A total of 200 regions were selected and 10,000 individuals aged over 1 year old were selected. The health examination and interview were performed in specially equipped buses, and the nutrition survey was conducted at home 1 week after the health interview. Trained professionals including nurses, dietitians, and health scientists conducted face-to-face interviews with structured questionnaires.
SUBJECTS
A total of 8058 subjects were included in the 2012 Korean National Health and Nutrition Examination Survey. We only selected men and women aged ≥40 years old, and thus 3556 subjects were excluded. Among the 4502 participants, 381 subjects who did not fast for at least 8 hours before the blood test, 373 subjects who did not give sufficient dental data, and 159 participants who did not give sufficient information to extract data for MS were excluded. Finally, a total of 3589 adults (1511 men and 2078 women) were included in this study. The study was approved by the institutional review board of the Korean Center for Disease Control and Prevention (2012-01EXP-01-2C), and was conducted according to the Ethical Principles for Medical Research Involving Human Subjects as defined by the Helsinki Declaration.44 All the subjects provided written informed consent.
Anthropometric and Blood Pressure Measurements
The WC was measured at the end of a normal expiration to within 0.1 cm on a horizontal plane at the midpoint between the iliac crest and the costal margin while the subject was wearing loose fitting clothing. The subject's height was measured to within 0.1 cm and their weight without shoes or heavy clothing was recorded using a digital scale to within 0.1 kg. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). Each subject was seated and rested for at least 5 minutes before their BP was taken. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a mercury sphygmomanometer (Baumanometer; W. A. Baum Co., Inc., Copiague, NY). The final BP value was determined by averaging the 2nd and 3rd BP values.
Biochemical Measurements
Blood samples were taken from subjects after they had fasted for at least 8 hours. Total cholesterol, low-density lipoprotein-cholesterol, HDL-C, TG, and FBG were measured enzymatically using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) at the Central Testing Institute in Seoul, Korea.
Socio-Demographic and General Health Behaviors
Socio-demographic and general health characteristics were evaluated using self-administered questionnaires regarding age, sex, smoking, alcohol consumption, physical activity, level of education, and household income. Nonsmokers were defined as those who never smoked or had smoked less than 100 cigarettes in their whole life. Ex-smokers were defined as those who had smoked more than 100 cigarettes in their whole life, but had stopped smoking at the time of the study. Current smokers were those who were smokers at the time of the study. Mild to moderate drinkers were defined as those who drank less than 3 glasses per day (15–30 g/day) and heavy drinkers were defined as those who drank more than 3 glasses per day (≥30 g/day). Physical activity was assessed using the International Physical Activity Questionnaire.45 Regular exercise was defined as exercise more than 5 times a week and for 30 min/session, or strenuous exercise more than 3 times a week and for 20 min/session. Education level was classified as either high school graduate (≥13 years) or not, and household income was divided into quartiles after adjusting for the number of family members.
Oral Health Behavior
We recorded the time of day when tooth-brushing occurred and whether secondary oral hygiene products including dental gargle, dental floss, interdental brush, and electric toothbrushes were used. The time of day was categorized as before or after breakfast, lunch, dinner, after snacks, and before bedtime. The frequency of daily tooth-brushing was calculated as the total number of times the subject cleaned their teeth in 1 day.
Measurement of Remaining Teeth
We categorized the number of remaining teeth into 3 groups; 0 to 19, 20 to 27, and 28 teeth. People with complete dentition have 28 teeth, excluding the 4 third molars. The subjects who had lost at least 1 of their natural teeth were classified to the other 2 groups.
Definition of MS
The American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement defined the criteria for MS in Asians.17,18 Subjects with 3 or more of the following 5 medical conditions were defined as having MS: abdominal obesity or a WC more than 90 cm in men and more than 80 cm in women, BP more than 130/85 mm Hg or the use of an antihypertensive drug, FBG more than 100 mg/dL or the use of an antidiabetic drug, an HDL-C level less than 40 mg/dL in men and 50 mg/dL in women or the use of an antidyslipidemic drug, and a TG level more than 150 mg/dL or use of an antidyslipidemic drug.
Statistical Analysis
Data are given as the mean ± standard error for continuous variables or as a percentage (standard error) for categorical variables. The general characteristics of subjects were analyzed using an unpaired t test for continuous variables and the Chi-square test for categorical variables. The latter was used to analyze the prevalence of MS and each component of MS among the 3 remaining teeth groups in men and women, and to analyze the proportion of each remaining teeth group according to the number of MS components in men and women. A multivariable logistic regression analysis was used to determine the odds ratio (OR) and 95% confidence interval (CI) of the 5 MS components among the 3 remaining teeth groups after adjusting for the following covariates: age, sex, and BMI in model 1; covariates of model 1 plus smoking, alcohol, regular exercise, education level, and household income in model 2; covariates of model 2 plus the use of secondary oral products and frequency of tooth brushing in model 3. The SAS software package version 9.2 (SAS institute, Cary, NC) was used for statistical analysis. All the statistical tests were 2-tailed and differences were considered to be statistically significant when the P value was less than 0.05.
RESULTS
The general characteristics of subjects with or without MS are summarized in Table 1. Both men and women with MS were older than those without MS (P < 0.05). However, BMI, WC, SBP, DBP, FBG, and TG were all higher and HDL-C was lower in subjects with MS compared to those without MS in both men and women (all P < 0.001). A significantly greater proportion of women with MS were in the lowest income quartile (Q1), but a significantly smaller proportion of these women had an education level ≥13 years (P < 0.001 for both). Periodontitis was more common in women with MS compared to those without MS (19.9% ± 1.6% vs 32.9% ± 2.3%), and women without MS also had a significantly greater number of remaining teeth compared to those with MS (24.5 ± 0.2 vs 21.0 ± 0.3, respectively) and the frequency of tooth brushing and the use of secondary oral products were also significantly different only among the female subjects (P < 0.001 for all).
TABLE 1.
General Characteristics of Subjects With or Without Metabolic Syndrome in the KNHANES
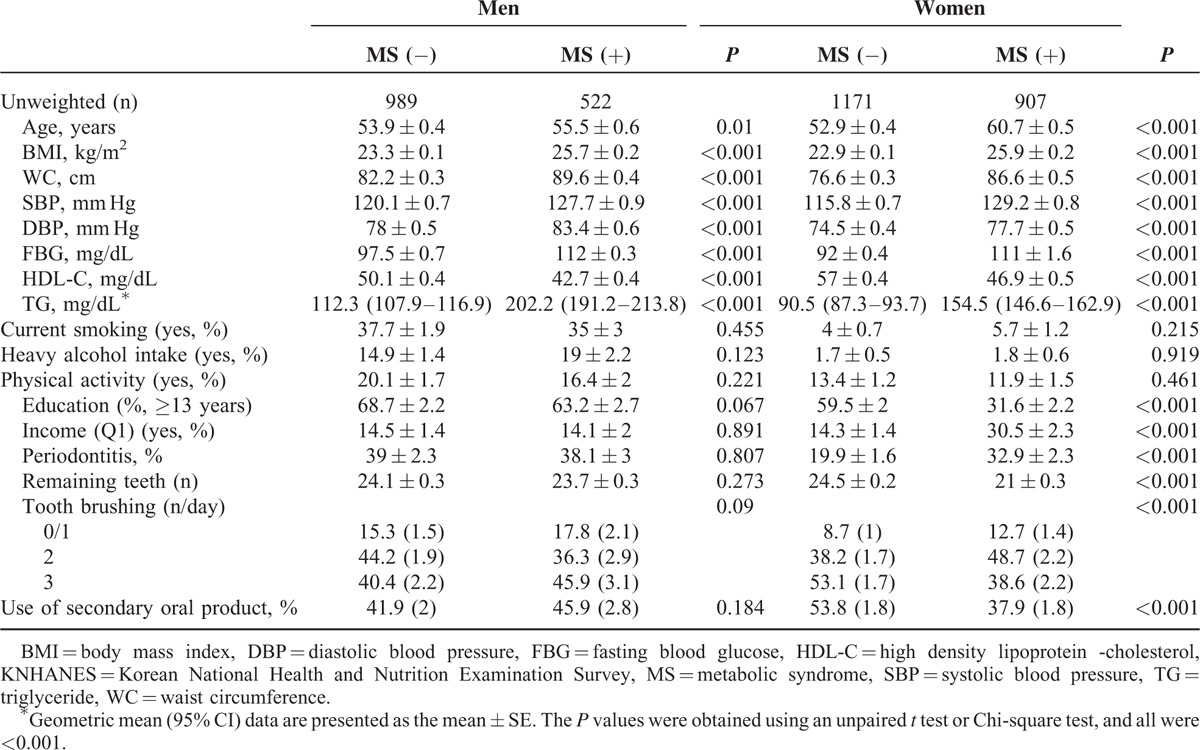
The prevalence of MS and each MS component among the 3 remaining teeth groups is given in Table 2. Men showed significant differences in the prevalence of high BP and high FBG levels (P = 0.003 and P < 0.001, respectively), although the prevalence of MS and all MS components were significantly different in women (P < 0.001 for all). Men with 0 to 19 remaining teeth had the highest prevalence of high BP and high FBG levels, and women with 0 to 19 remaining teeth had the highest prevalence of MS and each MS component.
TABLE 2.
Prevalence of MS and MS Components in the 3 Remaining Teeth Groups in Men and Women
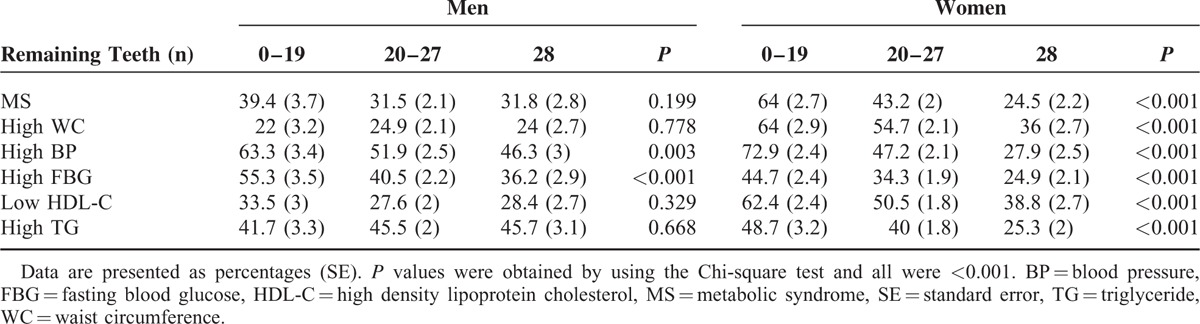
The relationship between the number of remaining teeth and the number of MS components is summarized in Figure 1. The proportion of subjects in each remaining teeth group was significantly different in men and women (P = 0.017 and P < 0.001, respectively), although the proportion of women in the 28 and the 0 to 19 remaining teeth groups increased and decreased, respectively, with an increasing number of MS components (P for trend < 0.001).
FIGURE 1.
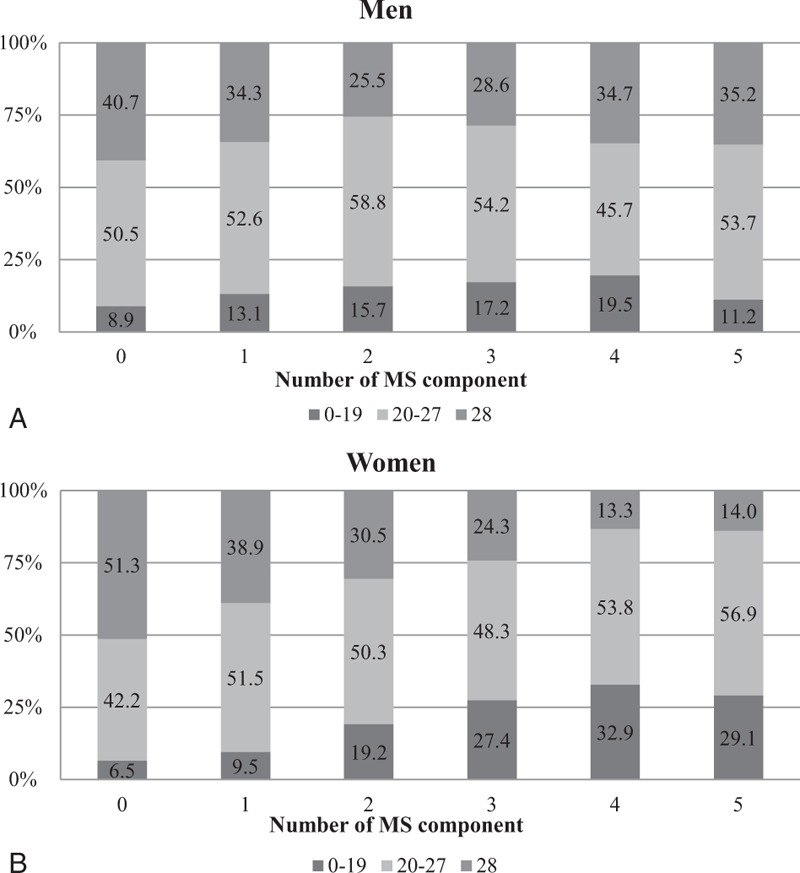
(A) Proportion of each remaining teeth group according to the number of metabolic syndrome (MS) components in men. (P = 0.017, P for trend <0.01). (B) Proportion of each remaining teeth group according to the number of MS components in women (P < 0.001, P for trend <0.001).
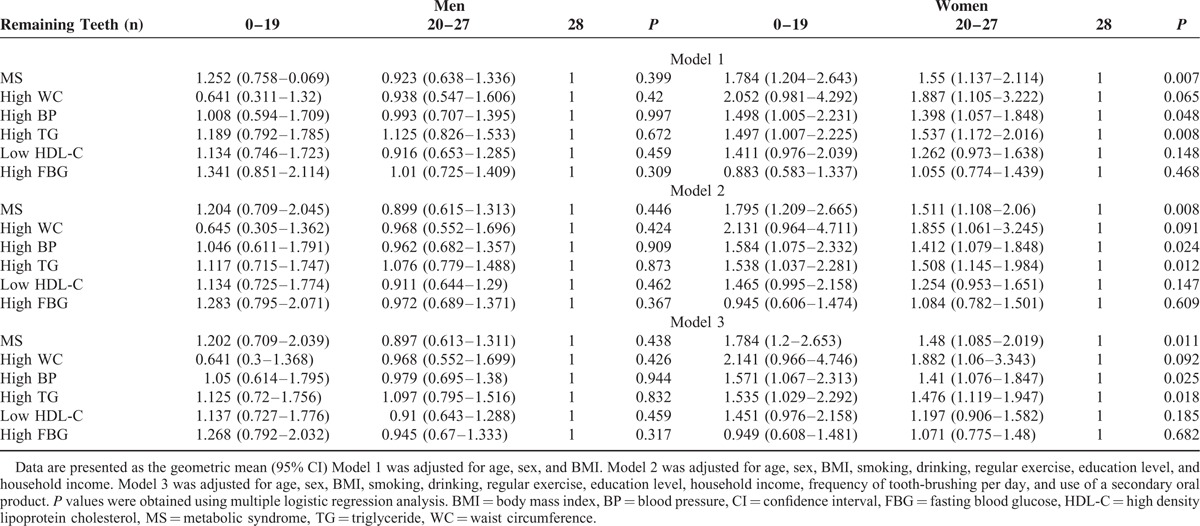
Compared to the 28 remaining teeth group, the OR for the prevalence of MS and each MS component in the 0 to 19 and 20 to 27 remaining teeth groups were not significantly different in men after adjusting for the covariates (Table 3). In women, the OR (95% CI) for the prevalence of MS was 1.784 (1.200–2.653) in the 0 to19 remaining teeth group and 1.480 (1.085–2.019) in the 20 to 27 remaining teeth group after adjusting for covariates. The prevalence of a high WC was high only in the 20 to27 remaining teeth group (OR [95% CI], 1.882 [1.060–3.343]). The prevalence of high BP was higher in both the 0 to 19 and 20 to 27 remaining teeth groups (OR [95% CI], 1.571 [1.067–2.313] and 1.410 [1.076–1.847], respectively) and the prevalence of high TG levels was also higher in both the 0 to 19 and 20 to 27 remaining teeth groups (OR [95% CI], 1.535 [1.029–2.292] and 1.476 [1.119–1.947], respectively) in women after adjusting for covariates.
TABLE 3.
Prevalence of MS and Each MS Component Among the 3 Remaining Teeth Groups in Men and Women
DISCUSSION
In this study, women with MS had significantly fewer remaining teeth than women without MS. The proportion of women with 28 and 0 to 19 remaining teeth decreased and increased, respectively, with an increasing number of MS components. The prevalence of MS and MS components was not associated with the number of remaining teeth in men. However, the prevalence of MS, high BP, and high TG levels was higher in women with 0 to 19 and 20 to 27 remaining teeth compared to those with 28 remaining teeth.
Several studies have shown an association between tooth loss and MS. A self-reported study of 947 Swedish subjects aged over 70 years old found that the number of remaining teeth was inversely related to the prevalence of MS.12 Another study that involved a total of 1354 middle-aged Finnish men showed an increased prevalence of MS in subjects with more than 4 missing teeth, although the third molars were also included and there was no adjustment for covariates.13 Similarly, a US population-based study of adults aged 20 years or older also showed an association between tooth loss and MS.14
A number of studies also found a relationship between tooth loss and MS components. An English study of 1958 adults aged 25 to 74 years old found that tooth loss was positively associated with serum TG levels in women,46 and another study showed similar results in that subjects with more than 6 missing teeth had significantly higher serum TG levels than subjects with less than 6 missing teeth.47 Two studies showed that SBP was associated with tooth loss48,49 and another prospective clinical trial showed a negative association between DBP and tooth loss in postmenopausal women.50 It was also reported that there was a negative association between the number of remaining teeth and SBP in men,49 although we could not find any association between tooth loss and MS in men.
In this study, abdominal obesity was more frequent in the 20 to 27 remaining teeth group in women. One cross-sectional study of 1720 adults aged 20 to 59 years old in Brazil demonstrated that the prevalence of abdominal obesity increased when adults had less than 10 remaining teeth in at least 1 arch compared to those with 10 or more teeth in both arches including the maxilla and mandible, although this difference was not significant after adjusting for age.51 Another Brazilian study of 471 elderly subjects aged between 60 and 89 years old also reported that subjects with fewer (1–8) remaining teeth had a significantly higher prevalence of abdominal obesity.52 Similarly, Swedish subjects younger than 60 years old showed an increased prevalence of abdominal obesity when they had less than 20 remaining teeth adjusting for age, sex, socioeconomic status, life-style, and comorbidities.53
A possible mechanism underlying the relationship between tooth loss and MS may involve elevated systemic inflammation. Several studies suggested that high sensitivity C-reactive protein and proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, and IL-6, were elevated in patients with MS.54–56 These inflammatory proteins may cause local periodontal destruction and subsequent tooth loss.57,58 TG is an another cause of inflammation,59 and the increased lipolysis of TG can give rise to endothelial inflammation through TNF-α release and the stimulation of reactive oxygen species production within cells.60 A prospective cohort study of 20,525 US middle-aged female adults found that increased C-reactive protein was associated with forthcoming hypertension,61 and hypertension itself also causes an increase in inflammatory markers.62–64
Obese people generally prefer a diet that is abnormally high in sugar, which might be involved in tooth loss.52 Conversely, subjects with few remaining teeth are likely to have a high calorie diet because of their compromised masticatory function.65 Taken together, this indicates that cumulative high-sugar diets may also increase the risk of both obesity and tooth loss,66 and abdominal obesity may accelerate tooth loss, or conversely, tooth loss may accelerate the development of abdominal obesity.
Poor oral health may affect all of these pathways. Regular tooth brushing is an effective means of removing plaque and maintaining healthy gingiva.67 A previous study of an adult Japanese population indicated that a low frequency of tooth brushing was associated with a high prevalence of MS,38 and a study of Korean adults also demonstrated that a low frequency of tooth brushing or no dental flossing may increase the likelihood of MS.37 Furthermore, local periodontitis as well as systemic inflammation may be worse in individuals with a poor oral health status.68 Consequently, poor oral health behavior may aggravate MS, giving rise to systemic and local inflammation, and ultimately tooth loss.
There was a sex-based difference in the relationship between tooth loss and MS in this study. This may be explained in part by the inclusion of women who were postmenopausal, defined as the cessation of menstruation for at least 1 year.69 Among the Korean population, women on average undergo the menopause when they are 46.9 ± 4.9 years old (median, 48 years).70 Menopause induces the ageing process and is associated with reduced estrogen synthesis. This hormonal change can evoke various unpleasant physical and psychological changes.34,71 A number of studies have shown that postmenopausal women have higher total cholesterol and TG levels, and lower HDL-C levels compared to premenopausal women.72,73 It has also been shown that systemic inflammation was accelerated in postmenopausal women with MS,36,74 which might explain the increased tooth loss in women compared to men in this study.
This study had a number of limitations that need to be considered. First, this was a cross-sectional study, and hence it is not possible to demonstrate a causal relationship between tooth loss and MS. Second, although this study enrolled a large representative sample, it was conducted in only a single Asian country. A multinational study involving subjects of other ethnicities is therefore needed for more generalizable results. Third, we did not measure the hormonal status of subjects, nor did we record their postmenopausal history, especially in women. Fourth, there were no descriptions of other causal factors for tooth loss such as dental caries and trauma. This study also has a number of notable strengths though. We used a representative sample of the whole South Korean population, and to the best of our knowledge, this is the first study to examine the relationship between tooth loss and MS in such a population. We also adjusted for many covariates such as age, sex, BMI, smoking, drinking, regular exercise, education level, household income, frequency of tooth-brushing, and the use of secondary oral products.
In conclusion, we found that tooth loss was associated with an increased prevalence of MS in South Korean women. Physicians should be aware that women with fewer remaining teeth may have an increased risk of MS. Further prospective, well-designed studies are needed to reveal the causal relationship between tooth loss and MS.
Acknowledgements
The authors thank the Korean Center for Disease Control and Prevention, which performed the KNHANES. The authors also thank the subjects who participated in the KNHANES.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, CRP = C-reactive protein, DBP = diastolic blood pressure, FBG = fasting blood glucose, HDL-C = high-density lipoprotein cholesterol, IL = interleukin, LDL-C = low-density lipoprotein-cholesterol, MS = metabolic syndrome, OR = odds ratio, SBP = systolic blood pressure, TG = triglyceride, TNF-α = tumor necrosis factor alpha, WC = waist circumference.
Ethical approval: The current study was approved by the institutional review board of the Korean Center for Disease Control and Prevention (2012-01EXP-01-2C).
ISS and YHK contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Al-Shammari KF, Al-Khabbaz AK, Al-Ansari JM, et al. Risk indicators for tooth loss due to periodontal disease. J Periodontol 2005; 76:1910–1918. [DOI] [PubMed] [Google Scholar]
- 2.Muller F, Naharro M, Carlsson GE. What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe? Clin Oral Implants Res 2007; 18 Suppl 3:2–14. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg T, Carlsson GE, Sundh V. Trends and prognoses of dental status in the Swedish population: analysis based on interviews in 1975 to 1997 by Statistics Sweden. Acta Odontol Scand 2000; 58:177–182. [DOI] [PubMed] [Google Scholar]
- 4.Ainamo A, Osterberg T. Changing demographic and oral disease patterns and treatment needs in the Scandinavian populations of old people. Int Dent J 1992; 42:311–322. [PubMed] [Google Scholar]
- 5.Dye B, Thornton-Evans G, Li X, et al. Dental caries and tooth loss in adults in the United States 2011–2012. NCHS Data Brief 2015; 197:1–8. [PubMed] [Google Scholar]
- 6.Desvarieux M, Demmer RT, Rundek T, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque The Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke 2003; 34:2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tramini P, Montal S, Valcarcel J. Tooth loss and associated factors in long-term institutionalised elderly patients. Gerodontology 2007; 24:196–203. [DOI] [PubMed] [Google Scholar]
- 8.Shimazaki Y, Soh I, Saito T, et al. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res 2001; 80:340–345. [DOI] [PubMed] [Google Scholar]
- 9.Kocher T, Dentf M, Biffar R, et al. The impact of tooth loss on general health related to quality of life among elderly Pomeranians: results from the study of health in Pomerania (SHIP-0). Int J Prosthodont 2005; 18:414–419. [PubMed] [Google Scholar]
- 10.Aida J, Ando Y, Akhter R, et al. Reasons for permanent tooth extractions in Japan. J Epidemiol 2006; 16:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chestnutt IG, Binnie VI, Taylor MM. Reasons for tooth extraction in Scotland. J Dent 2000; 28:295–297. [DOI] [PubMed] [Google Scholar]
- 12.Holmlund A, Hulthe J, Lind L. Tooth loss is related to the presence of metabolic syndrome and inflammation in elderly subjects: a prospective study of the vasculature in Uppsala seniors (PIVUS). Oral Health Prev Dent 2007; 5:125. [PubMed] [Google Scholar]
- 13.Hyvärinen K, Salminen A, Salomaa V, et al. Systemic exposure to a common periodontal pathogen and missing teeth are associated with metabolic syndrome. Acta Diabetol 2014; 52:179–182. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Hollis JH. Associations between the number of natural teeth and metabolic syndrome in adults. J Clin Periodontol 2015; 42:113–120. [DOI] [PubMed] [Google Scholar]
- 15.Alberti K, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Med 2006; 23:469–480. [DOI] [PubMed] [Google Scholar]
- 16.Cleeman JI, Grundy SM, Becker D, et al. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). J Am Med Assoc 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 18.Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 19.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes care 2001; 24:683–689. [DOI] [PubMed] [Google Scholar]
- 20.Lakka H-M, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. J Am Med Assoc 2002; 288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003; 108:414–419. [DOI] [PubMed] [Google Scholar]
- 22.Park Y-W, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2003; 163:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterhouse DF, McLaughlin AM, Sheehan F, et al. An examination of the prevalence of IDF- and ATPIII-defined metabolic syndrome in an Irish screening population. Ir J Med Sci 2009; 178:161–166. [DOI] [PubMed] [Google Scholar]
- 24.Cameron AJ, Magliano DJ, Zimmet PZ, et al. The metabolic syndrome in Australia: prevalence using four definitions. Diabetes Res Clin Pract 2007; 77:471–478. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 2015; 16:1–12. [DOI] [PubMed] [Google Scholar]
- 26.Gu DF, Reynolds K, Wu XG, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005; 365:1398–1405. [DOI] [PubMed] [Google Scholar]
- 27.Deepa M, Farooq S, Datta M, et al. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-34). Diabetes Metab Res Rev 2007; 23:127–134. [DOI] [PubMed] [Google Scholar]
- 28.Lee WY, Park JS, Noh SY, et al. Prevalence of the metabolic syndrome among 40,698 Korean metropolitan subjects. Diabetes Res Clin Pract 2004; 65:143–149. [DOI] [PubMed] [Google Scholar]
- 29.Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE family study. Med Sci Sports Exerc 2003; 35:1703–1709. [DOI] [PubMed] [Google Scholar]
- 30.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes. Diabetes Care 2010; 33:2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xi B, He D, Zhang M, et al. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev 2014; 18:293–297. [DOI] [PubMed] [Google Scholar]
- 32.Edwardson CL, Gorely T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLos One 2012; 7:e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He D, Xi B, Xue J, et al. Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine 2014; 46:231–240. [DOI] [PubMed] [Google Scholar]
- 34.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003; 88:2404–2411. [DOI] [PubMed] [Google Scholar]
- 35.Cho GJ, Lee JH, Park HT, et al. Postmenopausal status according to years since menopause as an independent risk factor for the metabolic syndrome. Menopause 2008; 15:524–529. [DOI] [PubMed] [Google Scholar]
- 36.Kim HM, Park J, Ryu SY, et al. The effect of menopause on the metabolic syndrome among Korean women the Korean National Health and Nutrition Examination Survey, 2001. Diabetes Care 2007; 30:701–706. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Kim DH, Lim KS, et al. Oral health behaviors and metabolic syndrome: the 2008–2010 Korean National Health and Nutrition Examination Survey. Clin Oral Investig 2014; 18:1517–1524. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi Y, Niu K, Guan L, et al. Oral health behavior and metabolic syndrome and its components in adults. J Dent Res 2012; 91:479–484. [DOI] [PubMed] [Google Scholar]
- 39.Andriankaja O, Sreenivasa S, Dunford R, et al. Association between metabolic syndrome and periodontal disease. Aust Dent J 2010; 55:252–259. [DOI] [PubMed] [Google Scholar]
- 40.Li P, He L, Sha Y-q, et al. Relationship of metabolic syndrome to chronic periodontitis. J Periodontol 2009; 80:541–549. [DOI] [PubMed] [Google Scholar]
- 41.Shimazaki Y, Saito T, Yonemoto K, et al. Relationship of metabolic syndrome to periodontal disease in Japanese women: the Hisayama Study. J Dent Res 2007; 86:271–275. [DOI] [PubMed] [Google Scholar]
- 42.Bays HE, Jones PH, Orringer CE, et al. National Lipid Association Annual Summary of Clinical Lipidology 2016. J Clin Lipidol 2016; 10 (1 Suppl):S1–S43. [DOI] [PubMed] [Google Scholar]
- 43.Akilen R, Pimlott Z, Tsiami A, et al. The use of complementary and alternative medicine by individuals with features of metabolic syndrome. J Integr Med 2014; 12:171–174. [DOI] [PubMed] [Google Scholar]
- 44.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human, subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 45.Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 2006; 9:755–762. [DOI] [PubMed] [Google Scholar]
- 46.Lowe G, Woodward M, Rumley A, et al. Total tooth loss and prevalent cardiovascular disease in men and women: possible roles of citrus fruit consumption, vitamin C, and inflammatory and thrombotic variables. J Clin Epidemiol 2003; 56:694–700. [DOI] [PubMed] [Google Scholar]
- 47.Ylostalo PV, Jarvelin MR, Laitinen J, et al. Gingivitis, dental caries and tooth loss: risk factors for cardiovascular diseases or indicators of elevated health risks. J Clin Periodontol 2006; 33:92–101. [DOI] [PubMed] [Google Scholar]
- 48.Peres MA, Tsakos G, Barbato PR, et al. Tooth loss is associated with increased blood pressure in adults–a multidisciplinary population-based study. J Clin Periodontol 2012; 39:824–833. [DOI] [PubMed] [Google Scholar]
- 49.Volzke H, Schwahn C, Dorr M, et al. Gender differences in the relation between number of teeth and systolic blood pressure. J Hypertens 2006; 24:1257–1263. [DOI] [PubMed] [Google Scholar]
- 50.Taguchi A, Sanada M, Suei Y, et al. Tooth loss is associated with an increased risk of hypertension in postmenopausal women. Hypertension 2004; 43:1297–1300. [DOI] [PubMed] [Google Scholar]
- 51.Bernardo Cde O, Boing AF, Vasconcelos Fde A, et al. Association between tooth loss and obesity in Brazilian adults: a population-based study. Rev Saude Publica 2012; 46:834–842. [DOI] [PubMed] [Google Scholar]
- 52.De Marchi RJ, Hugo FN, Hilgert JB, et al. Number of teeth and its association with central obesity in older Southern Brazilians. Community Dent Health 2012; 29:85–89. [PubMed] [Google Scholar]
- 53.Ostberg A-L, Nyholm M, Gullberg B, et al. Tooth loss and obesity in a defined Swedish population. Scand J Public Health 2009; 37:427–433. [DOI] [PubMed] [Google Scholar]
- 54.Ishikawa S, Kayaba K, Gotoh T, et al. Metabolic syndrome and C-reactive protein in the general population: JMS Cohort Study. Circ J 2007; 71:26–31. [DOI] [PubMed] [Google Scholar]
- 55.Saisho Y, Hirose H, Roberts R, et al. C-reactive protein, high-molecular-weight adiponectin and development of metabolic syndrome in the Japanese general population: a longitudinal cohort study. PLoS One 2013; 8:e73430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salmenniemi U, Ruotsalainen E, Pihlajamaki J, et al. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation 2004; 110:3842–3848. [DOI] [PubMed] [Google Scholar]
- 57.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 2003; 74:391–401. [DOI] [PubMed] [Google Scholar]
- 58.Howells GL. Cytokine networks in destructive periodontal disease. Oral Dis 1995; 1:266–270. [DOI] [PubMed] [Google Scholar]
- 59.März W, Scharnagl H, Winkler K, et al. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease the Ludwigshafen Risk and Cardiovascular Health study. Circulation 2004; 110:3068–3074. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Gill R, Pedersen TL, et al. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res 2009; 50:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sesso HD, Wang L, Buring JE, et al. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 2007; 49:304–310. [DOI] [PubMed] [Google Scholar]
- 62.Chae CU, Lee RT, Rifai N, et al. Blood pressure and inflammation in apparently healthy men. Hypertension 2001; 38:399–403. [DOI] [PubMed] [Google Scholar]
- 63.Ishibashi M, Hiasa K-i, Zhao Q, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res 2004; 94:1203–1210. [DOI] [PubMed] [Google Scholar]
- 64.Marvar PJ, Thabet SR, Guzik TJ, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II–induced hypertension. Circ Res 2010; 107:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahyoun NR, Lin C-L, Krall E. Nutritional status of the older adult is associated with dentition status. J Am Diet Assoc 2003; 103:61–66. [DOI] [PubMed] [Google Scholar]
- 66.Karjalainen S. Eating patterns, diet and dental caries. Dent Update 2007; 34 5:295–298.300. [DOI] [PubMed] [Google Scholar]
- 67.Lang NP, Cumming BR, Loe H. Toothbrushing frequency as it relates to plaque development and gingival health. J Periodontol 1973; 44:396–405. [DOI] [PubMed] [Google Scholar]
- 68.Montebugnoli L, Servidio D, Miaton RA, et al. Poor oral health is associated with coronary heart disease and elevated systemic inflammatory and haemostatic factors. J Clin Periodontol 2004; 31:25–29. [DOI] [PubMed] [Google Scholar]
- 69.Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause 2005; 12:128–135. [DOI] [PubMed] [Google Scholar]
- 70.Hong JS, Yi S-W, Kang HC, et al. Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas 2007; 56:411–419. [DOI] [PubMed] [Google Scholar]
- 71.Llaneza P, Garcia-Portilla MP, Llaneza-Suarez D, et al. Depressive disorders and the menopause transition. Maturitas 2012; 71:120–130. [DOI] [PubMed] [Google Scholar]
- 72.Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas 1990; 12:321–331. [DOI] [PubMed] [Google Scholar]
- 73.Campos H, McNamara JR, Wilson PW, et al. Differences in low density lipoprotein subfractions and apolipoproteins in premenopausal and postmenopausal women. J Clin Endocrinol Metab 1988; 67:30–35. [DOI] [PubMed] [Google Scholar]
- 74.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008; 60:10–18. [DOI] [PubMed] [Google Scholar]


