Abstract
CD4+ T helper (Th) cells are reported to be essential for initiating and maintaining an effective immune response to hepatitis B virus (HBV) infection. Th9 cells are a new subset of CD4+ Th cells that produce interleukin (IL)-9 and IL-10. The present study aimed to investigate the percentage of Th9 cells relative to the number of CD4+ cells in peripheral blood.
We also measured serum IL-9 and IL-10 levels in different stages of HBV infection and their relationship with progress and prognosis of liver disease. Whole blood samples from 111 patients with HBV infection, including 39 chronic hepatitis B (CHB), 25 HBV-liver cirrhosis (HBV-LC), 21 acute-on-chronic liver failure (ACLF) patients, and 26 healthy controls were collected.
The percentage of Th9 cells and serum IL-9 and IL-10 levels were determined. There was no significant difference in the percentage of Th9 cells and serum IL-9 and IL-10 levels among different groups, nor were these related to hepatitis B e antigen status, complications of cirrhosis, inflammation index, or prognosis indexes. There was no change in the percentage of Th9 cells before and after antiviral treatment in CHB patients. There was no correlation of Th9 cells with survival of ACLF patients. However, IL-9 and IL-10 levels were significantly higher in the nonsurvived ACLF patients compared to survived ACLF patients. Furthermore, baseline IL-9 level predicted the prognosis of ACLF patients with 87.5% sensitivity and 61.5% specificity.
Thus, our data indicate that Th9 cells were unlikely involved in the pathogenesis of HBV infection, but elevation in IL-9 and IL-10 may signal poor prognosis for ACLF.
INTRODUCTION
Hepatitis B virus (HBV) infection is a severe public health burden, and approximately one-third of the world population has serological evidence of resolved or ongoing infection.1,2 HBV infection causes a broad-spectrum of liver diseases ranging from acute hepatitis B, chronic infection with no biochemical evidence of liver injury to progressive chronic hepatitis B (CHB), which may advance to liver cirrhosis (LC), liver failure (LF), and hepatocellular carcinoma.1,3 HBV is thought to be noncytopathic during infection, and the host's immune response to HBV infection is responsible for viral pathogenesis and clearance of HBV infection.4,5
Many cytokines involved in the host's innate and adaptive immune responses have been suggested to contribute to effective antiviral immunity and outcomes of HBV infection.6 HBV-specific cytotoxic T lymphocytes and CD4+ T helper (Th) lymphocytes are 2 major components of the HBV-specific immune response.7,8 CD4+ Th cells are a group of lymphocytes that produce cytokines regulating strength and duration of immunity and inflammation. CD4+ Th cells can be classified into Th1 that secretes interleukin (IL)-2 and interferon (IFN)-gamma, Th2 that produces IL-4, Th17 (IL-17), Th22 (IL-22), and the newly discovered Th9 (IL-9) cells.9–11 Several studies have demonstrated that an imbalance in Th1/Th2 and/or T regulatory (Treg)/Th17 was involved in the progression of HBV infection and is attributed to liver injury and fibrosis.12–15
The Th9 cell is a new subset of Th cells. It secretes IL-9 and IL-10 and functions mainly through IL-9.10,16 The Th9 was reported to contribute to various diseases, such as allergic disease,17–19 autoimmune disease,20,21 cancer,22 transplant rejection,23 and parasitic infection.24 IL-9 has also been suggested to be involved in the pathogenesis of respiratory syncytial virus and EB virus infection.25,26 A recent study27 showed that the percentage of Th9 cells in peripheral blood mononuclear cells (PBMCs) and serum IL-9 level were significantly elevated in HBV-LC patients compared to healthy individuals. Furthermore, a higher expression of CCL20 and CC chemokine receptor 6 (CCR6) was detected on Th9 cells, suggesting that Th9 cells may contribute to the pathogenesis of HBV-LC through the CCL20/CCR6 axis. Our previous study also showed that the serum IL-9 level was significantly higher in HBV-LC than in healthy individuals.28 These observations led us to hypothesize that Th9 cells, as well as IL-9 and IL-10, are immune components participating in the pathogenesis of HBV infection. This study aimed to understand the involvement of Th9 cells in HBV infection and their relationship with progress and prognosis of liver disease. Specifically, we measured the percentage of Th9 cells and serum IL-9 and IL-10 levels in different stages of HBV infection. We also analyzed the kinetic changes in Th9 cells, IL-9, and IL-10 levels over time in CHB patients.
PATIENTS AND METHODS
Patients
This study retrospectively enrolled 85 chronic HBV infected patients including 39 CHB, 25 HBV-LC and 21 acute on chronic LF (ACLF) patients, who were hospitalized or followed from July 2012 to January 2013 in the Department of Infectious Diseases of the First Hospital of Quanzhou, affiliated with Fujian Medical University. The criteria for diagnoses of CHB,29 HBV-LC30 and ACLF31 have been previously described. Patients were excluded if they were (1) treated with antivirals or immunomodulating agents, such as thymosin and glucocorticoid hormones; (2) concomitantly infected with hepatitis A virus, hepatitis C virus (HCV), hepatitis D virus (HDV), or human immunodeficiency virus (HIV); or (3) concomitantly infected with alcohol- or drug-induced liver diseases. Blood samples from 26 healthy individuals were collected as health controls (HC). The study protocol was approved by the Ethics Committee of the First Hospital of Quanzhou affiliated with Fujian Medical University ([2013]60), and written informed consent was obtained from each participant.
Treatment and Follow-up
Out of the 39 CHB patients, 23 were treated with nucleos(t)ide analogs (NAs, i.e., entecavir, telbivudine, lamivudine, or adefovir dipivoxil) and were followed for 7.2 ± 3.4 months. A total of 21 ACLF patients were followed for >6 months. The patients were divided into survived (n = 13) and nonsurvived (n = 8) groups based on the final outcomes at the time this study was completed. The detailed characteristics of the 2 groups were described in a previous study.32
Laboratory Data
Blood samples were collected from all patients the next morning after admission and at the end of follow-up for the 23 CHB patients who were treated with NAs. All biochemical and serological markers were detected in the Department of Laboratory of the First Hospital of Quanzhou affiliated with Fujian Medical University, and the details of them have been described in our previous study.33,34 The Child–Pugh35 score and the model for end-stage liver disease (MELD)36 score were calculated using clinical and experimental parameters.
Cell Preparation and Flow Cytometry
PBMCs were isolated from 3 mL of blood by ficoll density gradient centrifugation and adjusted to a density of 2 × 106/mL in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA). Two milliliters of PBMCs were seeded on to a 6-well plate and maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY), 25 ng/mL phorbol myristate acetate, 1 μg/mL ionomycin, and 1.7 μg/mL monensin (all from eBioscience, San Diego, CA), and incubated at 37 °C and 5% CO2 conditions for 5 hours. Then the cells were collected and incubated with monoclonal antibodies CD3-PC5 and CD8-ECD (Beckman Coulter Immunotech, Brea, CA) for 15 minutes. The cells were permeabilized with FIX and PERM reagent A (Invitrogen, Carlsbad, CA) for 15 minutes, and FIX and PERM reagent B (Invitrogen, Carlsbad, CA) for 10 minutes. Then the cells were incubated with anti-IL-17A-FITC and anti-IL-9A-PE (eBioscience, San Diego, CA) for 20 minutes. After washing with phosphate buffer saline, the phenotypes of the stained cells were analyzed by flow cytometry (COULTER Epics XL, Beckman, Brea, CA). Cells with CD3+CD8– markers were recognized as CD4+ T cells and cells with IL-9+IL-17– markers among the T cells were recognized as Th9 cells. The number of CD3+CD8–IL-9+IL-17– cells divided by the total number of CD3+CD8– cells indicated the percentage of Th9 cells in CD4+ cells.
Enzyme Linked Immunosorbent Assays (ELISA)
Serum IL-9 and IL-10 levels were determined using commercial ELISA kits according to the manufacturer's instructions (Market Inc, San Jose, CA). The colorimetric changes in the wells were read at a wavelength of 450 nm in a microplate reader (ELx800, BioTek Instruments, Inc., Winooski, Vermont).
Statistical Analysis
All data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL). Continuous variables are expressed as the median (10th–90th percentile) unless specified. Comparison of differences was performed using the Kruskal–Wallis H test, Mann–Whitney nonparametric U test and Chi-square test. Paired comparisons were performed using Wilcoxon signed-rank test. The possible relationship between variables was evaluated using Spearman's rank correlation. The diagnostic value of variables was assessed by the area under the receiver operating characteristic (ROC) curves. A 2-side P value < 0.05 was considered significant.
RESULTS
Clinical Characteristics of Enrolled Subjects
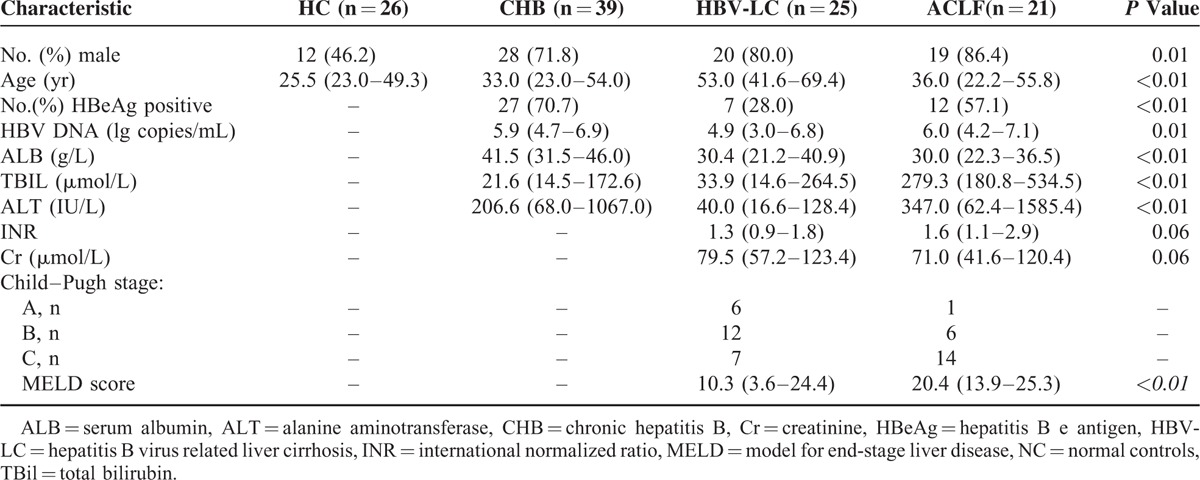
Clinical characteristics of the enrolled subjects are summarized in Table 1. Patients with HBV-LC were older than those with CHB, ACLF, and HC (P < 0.01). The hepatitis B e (HBe) antigen (HBeAg) positive percentage, HBV DNA, and alanine aminotransferase (ALT) levels were significantly lower in HBV-LC patients (P < 0.01) compared to CHB and ACLF patients. Serum albumin (ALB) levels were lower in HBV-LC and ACLF patients compared to CHB patients. Total bilirubin (TBil) were significantly higher in ACLF patients compared to CHB and HBV-LC patients (P < 0.01). The MELD scores were higher in ACLF patients compared to HBV-LC patients (P < 0.01).
TABLE 1.
Clinical Characteristics of the Subjects Enrolled in the Study
Th9 Cells, IL-9, and IL-10 Were not Related to Progression of HBV-Related Liver Injury
Th9 percentages in CD4+ T cells and serum IL-9 and IL-10 levels were analyzed in all groups. No significant differences in both the percentages of Th9 cells and the serum IL-9 and IL-10 levels were observed between the HC group and HBV-infected patients (all P > 0.05) (Figure 1B). However, the Th9 percentage was slightly higher in the CHB group compared to the other 3 groups (0.15% [0.04–0.37%] in the CHB group vs 0.12% [0.02–0.26%] in the HC group, 0.11% [0.05–0.45%] in the HBV-LC group, and 0.10% [0.03–0.23%] in the ACLF group; Figure 1B).
FIGURE 1.
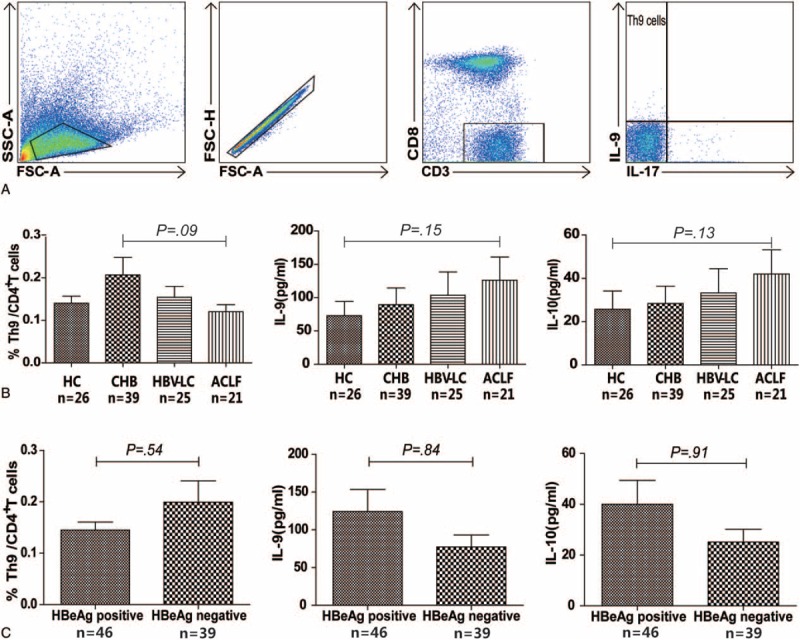
Frequencies of Th9 cells and serum levels of IL-9 and IL-10 in different stages of HBV infection. Sequential gating strategy for Th9 identification (CD3+CD8–IL-9+IL-17–) using flow cytometry from freshly isolated PBMC (A). The percentage of Th9 cells and the serum level of IL-9 and IL-10 were analyzed in HC, CHB, HBV-LC, and ACLF groups (B), and in HBeAg-positive and HBeAg-negative groups (C) using the Mann–Whitney U test. ACLF = acute-on-chronic liver failure; CHB = chronic hepatitis B; HBV-LC = hepatitis B virus-related liver cirrhosis, HC = health controls, IL = interleukin; .
The Th9 percentages and the serum IL-9 and IL-10 levels were further analyzed based on the HBeAg status of all patients. The percentage of Th9 cells was slightly lower in HBeAg-positive group compared to the HBeAg-negative group (0.12% [0.05–0.28%] vs 0.13% [0.03–0.45%], P = 0.54), whereas the serum IL-9 and IL-10 levels were slightly higher in the HBeAg-positive groups compared to the HBeAg-negative groups (IL-9: 37.7 [4.0–495.1] pg/mL vs 32.5 [7.7–197.7] pg/mL, P = 0.84; IL-10: 14.8 [1.8–165.0] pg/mL vs 12.2 [2.9–77.5] pg/mL, P = 0.91) (Figure 1C).
Th9 Cells, IL-9, and IL-10 Levels Were not Related to Severity of Liver Injury in Patients With HBV Infection
Previous studies showed that Treg and Th17 cells were related to the extent of liver injury.37,38 Extent of liver damage and the impact on liver functions can be reflected by elevated ALT, TBil, and reduced ALB levels. The relationship of Th9 cells, as well as IL-9 and IL-10 levels, with these biochemical parameters were analyzed. There was no obvious correlation between the percentage of Th9 cells, levels of IL-9, and IL-10 with age, TBil, ALB, ALT, and HBV DNA levels (P > 0.05) (Table 2). Also, there was no clear association of the Th9 percentages and the serum levels of IL-9 and IL-10 with the Child–Pugh and MELD scores (Figure 2A and B).
TABLE 2.
Correlation Between Th9 Cells, IL-9, and IL-10 and Laboratory Parameters
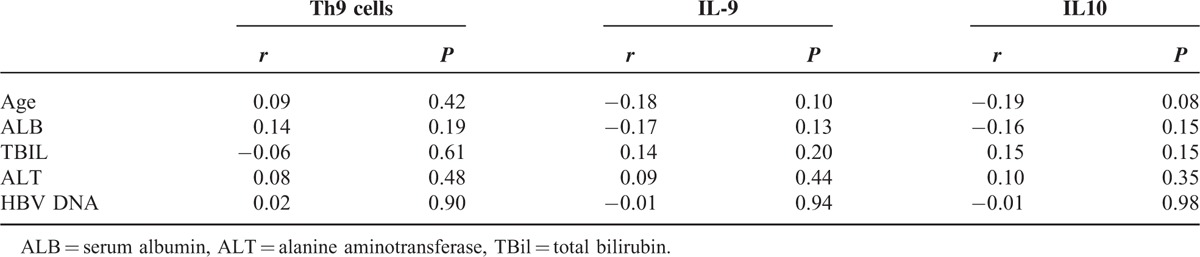
FIGURE 2.
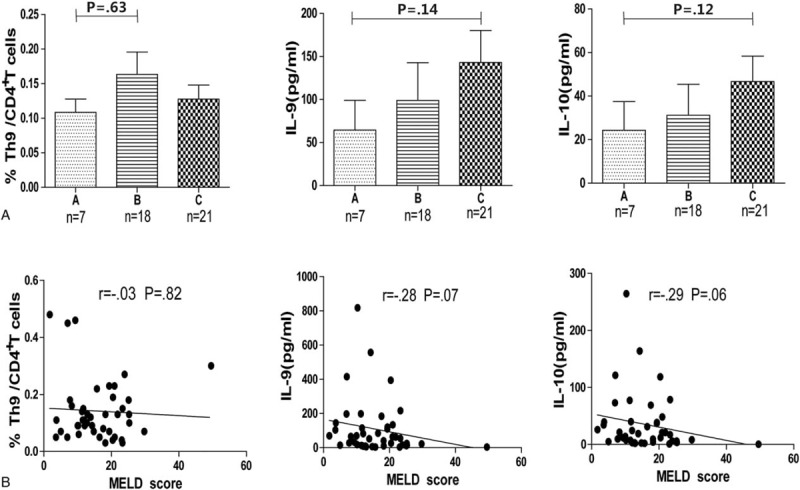
Frequencies of Th9 cells and serum levels of IL-9 and IL-10 did not correlate with disease progression in HBV-LC and ACLF patients. Correlations between the frequencies of Th9 cells, levels of IL-9 and IL-10, MELD, and Child–Pugh scores were analyzed using the Spearman's rank correlation test. ACLF = acute-on-chronic liver failure; HBV-LC = hepatitis B virus-related liver cirrhosis; MELD = model for end-stage liver disease.
Th9 Cells, IL-9, and IL-10 Were not Related to the Progression of HBV-LC
HBV-LC patients were divided into 2 groups to determine if there was a possible relationship between Th9 cells and serum IL-9 and 10 levels, based on the presence or absence of complications. As shown in Figure 3, there was no significant difference in Th9 cell frequencies and IL-9 and IL-10 levels between the HBV-LC patients with and without complications (P > 0.05). No significant difference was noted in Th9 cell frequencies, IL-9, and IL-10 levels between patients with and without HCC (P = 0.12, 0.32, and 0.42, respectively) (Figure 3).
FIGURE 3.
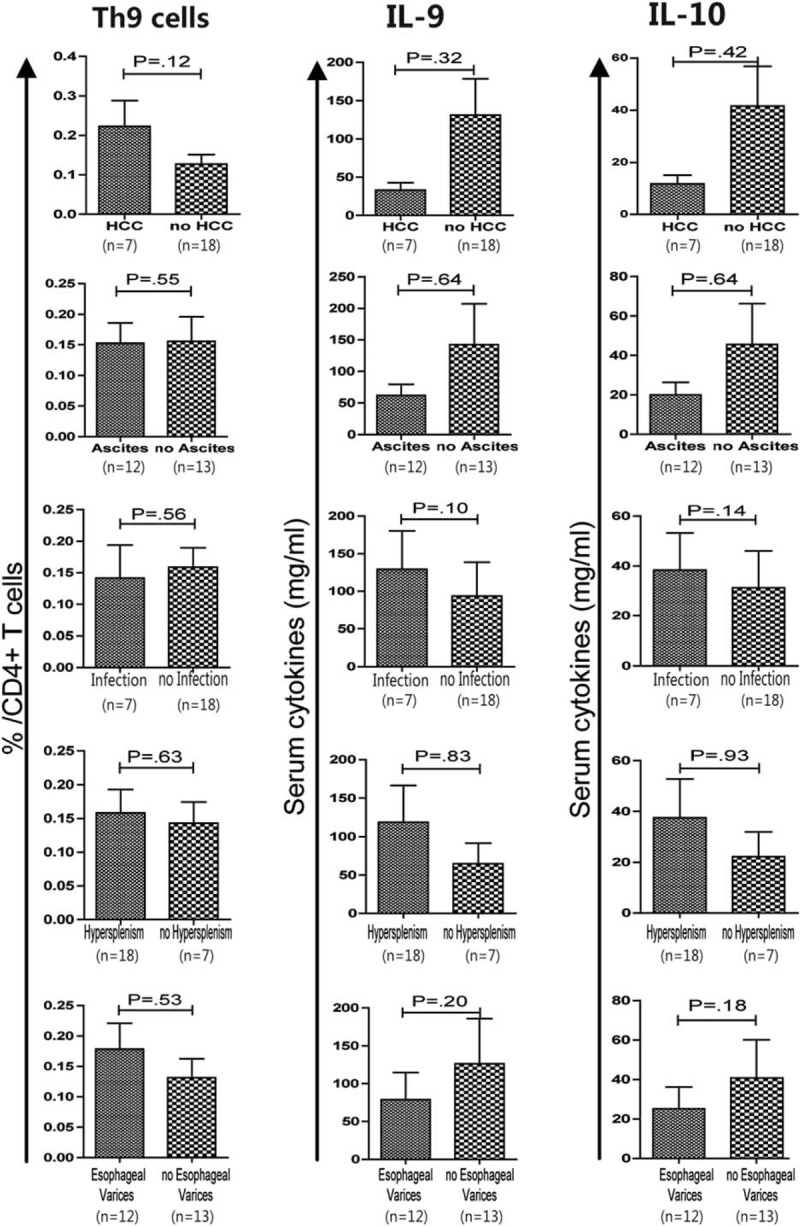
No association was found between the frequencies of Th9 cells and serum levels of IL-9 and IL-10 with HBV-LC patients with and without complications. Correlations of Th9 cell frequency, and IL-9 and IL-10 levels in patients with or without HCC, ascites, infection, hypersplenism, and esophageal varices were analyzed using the Mann–Whitney U test. HBV-LC = hepatitis B virus-related liver cirrhosis; HCC = hepatocellular carcinoma.
IL-9 and IL-10, but not Th9 Cells, Increased After Treatment in CHB Patients
A total of 23 CHB patients with an average age of 35.9 ± 9.7 (23–59) years were treated with NAs for 7.2 ± 3.4 months, and 17 of them were males. At the end of follow-up, ALT level and HBV DNA load were markedly decreased compared to baseline levels (all P < 0.01). IL-9 and IL-10 levels were significantly increased (P < 0.01 and 0.02, respectively), whereas the Th9 proportion was slightly reduced after treatment (P = 0.34) (Figure 4).
FIGURE 4.
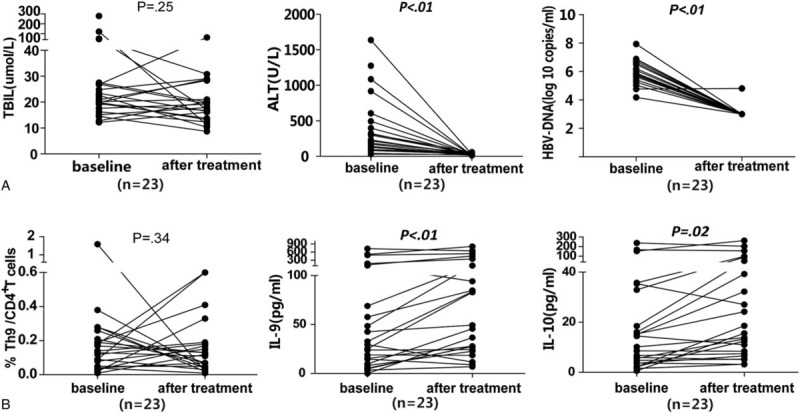
Changes in the Th9 cell frequencies and the serum levels of cytokines and laboratory parameters before and after nucleos(t)ide analog antiviral treatment in 23 patients with CHB. Data were analyzed with the Wilcoxon signed-rank test. ALT = alanine aminotransferase; CHB = chronic hepatitis B; TBil = total bilirubin.
Serum IL-9 and IL-10 Levels, but not the Percentage of Th9 Cells, Predicted the Prognosis of ACLF Patients
Our previous study indicated that the ratio of Treg to Th17 cells was associated with survival of ACLF patients.13 We wondered if the percentage of Th9 cells was also related to survival of ACLF patients. In this study, the percentage of Th9 cells was slightly lower in the nonsurvived group compared to the survived group (0.09 [0.03–0.19] pg/mL vs 0.12 [0.04–0.25] pg/mL, P = 0.29), but the serum levels of IL-9 and IL-10 were significantly higher in the nonsurvived group compared to the survived group (IL-9: 200.2 [26.6–556.7] pg/mL vs 40.3 [4.6–126.1] pg/mL, P = 0.01; IL-10: 73.8 [11.9–171.5] pg/mL vs 9.3 [2.1–44.8] pg/mL, P = 0.01) (Figure 5A). The possible predictive values of IL-9 and IL-10 were further explored by ROC curve analysis. Although the same area under the curve (AUC) value (0.84, P = 0.01) was reached for both IL-9 and IL-10, only IL-9 showed a high sensitivity and specificity for predicting the survival of ACLF patients (87.5% and 61.54% at the cutoff value of 53.19 pg/mL, respectively) (Figure 5B).
FIGURE 5.
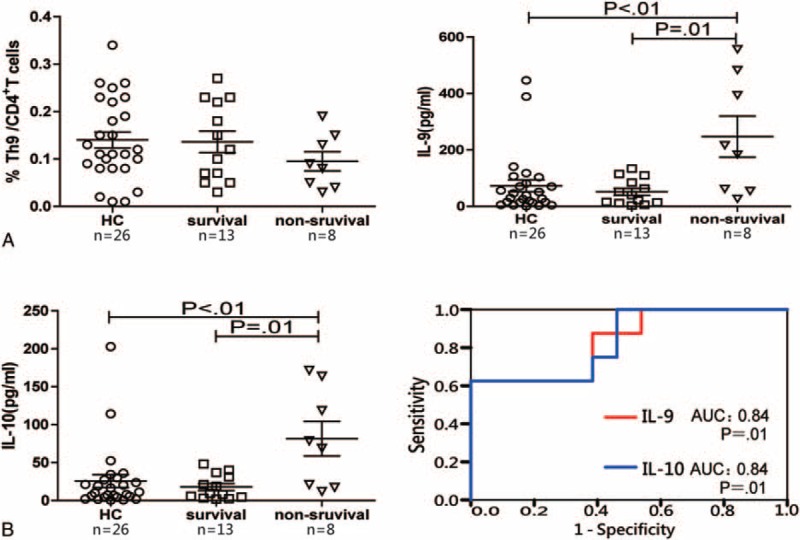
Correlations of frequencies of Th9 cells and serum levels of cytokines with survival of ACLF patients. (A) Frequencies of Th9 cells, serum levels of IL-9 and IL-10 in the survived and nonsurvived ACLF patients were analyzed using the Mann–Whitney U test. (B) Receiver operating characteristic curve of IL-9 and IL-10 for the prognosis prediction in ACLF patients. ACLF = acute-on-chronic liver failure; HC = health controls.
DISCUSSION
In this study, the percentage of Th9 cells in the total CD4+ T cells and the serum levels of IL-9 and IL-10 were analyzed in different stages of HBV infection. We found that Th9 cells, and IL-9 and IL-10 levels were not related to the severity of liver injury, progression, and prognosis of HBV-related chronic liver diseases. No significant change in Th9 cell frequency was observed before and after NAs treatment in CHB patients.
Our findings were different from a published study27 that detected the frequency of Th9, serum levels of IL-9 and CCL-20, and CCL20 and CCR6 expression in liver tissues in 18 HBV-LC patients and 6 healthy individuals. This study found that Th9 cell percentages and IL-9 level were significantly higher in HBV-LC patients compared to healthy individuals. The study also suggested that these factors were positively related to the severity of liver fibrosis, indicating that Th9 cells may be involved in the pathogenesis of HBV-LC through the CCL20/CCR6 axis. The difference between these 2 studies may be attributed to different sample size and severity of liver cirrhosis.
HBV-related ACLF is the most severe complication triggered by flare-ups of liver injury and is accompanied by high mortality. Cytokine storm is considered one of factors in the pathologic process of ACLF.39 Several studies have shown the association of Treg and Th17 cells and the cytokines they secrete with development of ACLF.13,40 However, it is unclear whether Th9 cells are also involved in the pathogenesis of ACLF. The present study showed that the percentage of Th9 cells was lower in ACLF compared to HC, and it was lower in the nonsurvived ACLF than the survived ACLF, although the difference was not significant. The data indicated that Th9 cells were unlikely involved in the pathogenesis of ACLF, or the Th9 cells migrated to the livers in response to severe liver injury and inflammation.41
However, serum levels of IL-9 and IL-10 were significantly higher in the nonsurvived ACLF patients compared to the survived ACLF patients and HC. In fact, the IL-9 level was predictive of ACLF prognosis, suggesting a possible involvement of IL-9 and IL-10 in the progression of ACLF. The increased IL-9 and IL-10 levels in the nonsurvived ACLF patients could be caused by other immune cells that also secrete IL-9 and IL-10 as part of the cytokine storm, in addition to Th9 cells. Our results suggest that a significant elevation in cytokine production is a sign for high mortality in ACLF patients.
A previous study reported that the IL-9 level was higher in CHB patients, but decreased after 6 months of telbivudine treatment.42 In this study, the IL-9 and IL-10 levels were significantly increased after treatment. This discrepancy may be explained by the number of T cells that produce IL-9 and IL-10. Other Th cells, such as Th2 and Th17, were activated to secrete more IL-9 and IL-10 once HBV replication was profoundly inhibited by NAs therapy.10 This hypothesis was supported by our previous study that Th17 cell frequencies and IL-17 level were increased in CHB patients who mounted a complete immune response.33
We found no clear correlation of Th9 cells with HBV infection and related liver injury. This may not only reflect the fact that there was a very low percentage of Th9 cells in the peripheral blood,20 which was as low as 0.01% (range 0.01–1.59%) in the present study, but also IL-9 and IL-10 can be secreted by other CD4+ T cells. Thus, the changes in the serum levels of IL-9 and IL-10 in HBV infection may not necessarily reflect the function of the Th9 cells.
There are several limitations in the present study. First, we did not measure Th9 cells in the livers because liver samples are not available. Second, Th9 cell-related transcription activity was not analyzed to determine the IL-9 and IL-10 mRNA levels. Third, other CD4+ T cells, such as Th2, Th17, and Treg that can also secrete IL-9, were not measured, and we were unable to determine if the elevated IL-9 and IL-10 levels were secreted by the Th9 cells alone or by other Th cells. We hope to address these limitations in future studies.
In conclusion, we found that Th9 cells and IL-9 and IL-10 were unlikely related to HBV infection, degree of liver injury, and progression of HBV infection-related cirrhosis. However, serum levels of IL-9 and IL-10 appeared to be predicative of ACLF prognosis in patients.
Acknowledgments
The authors are grateful for the assistance of all the medical staff for collecting clinical specimens, in the department of infectious diseases of the first hospital of Quanzhou affiliated to Fujian Medical University.
Footnotes
Abbreviations: ALB = serum albumin, ALT = alanine aminotransferase, CHB = chronic hepatitis B, ELISA = enzyme-linked immunosorbent assay, HAV = hepatitis A virus, HBV = hepatitis B virus, HBV-ACLF = hepatitis B virus-related acute-on-chronic liver failure, HBV-LC = hepatitis B virus-related liver cirrhosis, HC = health controls, HCC = hepatic carcinoma, HCV = hepatitis C virus, HDV = hepatitis D virus, HIV = human immunodeficiency virus, MELD = model for end-stage liver disease, TBil = total bilirubin.
Financial disclosure: this study was supported by National Natural Science Foundation of China (81400625), Natural Science Foundation of Fujian province (2014J01392, 2015J01413), Fujian Medical University Research and Development Project (FZS13029Y), Fujian Provincial Health Bureau Youth Research Project (2013-1-45) and Quanzhou Major Science and Technology Plan Project (2013Z53).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Papatheodoridis G, Buti M, Cornberg M, et al. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 2.Jindal A, Kumar M, Sarin SK. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int 2013; 33 Suppl 1:164–175. [DOI] [PubMed] [Google Scholar]
- 3.Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010; 58:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isogawa M, Tanaka Y. Immunobiology of hepatitis B virus infection. Hepatol Res 2015; 45:179–189. [DOI] [PubMed] [Google Scholar]
- 5.Hui CK, Lau GK. Immune system and hepatitis B virus infection. J Clin Virol 2005; 34 Suppl 1:S44–48. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Liu X, Tian L, et al. Cytokine-mediated immunopathogenesis of hepatitis B virus infections. Clin Rev Allergy Immunol 2016; 50:41–54. [DOI] [PubMed] [Google Scholar]
- 7.Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis 2002; 2:43–50. [DOI] [PubMed] [Google Scholar]
- 8.Ge J, Wang K, Meng QH, et al. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol 2010; 30:60–67. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol 2014; 35:61–68. [DOI] [PubMed] [Google Scholar]
- 10.Zhao P, Xiao X, Ghobrial RM, et al. IL-9 and Th9 cells: progress and challenges. Int Immunol 2013; 25:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Rostami A. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol 2010; 5:198–209. [DOI] [PubMed] [Google Scholar]
- 12.Song XW, Wang XW, Gao HL, et al. Apoptosis is related to imbalance of Th1/Th2-type cytokine in peripheral blood mononuclear cells of patients with chronic hepatitis B. Acta Virol 2012; 56:153–154. [DOI] [PubMed] [Google Scholar]
- 13.Yu XP, Guo R, Ming D, et al. Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor-beta1/interleukin-17 to be associated with the development of hepatitis B virus-associated liver cirrhosis. J Gastroenterol Hepatol 2014; 29:1065–1072. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Wang FP, She WM, et al. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat 2014; 21:129–140. [DOI] [PubMed] [Google Scholar]
- 15.Silva LD, Rocha AM, Rocha GA, et al. The presence of Helicobacter pylori in the liver depends on the Th1, Th17 and Treg cytokine profile of the patient. Mem Inst Oswaldo Cruz 2011; 106:748–754. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev 2013; 252:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoppenot D, Malakauskas K, Lavinskiene S, et al. Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina (Kaunas) 2015; 51:10–17. [DOI] [PubMed] [Google Scholar]
- 18.Froidure A, Shen C, Gras D, et al. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy 2014; 69:1068–1076. [DOI] [PubMed] [Google Scholar]
- 19.Ye ZJ, Yuan ML, Zhou Q, et al. Differentiation and recruitment of Th9 cells stimulated by pleural mesothelial cells in human Mycobacterium tuberculosis infection. PLoS One 2012; 7:e31710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciccia F, Guggino G, Rizzo A, et al. Potential involvement of IL-9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2015; 54:2264–2272. [DOI] [PubMed] [Google Scholar]
- 21.Schlapbach C, Gehad A, Yang C, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 2014; 6:219ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu ZW, Wang YX, Cao ZW. T-helper type 9 cells play a central role in the pathogenesis of respiratory epithelial adenomatoid hamartoma. Medicine (Baltimore) 2015; 94:e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Fan H, Jiang S. CD4(+) T-cell subsets in transplantation. Immunol Rev 2013; 252:183–191. [DOI] [PubMed] [Google Scholar]
- 24.Anuradha R, George PJ, Hanna LE, et al. IL-4-, TGF-beta-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. J Immunol 2013; 191:2466–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara PS, Flanagan BF, Baldwin LM, et al. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 2004; 363:1031–1037. [DOI] [PubMed] [Google Scholar]
- 26.Samanta M, Takada K. Modulation of innate immunity system by Epstein-Barr virus-encoded non-coding RNA and oncogenesis. Cancer Sci 2010; 101:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JX, Guo XY, Jiang HX, et al. Circulatory Th9 cells in patients with hepatitis B associated liver cirrhosis. In Chinese. Shijie Huaren Xiaohua Zazhi 2015; 23:1736–1744. [Google Scholar]
- 28.Deng Y, Su ZJ, Yu XP, et al. IL-9, IL-10 and TGFβ1 in the peripheral blood of patients with chronic hepatitis B. In Chinese. Chin J viral Dis 2012; 2:296–300. [Google Scholar]
- 29.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662. [DOI] [PubMed] [Google Scholar]
- 30.Sun HQ, Zhang JY, Zhang H, et al. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat 2012; 19:396–403. [DOI] [PubMed] [Google Scholar]
- 31.Sun QF, Ding JG, Xu DZ, et al. Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat 2009; 16:464–470. [DOI] [PubMed] [Google Scholar]
- 32.Zhai S, Zhang L, Dang S, et al. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol 2011; 24:303–310. [DOI] [PubMed] [Google Scholar]
- 33.Su ZJ, Yu XP, Guo RY, et al. Changes in the balance between Treg and Th17 cells in patients with chronic hepatitis B. Diagn Microbiol Infect Dis 2013; 76:437–444. [DOI] [PubMed] [Google Scholar]
- 34.Yu XP, Guo R, Ming D, et al. The transforming growth factor beta1/interleukin-31 pathway is upregulated in patients with hepatitis B virus-related acute-on-chronic liver failure and is associated with disease severity and survival. Clin Vaccine Immunol 2015; 22:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 36.Salerno F, Merli M, Cazzaniga M, et al. MELD score is better than Child–Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol 2002; 36:494–500. [DOI] [PubMed] [Google Scholar]
- 37.Shiao YM, Chung HJ, Chen CC, et al. MCP-1 as an effector of IL-31 signaling in familial primary cutaneous amyloidosis. J Invest Dermatol 2013; 133:1375–1378. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JY, Zhang Z, Lin F, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010; 51:81–91. [DOI] [PubMed] [Google Scholar]
- 39.Perazella MA. Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr Opin Nephrol Hypertens 2009; 18:519–525. [DOI] [PubMed] [Google Scholar]
- 40.Kim HY, Jhun JY, Cho ML, et al. Interleukin-6 upregulates Th17 response via mTOR/STAT3 pathway in acute-on-chronic hepatitis B liver failure. J Gastroenterol 2014; 49:1264–1273. [DOI] [PubMed] [Google Scholar]
- 41.Wasmuth HE, Kunz D, Yagmur E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol 2005; 42:195–201. [DOI] [PubMed] [Google Scholar]
- 42.Nan XP, Zhang Y, Yu HT, et al. Inhibition of viral replication downregulates CD4(+)CD25(high) regulatory T cells and programmed death-ligand 1 in chronic hepatitis B. Viral Immunol 2012; 25:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


