Abstract
The aim of this study was to compare the efficacy and safety of interferon (IFN) combined with dacarbazine (DTIC) (experimental group) versus DTIC alone (control group) in cutaneous malignant melanoma.
After searching all available databases, eligible articles were identified and subjected to quality assessment. Meta-analysis was performed using RevMan 5.3; combined relative risk (RR) and 95% confidence intervals (95% CIs) were calculated for survival rates, response rates, and adverse events.
Eight randomized controlled trials published between 1990 and 2014 involving 795 patients were included in the meta-analysis. Compared with DTIC alone, IFN combined with DTIC significantly increased the overall response rate (RR = 1.59, 95% CI 1.21–2.08, P = 0.0008),the complete response rate (RR = 3.30, 95% CI 1.89–5.76, P < 0.0001), 2-year survival (RR = 1.59, 95% CI 0.99–2.54, P = 0.050) grade ≥3 hematologic toxicity (RR = 2.30, 95% CI 1.32–4.02, P = 0.003), neurotoxicity (RR = 18.15, 95% CI 5.34–61.74, P < 0.00001), and flu-like symptoms (RR = 6.31, 95% CI 1.95–20.39, P = 0.002). The partial response rate, grade ≥3 nausea and vomiting, treatment-related, and 1- and 3-year survival were not significantly different between IFN combined with DTIC and DTIC alone.
IFN combined with DTIC may moderately improve the complete response rate, but increases the incidence of adverse events and has no significant effect on 1- and 3-year survival in cutaneous malignant melanoma.
INTRODUCTION
Cutaneous malignant melanoma (CMM) is a relatively common, highly malignant tumor type that has a poor prognosis.1 According to data from the National Cancer Research Center, the incidence of CMM in the white American population increased from 7.5 per 100,000 in 1973 to 22.5 per 100,000 in 2011, and CMM accounts for 75% of all skin cancer-related mortalities in the United States.2 The incidence of CMM in China is approximately 0.3 per 100,000.3 The past decade has given rise to a variety of targeted therapies that hold great promise for the treatment of melanoma.4 For >30 years, Dacarbazine has been considered as the standard drug treatment for metastatic melanoma. It was the first5 chemotherapy drug to be approved for the treatment of metastatic melanoma and is still considered to be the drug with most activity against the condition. Interferons(IFNs), a class of glycoproteins produced by selected cells that have been exposed to a variety of agents, possess many antiproliferative and immunomodulatory effects,6,7 causing tumor cells to be more susceptible to the immune system. Interferons possess many antiproliferative and immunomodulatory effects,8 causing tumor cells to be more susceptible to the immune system. Immunomodulation, acting as a type of IFN, may be the basis for the action of interferon-α against melanoma. Specific actions may include the following: increased numbers of infiltrating cells5; stimulation of antibody production9,10; reduced Treg cell numbers in the circulation11; changes to the STAT1/STAT3 ratio both in the circulating lymphocytes12 and in the cells of the tumor; and changes in the blood cytokine levels.13 Some researchers consider that DTIC combined with interferon-α is more effective against metastatic melanoma than is DTIC alone. In this article, we will explore the safety and efficacy about the treatment of CMM.
Comprehensive individualized treatment may provide the optimal method for treating advanced CMM. Biochemotherapy, a combination of biotherapy and chemotherapy, represents a novel basis for the treatment of malignant tumors, and has undergone rapid development in recent years.14 Currently, biochemotherapy has shown promise for the treatment of esophageal cancer, stomach cancer, lung cancer, and breast cancer.14 The University of Texas MD Anderson Cancer Center introduced a biochemotherapy regimen based on a sequential combination of DTIC, cisplatin, vinblastine, IFN, and interleukin-2 for CMM, and patients treated using this regimen achieved a complete remission rate of 21% and median survival time increased to 6 months.15,16 However, controversies have emerged regarding the treatment effects of IFN combined with DTIC compared with DTIC alone in CMM. Pyrhonen et al17 also concluded that the combined therapy had a very good curative effect in CMM. However, research by Young et al18 did not confirm that the combined regimen improved patient survival. Therefore, the aim of this study was to perform a meta-analysis to compare the efficacy of DTIC combined with IFN versus DTIC alone in CMM to offer reference for treatment of CMM.
MATERIALS AND METHODS
Ethical Review
Meta-analysis does not involve ethical review.
Literature Search Strategy
In combination with a manual retrieval of relevant articles, we searched PubMed, EMBASE, the China journal full text database (CNKI), Wan Fang Data Resource, Chinese biomedical literature database (CBM), and VIP Chinese Journal Full Text Database using the search terms “dacarbazine(DTIC),” “melanoma,” and “IFN (interferon)”. No language restrictions were applied; the search was last updated on April 18, 2014. We identified all cohort studies published in China or elsewhere on DTIC with IFN and DTIC alone for the treatment of CMM.
Inclusion/Exclusion Criteria
The inclusion criteria were as follows: randomized controlled trials (RCTs) comparing DTIC combined with IFN and DTIC as a single drug; as a first-line treatment in patients diagnosed with CMM by clinical pathology. Studies in which the baseline studies of the experimental group and control group were inconsistent, in which the patients were not receiving their first-line treatment, or that repeated previously published data were excluded.
Data Extraction and Quality Evaluation
Two researchers independently identified relevant studies by reading the abstracts, and then extracted the data from the full-text versions of the relevant studies. The quality of each study was evaluated independently according to the RCT quality evaluation standards of the Cochrane review manual, based on the use of random sequence generation, allocation concealment, blinded methods, and follow-up duration. For each of these criteria, the studies were scored “yes” (low bias), “no,” (high bias) or “not clear” (lack of information or bias is uncertain). Studies that scored “yes” for all 4 quality standards are classified as “A level” (minimum possibility of bias); studies that partially satisfy 3 of the quality standards are classified as “B level”; studies which satisfy ≤2 quality standards are classified “C level.” When screening the literature and during the data extraction and quality evaluation process, all inconsistencies were reviewed by 3 researchers and a consensus was reached.
Outcome Indicators
Treatment Effects
Evaluation of the curative effect was performed with reference to the 1979 WHO standard.19 Complete response (CR) was defined as the disappearance of all symptoms and signs of all measurable disease, lasting for at least 4 weeks, without appearance of new lesions. Partial response (PR) was defined as a >50% reduction in the sum of the products of the perpendicular diameters of all measurable lesions, lasting for at least 4 weeks, without the appearance of new lesions or enlargement of existing lesions. Progressive disease (PD) was defined as an increase in the product of 2 perpendicular diameters of any measured lesion by >25% relative to study entry, or the appearance of new lesions. No change (NC) was defined as a <50% reduction in the sum of the products of the perpendicular diameters of all measurable lesions, or an increase in the product of any 2 perpendicular diameters of all measured lesions by <25%. Overall response rate (ORR) included CR and PR.
Adverse Effects
Adverse effects were assessed using the acute and subacute toxicity grading standards of the WHO and scored as: 0 (none), I (mild), II (moderate), III (severe), IV (threat to patient's life). The adverse effects assessed in this study are presented in Table 1.
TABLE 1.
The Types of Adverse Effects and Grade
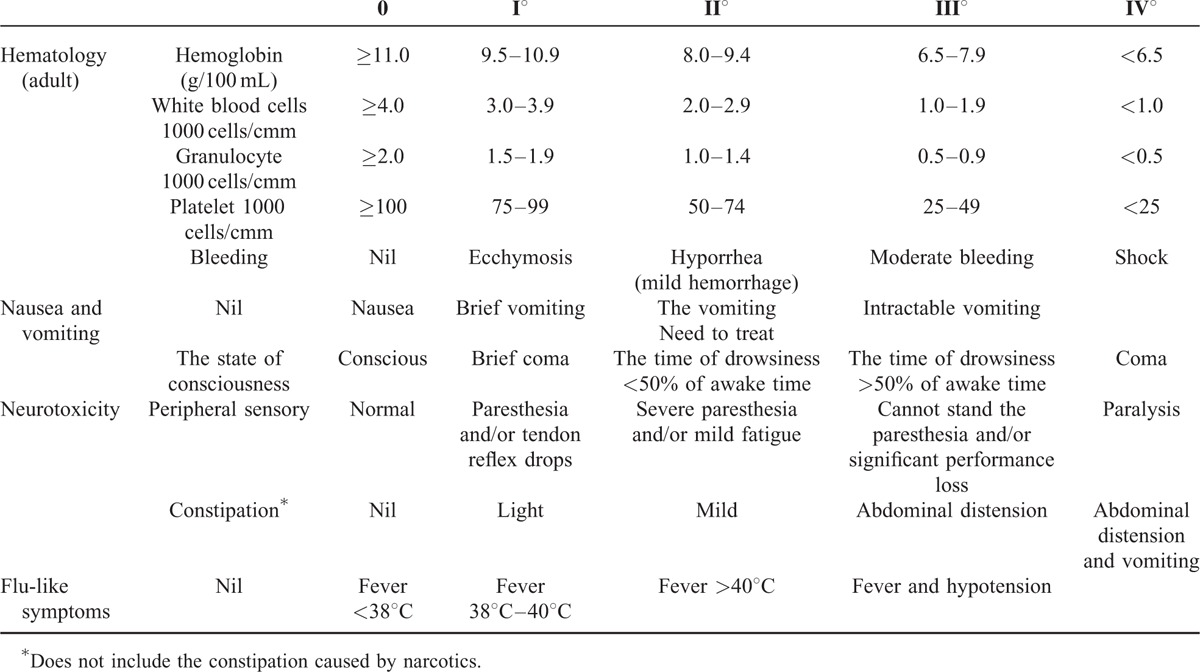
Survival rates
Overall survival was assessed at 1 , 2, and 3 years.
Statistical Analysis
This meta-analysis was performed using Review Manager Version 5.3 software. Summary measures of efficacy and safety were assessed using the relative risk (RR) for dichotomous variables and corresponding 95% confidence interval (95% CI). Between-studies heterogeneity was evaluated using the χ2 test, P values, and I2 statistics. If there was no significant heterogeneity (P > 0.10, I2 < 50%), the pooled RR was estimated by a fixed-effect model; if heterogeneity existed (P < 0.10, I2 ≥ 50%), subcategory analyses were performed to identify factors that may contribute to the heterogeneity. If there was statistical heterogeneity among studies without clinical heterogeneity or the difference was not clinically significant, a random-effects model was applied. If the heterogeneity between groups was too great, or sufficiently detailed data from the original trials were not available, a descriptive analysis could be adopted. Sensitivity analysis was performed to examine the influence of individual studies.
RESULTS
LiteratureSearch and Characteristics of the Trials Included
The initial search identified 76 potential citations, of which 35 were excluded after the titles and abstracts were reviewed and 33 were excluded after reading the full-text articles (18 studies have on control group, 15 are not RCTs). The 8 remaining qualitative studies were assessed for eligibility: all 8 articles included were “B level” and met the principles of randomization; all studies included reported the baseline condition of the patients. All 8 of these articles were included in the meta-analysis (Figure 1). The quality evaluations of the included studies are shown in Figure 2.
FIGURE 1.
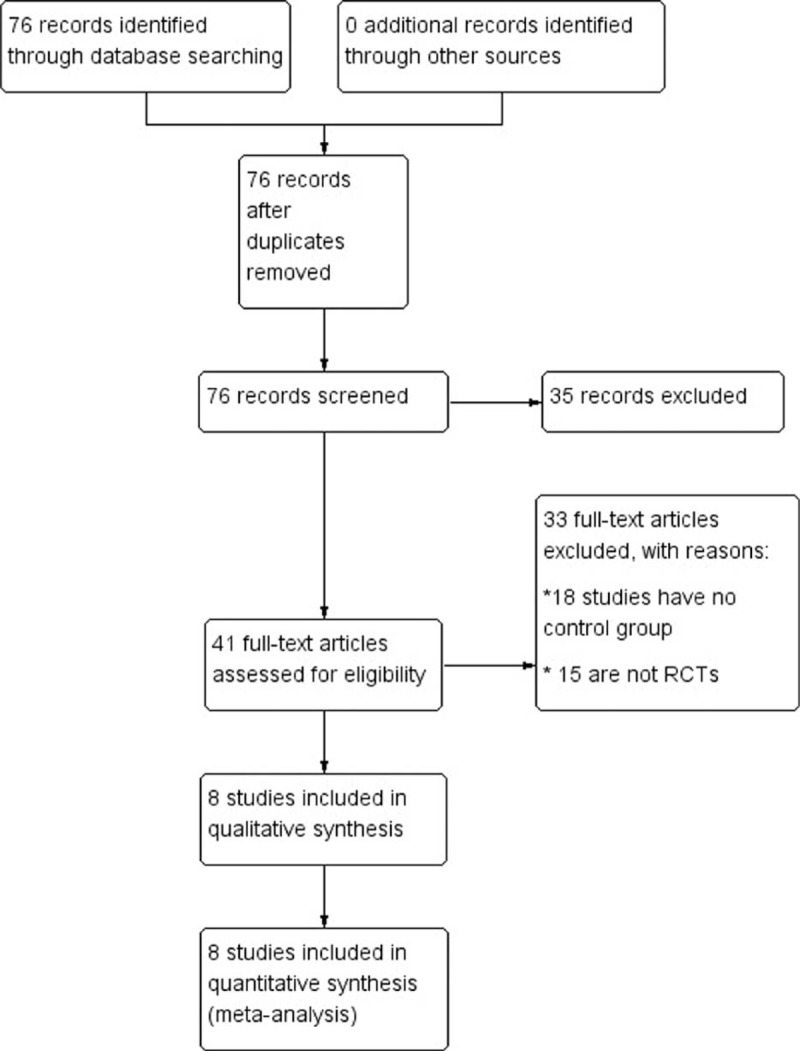
Flow diagram of identification and selection of eligible studies.
FIGURE 2.
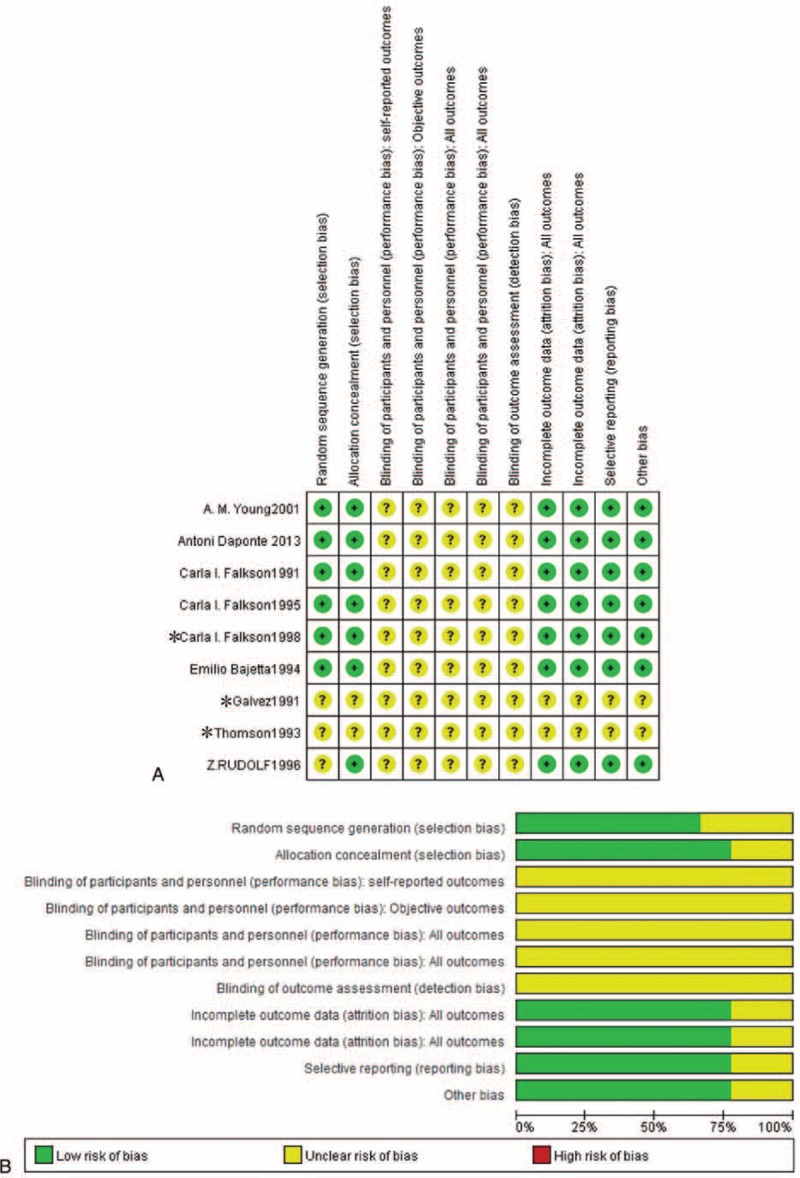
Risk of bias. (A) percentile chart and (B) summary diagram. ∗Falkson, 199520; Galvez and Bonamassa, 199121; Thompson et al, 199322.
Survival
Owing to significant heterogeneity (I2 = 42%, P = 0.11), a fixed-effects model was used for 1-year survival. The RR and 95% CI for 1-year survival were 1.10 and 0.92 to 1.32, respectively (Figure 3A), suggesting that the regimen had no significant effect on 1-year survival.
FIGURE 3.
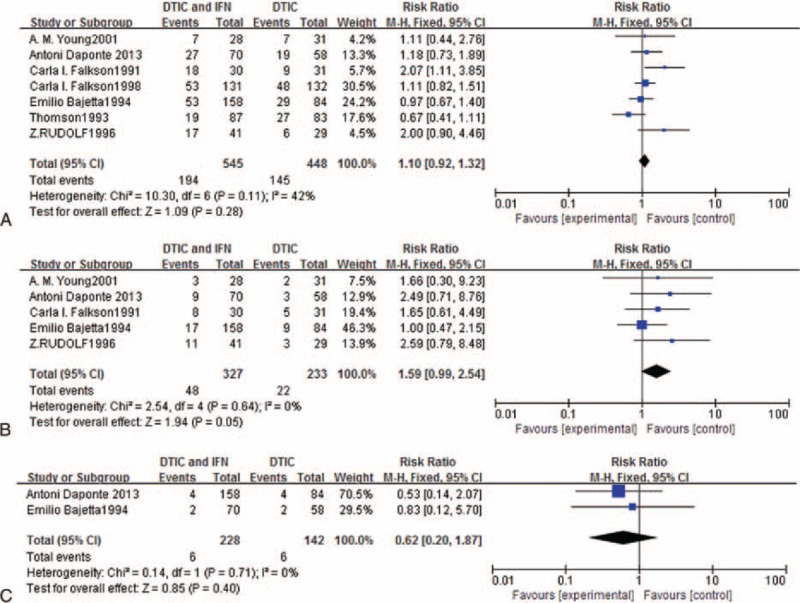
Meta-analysis of the effects of experimental group versus control group in terms of (A) overall response rate, (B) complete response, and (C) partial response.
The test for heterogeneity was not statistically significant for 2-year survival, allowing the datasets to be pooled using a random-effects model. Owing to P = 0.05, 2-year survival might be different for DTIC in combination with IFN compared with DTIC alone (RR = 1.59, 95% CI 0.99, 2.54; Figure 3B).
A fixed-effects model was used for 3-year survival owing to significant heterogeneity (I2 = 0%, P = 0.71). The RR and 95% CI for 3-year survival were 0.62 and 0.20 to 1.87, respectively (Figure 3C), suggesting that the regimen had no significant effect on 3-year survival.
Effectiveness and Safety
Overall response (OR), CR, and PR were evaluated using all 8 studies (all RCTs). The tests for heterogeneity for OR and CR were not statistically significant, allowing the datasets for each outcome to be pooled using random-effects models. The meta-analysis demonstrated a significant difference between the ORR for DTIC combined with IFN compared with DTIC alone (RR = 1.59, 95% CI 1.21–2.08, P < 0.05; Figure 4A). There was also a significant difference in the CR rate between DTIC combined with IFN and DTIC alone [RR = 3.30, 95% CI 1.89–5.76, P < 0.05; Figure 4B). A fixed-effects model was used for PR because of significant heterogeneity (I2 = 0%, P = 1.00). The RR and 95% CI for PR were 1.05 and 0.74 to 1.50, respectively (Figure 4C), indicating there was no significant difference in the PR rate between DTIC combined with IFN and DTIC alone.
FIGURE 4.
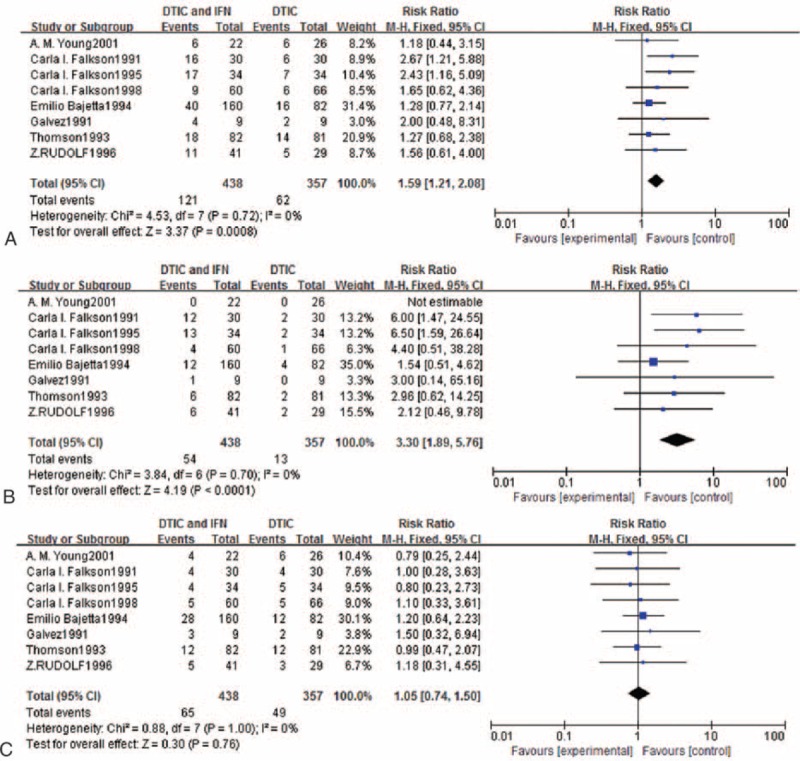
Meta-analysis of the effects of experimental group versus control group in terms of adverse reactions.
Adverse Reactions
The test for heterogeneity for hematologic toxicities was not statistically significant, allowing the 2 datasets to be pooled using a random-effects model. Grade 3 or worse hematologic toxicity was significantly more frequent for DTIC in combination with IFN than DTIC alone (RR = 2.30, 95% CI 1.32–4.02; Figure 5A).
FIGURE 5.
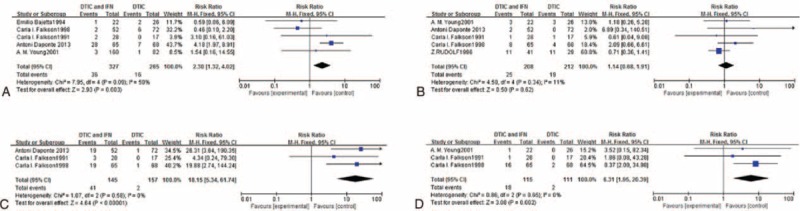
Meta-analysis results of the effects of experimental group versus control group in terms of treatment-related mortality.
A fixed-effects model was used for nausea and vomiting because of significant heterogeneity (I2 = 11%, P = 0.34). The RR and 95% CI for grade 3 or worse nausea and vomiting were 1.14 and 0.68 to 1.91, respectively (Figure 5B), indicating that there was no significant difference in the incidence of nausea and vomiting between DTIC in combination with IFN compared with DTIC alone.
The test for heterogeneity for neurotoxicity was not statistically significant, allowing the datasets to be pooled using a random-effects model. The meta-analysis revealed that grade 3 or worse neurotoxicity was significantly more frequent for DTIC in combination with IFN than DTIC alone (RR = 18.15, 95% CI 5.34–61.74; Figure 5C).
The test for heterogeneity for flu-like symptoms was not statistically significant, allowing the dataset results to be pooled using a random-effects model. Grade 3 or worse flu-like symptoms were significantly more frequent for DTIC in combination with IFN than DTIC alone (RR = 6.31, 95% CI 1.95–20.39; Figure 5D).
A fixed-effects model was used for treatment-related mortality owing to significant heterogeneity (I2 = 30%, P = 0.23). The RR and 95% CI for treatment-related mortality were 1.52 and 0.25 to 9.06, respectively (Figure 6), suggesting there was no significant difference in treatment-related mortality between DTIC in combination with IFN compared with DTIC alone.
FIGURE 6.
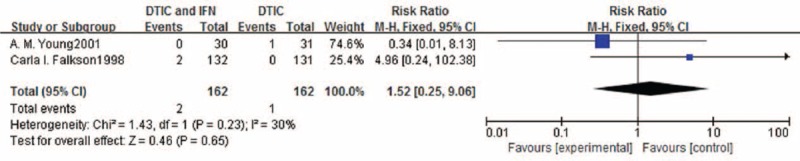
Meta-analysis of the effects of experimental group versus control group in terms of 1-, 2-, and 3-year survival.
Publication Bias
Using the inverted funnel plot method, a scatter diagram was created with the RR values as the abscissa and SE (log [RR]) values as the ordinate. As shown in Figure 7, a large proportion of the study datasets was concentrated in the center of the graph, with a few studies scattered at the bottom of the funnel plot. So it can be considered that there was no publication bias.
FIGURE 7.
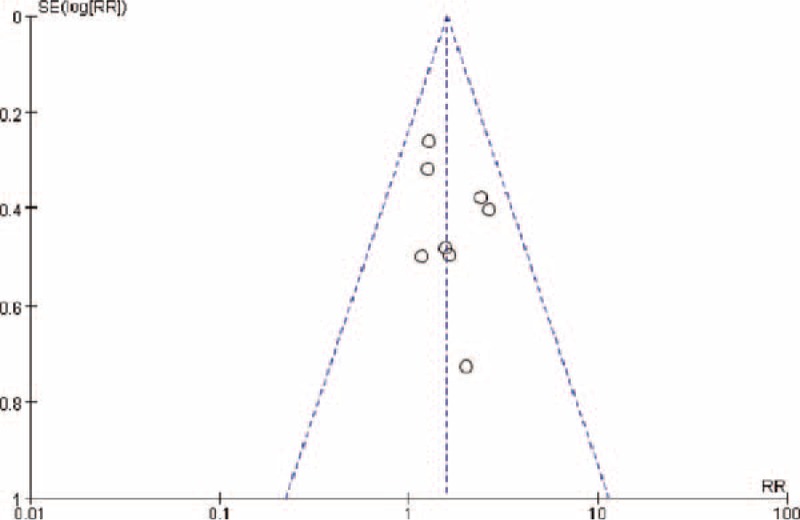
Funnel plot.
DISCUSSION
Many patients with early-stage CMM can be cured by surgery. Patients with advanced disease currently require surgery combined with other therapy. The past decade has given rise to a variety of therapies that hold great promise for the treatment of melanoma, such as immunotherapy with ipilimumab, a BRAF-inhibitor in BRAF-mutant, and so on. But immunotherapy and BRAF-mutant as the new therapy methods give the patients a greater financial burden. In contrast, DTIC is still the most commonly used cytotoxic chemotherapeutic agent for cutaneous and noncutaneous metastatic melanomas.
Biochemotherapy, which is highly cost-effective, is a novel comprehensive treatment mode of biotherapy combined with chemotherapy,14 and is considered to be a more effective adjuvant treatment for CMM than traditional chemotherapy alone. The use of IFN for the treatment of CMM is a research hotspot in biotherapy.14 There are 3 types of IFN, namely α, β, and γ, of which IFN α is the most widely used. To date, recombinant IFN-α including both α-2a and α-2b has been tested in clinical research to treat CMM.23 Five of the 8 eligible articles in our meta-analysis were research IFN-α 2b combined with DTIC versus DTIC alone, two of them were research IFN-α 2a combined with DTIC versus DTIC alone. And one of them was only described as IFN-α combined with DTIC versus DTIC alone.
This systematic review evaluated the efficacy of DTIC in combination with IFN compared with DTIC alone in CMM in terms of 1-, 2-, and 3-year overall survival, adverse reactions, and response rates (OR, CR, PR). The meta-analysis showed that IFN in combination with DTIC significantly increased the CR rate compared with DTIC alone, but did not significantly increase the ORR or PR rate. In agreement with our meta-analysis, Avril et al24 previously reported that IFN with DTIC tended to improve the CR rate in patients with CMM. This effect may be related to the mechanisms of action of IFN, which binds to specific cell surface receptors to promote a variety of cellular activities including the induction of certain enzymes, increased macrophage phagocyte activity, enhanced toxicity of lymphocytes toward target cells and inhibition of the growth of endothelial cells and angiogenesis, which exerts an antitumor effect.25 But clinical trials have shown that only high-dose IFN may be effective.26 However, high-dose IFN induces serious side effects, mainly hematological toxicities, flu-like symptoms, nausea, vomiting, chronic fatigue, and headaches.27
This meta-analysis failed to obtain a precise answer on the value of IFN in combination with DTIC on the survival outcomes of patients with CMM. We found that IFN combined with DTIC may increase the 2-year survival rate compared with DTIC alone, but had no significant effect on 1- and 3-year survival, in agreement with Young et al.18 This may be related to the fact that CMM is highly malignant and has a relatively poor prognosis.28
This meta-analysis showed that IFN combined with DTIC significantly increased the frequency of grade 3 or worse hematologic toxicity, neurotoxicity, and flu-like symptoms compared with DTIC alone. Falkson et al29 also reported that IFN combined with DTIC significantly increased the risk of these toxicities. IFN may increase hematological toxicities for 2 reasons. First, IFN nonspecifically inhibits cell proliferation and exerts a delirious effect on bone marrow cells. Second, IFN induces peripheral blood cell redistribution. IFN also directly and indirectly affects the hypothalamus, alters the hypothalamic-pituitary-gonadal axis, and increases or decreases the levels of neurotransmitters, which lead to neurotoxicity. As an endogenous spyrogen, IFN can also activate the hypothalamic thermoregulatory center by stimulating the synthesis of prostaglandin E, which leads to flu-like symptoms. However, this meta-analysis showed that grade 3 or worse nausea and vomiting were not significantly different between the patients treated with IFN combined with DTIC compared with DTIC alone, consistent with the study of Rudolf et al.30 It is possible that DTIC may induce serious gastrointestinal reactions that cause significant nausea and vomiting, and although IFN can also induce nausea and vomiting, the combination of these treatments may not aggravate this effect. Overall, this study indicates that IFN combined with DTIC could lead to a higher rate of adverse reactions. There was no significant difference in treatment-related mortality between the 2 therapeutic regimens, consistent with the results of previous studies.29,31,32 This may be explained by the fact that when using IFN in combination with DTIC, adverse reactions can be controlled by certain measures such as changing the dose or duration of drug use. Overall, this meta-analysis indicates it is relatively safe to use IFN in patients with CMM.
This systematic review shows that although IFN combined with DTIC can improve the short-term response rate, it does not improve survival and leads to a higher incidence of adverse reactions, consistent with the meta-analysis by Sasse et al.33 The anti-tumor effects of IFN may increase the rate of complete remission in CMM; however, IFN does not significantly prolong survival because of the highly malignant nature of this disease. Additionally, IFN also exerts other physiological effects such as bone marrow suppression, which lead to adverse reactions. Therefore, oncologists should pay close attention to the physical condition of patients with CMM treated with IFN combined with DTIC, to stop or prevent adverse reactions as quickly as possible.
This study failed to confirm a survival advantage for IFN combined with DTIC compared with DTIC alone in the treatment of CMM; this may be because of the limited number of eligible studies and relatively small numbers of patients. In addition, each study in this meta-analysis used a different dose of IFN, which may influence the analysis, especially in terms of adverse reactions. Therefore, the exact therapeutic effects of IFN combined with DTIC compared with DTIC alone in CMM remain to be studied further. There still remains a significant need for better therapies with improved long-term efficacy and decreased toxicity.
Acknowledgments
This project is supported by grants from the National Natural Science Foundation of China (No. 81372916), the ‘Six Talent Peaks’ Project of Jiangsu Province (No. 2013-WSN-014), the Natural Science Foundation of Jiangsu province (No. BK20131131), the Science and Technology Department of Jiangsu province (No. BK20141142), the Science and Technology Project of Xuzhou city (No. KC15SH010), the Innovation of Graduate Student Training Projects in Jiangsu Province of China (No. SJLX15-0726, NO. KYLX15_1477) and Xuzhou Medical Young Talents Project.
Footnotes
Abbreviations: CI = confidence intervals, CMM = cutaneous malignant melanoma, CR = complete response, DTIC = dacarbazine, IFN = interferon, ORR = overall response rate, PD = Progressive disease, PR = Partial response, PR = Partial response, RCTs = randomized controlled trials, RR = relative risk.
YX and QH contributed equally to this article.
The authors report no conflicts of interest.
REFERENCES
- 1.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol 2009; 129:1666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 3.Huang V, Li W, Tsai J, et al. Cancer mortality among Asians and Pacific Islanders in New York City, 2001-2010. J Cancer Epidemiol 2013; 2013:986408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maverakis E1, Cornelius LA, Bowen GM, et al. Metastatic melanoma - a review of current and future treatment options. Acta Derm Venereol 2015; 95:516–524. [DOI] [PubMed] [Google Scholar]
- 5.Daponte A1, Signoriello S, Maiorino L, et al. Phase III randomized study of fotemustine and dacarbazine versus dacarbazine with or without interferon-α inadvanced malignant melanoma. J Transl Med 2013; 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torrence PF, De Clerq E. Inducers and induction of interferon. Pharmacol Ther 1977; 2:1–88. [Google Scholar]
- 7.Berman B, Frankfort HM. The human interferon system. Int J Dermatol 1982; 21:12–18. [DOI] [PubMed] [Google Scholar]
- 8.Huncharek M, Caubet JF, McGarry R. Single-agent DTIC versus combination chemotherapy with or without immunotherapy in metastatic melanoma: a meta-analysis of 3273 patients from 20 randomized trials. Melanoma Res 2001; 11:75–81. [DOI] [PubMed] [Google Scholar]
- 9.Ives NJ, Stowe RL, Lorigan P, et al. Chemotherapy compared with biochemotherapy forthe treatment of metastatic melanoma: a meta-analysis of 18trials involving 2,621 patients. J Clin Oncol 2007; 25:5426–5434. [DOI] [PubMed] [Google Scholar]
- 10.Ascierto PA, Kirkwood JM. Adjuvant therapy of melanoma with interferon:lessons of the past decade. J Transl Med 2008; 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIb melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol 2006; 24:3164–3167. [DOI] [PubMed] [Google Scholar]
- 12.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 2006; 354:709–718. [DOI] [PubMed] [Google Scholar]
- 13.Ascierto PA, Napolitano M, Celentano E, et al. Regulatory T cell frequency in patients with melanoma with different disease stage and course, and modulating effects of high-dose interferon-alpha 2b treatment. J Transl Med 2010; 8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homet B, Ribas A. New drug targets in metastatic melanoma. J Pathol 2014; 232:134–141. [DOI] [PubMed] [Google Scholar]
- 15.Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res 2006; 12:2353s–2358s. [DOI] [PubMed] [Google Scholar]
- 16.Kirkwood JM, Jukic DM, Averbook BJ, et al. Melanoma in pediatric, adolescent, and young adult patients. Semin Oncol 2009; 36:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyrhönen S, Hahka-Kemppinen M, Muhonen T. A promising interferon plus four-drug chemotherapy regimen for metastatic melanoma. J Clin Oncol 1992; 10:1919–1926. [DOI] [PubMed] [Google Scholar]
- 18.Young AM, Marsden J, Goodman A, et al. Prospective randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon-alpha (IFN-alpha) in metastatic melanoma. Clin Oncol (R Coll Radiol) 2001; 13:458–465. [DOI] [PubMed] [Google Scholar]
- 19.WHO: WHO Handbook for Reporting Results of Cancer Treatment. WHO offset publication No. 48. Geneva, Switzerland, World Health Organization, 1979, pp 22–30. [Google Scholar]
- 20.Falkson CI. Experience with interferon alpha 2b combined with dacarbazine in the treatment of metastatic malignant melanoma. Med Oncol 1995; 12:35–40. [DOI] [PubMed] [Google Scholar]
- 21.Galvez CA, Bonamassa M. Advanced malignant melanoma: DTIC plus rIFN-alfa-2b vs DTIC alone. Eur J Cancer 1991; 27 (suppl 2; abstr 932):s155. [Google Scholar]
- 22.Thomson DB, Adena M, McLeod GR, et al. Interferon-alpha 2a does not improve response or survival when combined with dacarbazine in metastatic malignant melanoma: results of a multi-institutional Australian randomized trial. Melanoma Res 1993; 3:133–138. [PubMed] [Google Scholar]
- 23.Ascierto PA1, Palmieri G, Parasole R, et al. 3-year treatment with recombinant interferon-alpha as adjuvant therapy of cutaneous malignant melanoma. Int J Mol Med 1999; 3:303–306. [DOI] [PubMed] [Google Scholar]
- 24.Avril MF, Beerblock K, Dreno B, et al. Treatment of metastatic melanoma with dacarbazine recombinant interferon alfa 2A combination: results of multicentric study. Bull Cancer 1990; 77:1183–1191. [PubMed] [Google Scholar]
- 25.Petrella T, Verma S, Spithoff K, et al. Adjuvant interferon therapy for patients at high risk for recurrent melanoma: an updated systematic review and practice guideline. Clin Oncol (R Coll Radiol) 2012; 24:413–423. [DOI] [PubMed] [Google Scholar]
- 26.Mocellin S1, Lens MB, Pasquali S, et al. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev 2013; 6:CD008955.doi:10.1002/14651858.CD008955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozera C, Cappellini GA, D’Agostino G, et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma. J Transl Med 2015; 13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao M, Song F, Du X, et al. Advances in targeted therapy for unresectable melanoma: new drugs and combinations. Cancer Lett 2015; 359:1–8. [DOI] [PubMed] [Google Scholar]
- 29.Falkson CI, Ibrahim J, Kirkwood JM, et al. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: an Eastern Cooperative Oncology Group study. J Clin Oncol 1998; 16:1743–1751. [DOI] [PubMed] [Google Scholar]
- 30.Rudolf Z, Strojan P. DTIC vs. IFN-alpha plus DTIC in the treatment of patients with metastatic malignant melanoma. Neoplasma 1995; 43:93–97. [PubMed] [Google Scholar]
- 31.Bajetta E, Di Leo A, Zampino MG, et al. Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. J Clin Oncol 1994; 12:806–811. [DOI] [PubMed] [Google Scholar]
- 32.Falkson CI, Falkson G, Falkson HC. Improved results with the addition of interferon alfa-2b to dacarbazine in the treatment of patients with metastatic malignant melanoma. J Clin Oncol 1991; 9:1403–1408. [DOI] [PubMed] [Google Scholar]
- 33.Sasse A, Sasse E, Clark L, et al. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev 2007; CD005413. [DOI] [PubMed] [Google Scholar]


