Abstract
Intermedin (IMD), an autocrine/paracrine biologically active peptide, plays a critical role in maintaining vascular homeostasis. Recent research has shown that high plasma levels of IMD are associated with poor outcomes for patients with ST-segment elevation acute myocardial infarction. However, the prognostic utility of IMD levels in non-ST-segment elevation acute coronary syndrome (NSTE-ACS) has not yet been investigated. We hypothesized that the level of plasma IMD would have prognostic value in patients with NSTE-ACS.
Plasma IMD was determined by radioimmunoassay in 132 NSTE-ACS patients on admission to hospital and 132 sex- and age-matched healthy-control subjects. Major adverse cardiovascular events (MACEs), including death, heart failure, hospitalization, and acute myocardial infarction, were noted during follow-up. In total, 23 patients suffered MACEs during the follow-up period (mean 227 ± 118 days, range 2–421 days). Median IMD levels were higher in NSTE-ACS patients than control [320.0 (250.9/384.6) vs. 227.2 (179.7/286.9) pg/mL, P <0.001]. The area under the receiver-operating characteristic curve for IMD and N-terminal pro-B-type brain natriuretic peptide (NT-proBNP) did not significantly differ (0.73 and 0.79, both P <0.001, respectively; P = 0.946). ROC curve analysis revealed a cut-off value for IMD at 340.7 pg/mL. Cox regression analysis with cardiovascular risk variables and NT-proBNP showed that the risk of MACEs increased by a factor of 12.96 (95% CI, 3.26–49.42; P <0.001) with high IMD levels (at the cut-off value).
IMD has potential as a prognostic biomarker for predicting MACEs in patients with NSTE-ACS.
INTRODUCTION
Non-ST-segment elevation acute coronary syndrome (NSTE-ACS) refers to a spectrum of clinical presentations ranging from non-ST-segment elevation myocardial infarction (NSTEMI) to unstable angina. Each year, NSTE-ACS is responsible for approximately 2.5 million hospital admissions worldwide with an in-hospital mortality rate of approximately 5%.1,2 Early risk stratification can help identify high-risk patients who may benefit from early treatment with aggressive antithrombotic treatment, angiography, and coronary revascularization.2
Intermedin, also called adrenomedullin 2 (ADM2), is a recently defined member of the calcitonin gene-related peptide (CGRP) family that may have a range of biological effects because of its wide tissue distribution and nonselectivity for CGRP family receptors.3 Animal studies have indicated that intermedin (IMD) plays a critical role in the regulation of blood pressure and cardiac function, acceleration of angiogenesis, and has antiapoptotic functions.4–6 Moreover, IMD is involved in the pathogenesis of cardiovascular diseases such as hypertension, atherosclerosis, and vascular calcification.7–9 In humans, the plasma concentration of IMD is elevated in various cardiovascular diseases, including acute myocardial infarction (AMI)10 and ACS.11 A high transcription level of IMD has also been observed in the leukocytes of patients with chronic heart failure, correlating with clinical severity.12 A recent clinical trial showed that elevated plasma IMD level is associated with poor outcomes in patients with ST-segment elevation acute myocardial infarction (STEMI), and it has been added as a prognostic indicator to the Global Registry of Acute Coronary Events (GRACE) scoring system.13
Biomarkers such as N-terminal pro-B-type brain natriuretic peptide (NT-proBNP) have helped identify patients at risk of poor outcomes and are used to guide therapy.14–17 For better stratification, a multimarker strategy has become popular and may be more effective for risk assessment in NSTE-ACS.15 Thus, new peptides that provide prognostic information, particularly those independent of the abovementioned biomarkers, may be useful additions to a multimarker strategy for identifying patients at the highest risk and who have the most to gain from effective therapies.
The prognostic utility of IMD levels in NSTE-ACS has not yet been assessed, so we analyzed its prognostic performance relative to major adverse cardiovascular events (MACEs) in a cohort of patients with NSTE-ACS and compared its sensitivity to that of NT-proBNP, a peptide of established prognostic value in these patients.14,16,17
METHODS
Study Population
We recruited 132 NSTE-ACS patients admitted to Aerospace Central Hospital (a training hospital of Peking University, Haidian district, Beijing, China), between March and September 2014 who presented with at least 10 minutes of ischemic symptoms of unstable coronary artery disease and clear signs of myocardial ischemia, such as electrocardiographic changes (ST-segment decrease ≥0.1 mV or T-wave inversion ≥0.1 mV) or elevated levels of biochemical markers of myocardial necrosis.17 The exclusion criteria were known malignancy, surgery, or renal replacement therapy in the previous month. All patients received standard medical treatment, and revascularization was at the discretion of the attending physician. Plasma IMD levels were also measured in 132 healthy sex- and age-matched controls, who were given physical examinations at our hospital during the same period. Those with diabetes, hyperlipidemia, liver or kidney diseases, hypertension, and other cardiovascular diseases were excluded. The study complied with the Declaration of Helsinki and was approved by the ethics committees of the hospital and the Peking University Health Science Center; written informed consent was obtained from all patients.
Laboratory Methods
Routine laboratory variables including NT-proBNP, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), myoglobin (MYO), creative kinase MB (CK-MB), and troponin I were measured immediately after taking blood samples by standardized methods. The estimated glomerular filtration rate (eGFR) was calculated using the abbreviated equation for modification of diet in renal disease.18 For IMD testing, blood samples were placed into tubes containing disodium ethylenediamine tetraacetic acid (1 mg/mL) and aprotinin (500 KIU/mL; Sigma, St. Louis, MO) and centrifuged immediately at 3500 × g for 10 minutes at 4°C; plasma was stored at −80°C.
Radioimmunoassay Determination of IMD Level
Plasma samples were extracted using a SepPak C18 cartridge as described,19 and were assayed with an IMD radioimmunoassay kit (Phoenix Pharmaceuticals, Belmont, CA). The IC50 of human IMD was 100.10 pg per tube, and the cross-reactivity with human IMD was 100%, with no cross-reactivity between human CGRP and AMD (adrenomedullin). All biomarker testing was performed by staff who were blinded to the clinical outcomes.
ENDPOINT
The composite endpoint was MACEs, including death, hospitalization for heart failure, and AMI.16 Patients were individually contacted by telephone, and events were characterized using hospital charts and available outpatient information.
Statistical Analysis
Normally distributed variables are described using mean ± SD and abnormally distributed variables with median (25th and 75th quartiles). Categorical variables are expressed as percentages. Differences between categorical variables were analyzed by χ2 test, and continuous variables by ANOVA. Nonparametric tests were used for abnormally distributed data (Mann–Whitney U or Kruskal–Wallis test, Wilcoxon signed-rank test and Spearman [rs] correlations). Plasma level of IMD was categorized in tertiles. To evaluate effects over time, we performed Kaplan–Meier analysis according to IMD levels on admission (in tertiles). To compare the prognostic utility of levels of IMD and NT-proBNP, receiver-operating characteristic (ROC) curves were generated and the area under the ROC curve (AUC) was calculated. The cut-off value for IMD level was determined by the maximum value on the Youden index. To assess the relative prognostic accuracy of IMD, a Cox regression model introducing IMD levels as a categorical variable (using the cut-off value) adjusted for normative risk factors (age, sex, body mass index, hypertension, diabetes, hyperlipidemia, family history, current cigarette smoking, eGFR) and NT-proBNP level (NT-proBNP was log10-transformed; thus, the hazard ratio refers to a 10-fold increase in the level of NT-proBNP).20 In all tests, P <0.05 was considered statistically significant. SPSS 22.0 (IBM, Armonk, NY) was used for analysis.
RESULTS
Patient Characteristics
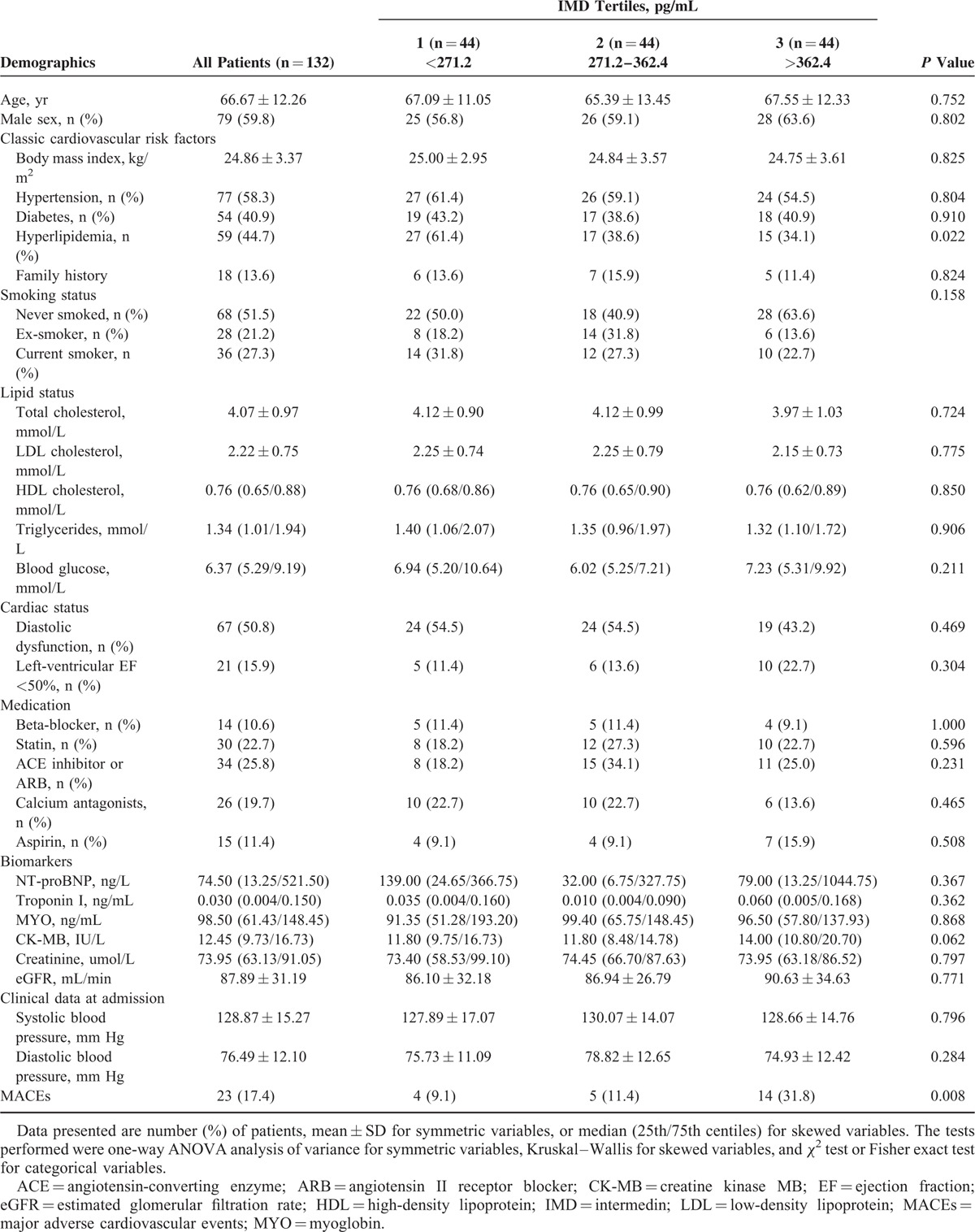
Patients’ demographic data are set out in Table 1. The study population was 100% ethnic Han Chinese. No patient was omitted from follow-up, whose mean duration was 227 ± 118 days (ranging from 2 to 421 days). During follow-up, 4 (3.0%) patients died, 6 (4.5%) had AMI, and 13 (9.8%) were readmitted to hospital.
TABLE 1.
Characteristics of the 132 NSTE-ACS Patients According to IMD Levels on Admission
Subject IMD Levels
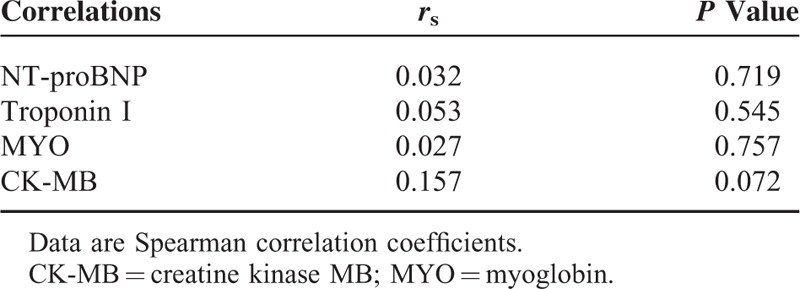
The median plasma level of IMD in patients with NSTE-ACS was 320.0 (range, 73.0–2121.9) pg/mL. Plasma IMD concentration was higher in NSTE-ACS patients than in the control group [320.0 (250.9/384.6) vs. 227.2 (179.7/286.9) pg/mL, P <0.001]. IMD was higher in patients with subsequent MACEs than in MACE-free survivors [380.9 (345.2/470.8) vs. 309.0 (241.2/371.6) pg/mL, P <0.001]. IMD concentrations were lower in patients with hyperlipidemia than in those without (P = 0.019), with no difference noted between other subgroups of patients (Table 2). Of the biomarkers NT-proBNP, Troponin I, MYO, and CK-MB, none correlated with IMD (Table 3).
TABLE 2.
Comparison of IMD Levels in Different Patient Subgroups
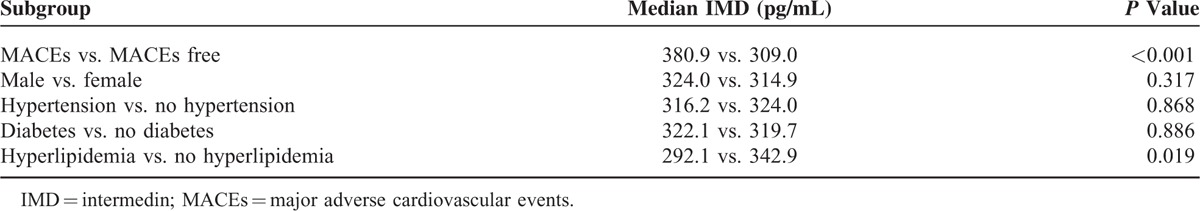
TABLE 3.
Correlation Between IMD Concentrations and Other Biomarkers
IMD Has Utility as a Predictor of MACEs in NSTE-ACS Patients
Kaplan–Meier analysis of plasma IMD level on admission, with the cohort separated into 3 groups by tertiles (<271.2; 271.2–362.4; >362.4 pg/mL), identified patients at low, medium, and high risk of MACEs (log-rank test: 10.10, P = 0.006), with early separation of the survival plot (Figure 1). A threshold effect was apparent, because the risk of MACEs was broadly similar in patients of the 2 lower 2 tertiles of IMD level, while a significantly increased risk was noted in subjects belonging to the upper tertile. The AUCs for the IMD and NT-proBNP levels were 0.73 (95% CI, 0.62–0.85, P <0.001) and 0.79 (95% CI 0.68–0.91, P <0.001) respectively, with no significant difference between the AUCs (P = 0.946) (Figure 2). ROC curve analysis with determination of the Youden index for the follow-up time revealed a cut-off value of 340.7 pg/mL, with a sensitivity of 82.6% and specificity of 64.2%, a negative likelihood ratio of (LR−) of 0.27 and a positive likelihood ratio (LR+) of 2.31.
FIGURE 1.

Kaplan–Meier plot of cumulative MACEs rates associated with IMD levels. IMD = intermedin; MACEs = major adverse cardiovascular events.
FIGURE 2.
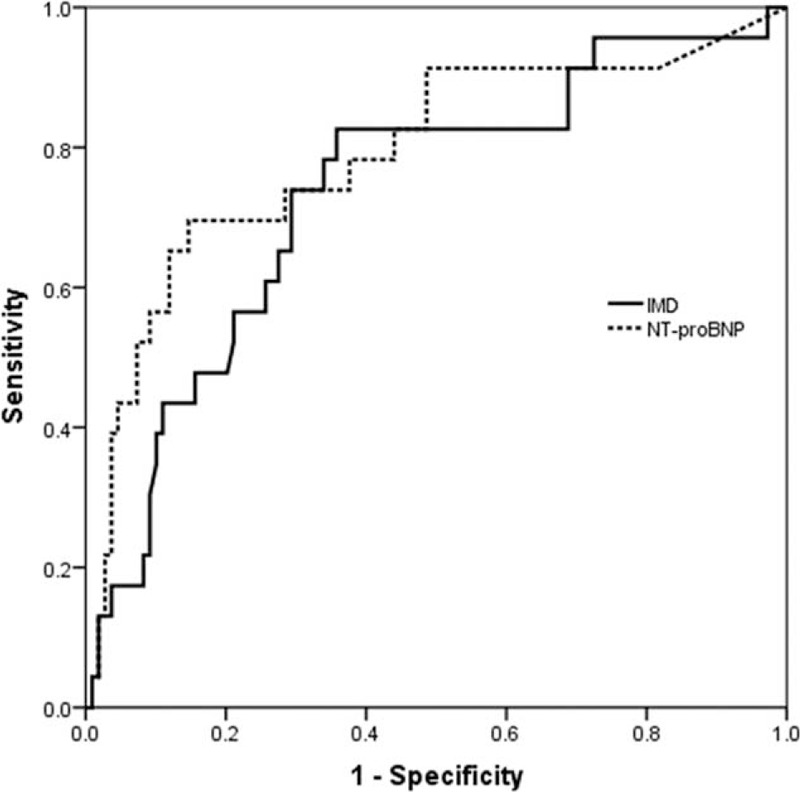
Receiver operating characteristic curve for IMD and NT-proBNP. IMD = intermedin.
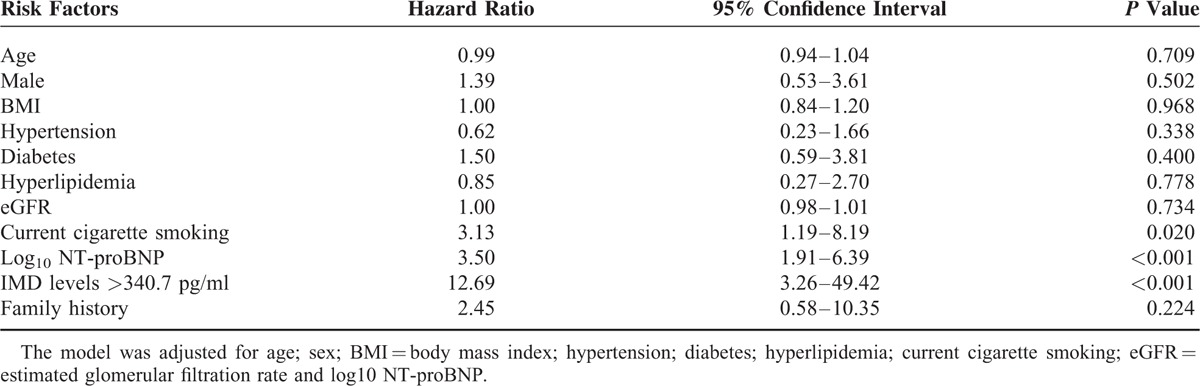
High IMD (> 340.7 pg/mL) NSTE-ACS subjects had an adjusted hazard ratio for MACEs of 12.96 (95% CI, 3.26–49.42; P <0.001) (Table 4). The correlation between IMD level and risk of MACEs was independent in the overall sample (Table 4).
TABLE 4.
Multivariate Cox Proportional Hazards Regression Model of MACEs
DISCUSSION
IMD is an autocrine/paracrine biologically active peptide, identified independently in 2004 by 2 groups of researchers,21,22 It is expressed in a wide range of tissues including the gastrointestinal tract, submaxillary gland, lungs, skin, cardiovascular and renal systems, hypothalamus, pituitary gland, placenta, pancreas, spleen, thymus, uterus, and ovaries.21–24 Notably, it is present in endothelial cells and is up-regulated in stressed myocardial cells.9,24–26 Since its discovery, IMD has attracted interest in cardiovascular research because of its multiple cardiovascular biological functions, which include induction of vasodilation and hypotension,21,24,27 increasing cardiac perfusion,28 and stabilizing endothelial barriers,29 attenuating myocardial infarction through activation of autophagy,30 as well as lowering serum LDL-C and total cholesterol levels,31 deposition of vascular calcium, alkaline phosphatase activity.7
This study assesses the prognostic utility of IMD levels in a prospective observational cohort of NSTE-ACS patients at a single center. The plasma level of IMD was higher in NSTE-ACS patients than in healthy controls. Plasma IMD levels were higher in patients with subsequent MACEs than without and were lower in patients with hyperlipidemia than in those without. Moreover, survival analysis showed that IMD level was an independent predictor of MACEs with a prognostic accuracy comparable to that of NT-proBNP level. The risk of MACEs in NSTE-ACS patients with high levels of plasma IMD increased 12.69-fold (with a cut-off value set at 340.7 pg/mL).
Several clinical studies have focused on the role of IMD in human cardiovascular diseases. Plasma IMD level has been found to be markedly elevated in ACS patients and correlative to BNP and CK-MB levels and Gensini scores, which reflect the severity of coronary stenosis.11 Plasma IMD levels were elevated in patients with stable coronary heart disease and AMI, and were higher in AMI patients with stenotic lesions ≥75% in all 3 coronary branches than in those patients with this level of lesions in just 1 or 2 coronary branches.10 Another prospective study of 128 STEMI patients showed that elevated plasma IMD level is an independent predictor of 6-month MACEs, with a predictive value similar to GRACE scores.13 In the present study, plasma IMD level was significantly elevated in NSTE-ACS patients. Higher levels of IMD were observed in patients who went on to present with MACEs than in those who were MACE-free throughout the follow-up period. Plasma IMD level retains its independent prognostic accuracy even after adjustment for NT-proBNP level and other normative risk factors in NSTE-ACS. IMD appears to play an important role in the progression of coronary heart disease and the plasma level may reflect its pathophysiologic roles in the natural course of coronary heart disease as well as correlating to the severity of the disease.
We found lower IMD levels in patients with than without hyperlipidemia. This finding agrees with earlier studies showing that IMD treatment can modify serum lipid profiles, decreasing serum total cholesterol and LDL-C levels and increasing the level of HDL-C,30 as well as it can reduce LDL uptake and intracellular cholesterol content in macrophages.8,32 The lower IMD levels in hyperlipidemic patients could be an indication of the beneficial effects of IMD on the lipid metabolism. However, plasma IMD and its receptor components are found elevated in hypercholesterolemia rats.33 This contradiction may be due to the course of hypercholesterolemia. In the early stage, up-regulated IMD may protect against hypercholesterolemia; with the progression of the disease, the secretion of IMD may somehow be impaired. Further studies are needed to better understand the role of IMD in hypercholesterolemia.
Unlike BNP, the primary source of plasma IMD remains unclear.21 Although there are studies demonstrating that IMD is involved in the gene expression of BNP in cardiac tissue, how IMD affects plasma BNP is not addressed yet. Study of Yuan et al found up-regulated gene expression of BNP in spontaneously hypertensive rats when given exogenous IMD. That may be a secondary response to the antidiuretic effect of IMD and synergize the cardioprotective effect of IMD.34 While another study showed that IMD significantly decreased BNP expression in heart of hypertrophic mouse and improved cardiac functions.35 In this study, we did not find any correlation between IMD and biomarkers of myocardial necrosis (troponin I, MYO, or CK-MB) or hemodynamic stress (NT-proBNP). This finding is consistent with that of Lv et al10 for AMI but contradicts that of Qin et al11 for ACS. However, these studies were conducted in a variety of clinical settings and with different criteria for inclusion and exclusion. It should also be noted that other mechanisms, beyond cell injury and hemodynamic stress, may be causative in the elevated levels of IMD in NSTE-ACS.
Our study has some limitations. First, this was a single-center study with very small cohort. Second, the incidence of MACEs was low in our NSTE-ACS population, and the follow-up period relatively short, thus our data may not fully reflect the prognostic utility of IMD levels over the longer term. Third, in this study the biochemical indicators of healthy controls were in the normal range, and subjects presenting with hypertension, diabetes, hyperlipidemia, liver or kidney diseases, and cardiovascular diseases were excluded, with the result that detailed parameters were not established. One further limitation is that some polymorphisms linked to endothelial dysfunction and CAD, such as CaMK4 (calmodulin-dependent protein kinase 4),36 PlA2 (phospholipase A2),37,38 and GRKs (G protein-coupled receptor kinases),39 are not discussed.
CONCLUSION
In summary, we found elevated plasma IMD levels in NSTE-ACS patients. NSTE-ACS patients with subsequent MACEs were found to have higher IMD plasma concentrations than those whose follow-up periods were MACE-free, while the risk of MACEs increased 12.96-fold in NSTE-ACS patients presenting with high IMD plasma levels (at the cut-off value of 340.7 pg/mL). IMD level could thus be a useful additional prognostic tool in cases of NSTE-ACS.
Footnotes
Abbreviations: ACE = angiotensin-converting enzyme, ACS = acute coronary syndrome, ADM2 = adrenomedullin 2, AMD = adrenomedullin, AMI = acute myocardial infarction, ARB = angiotensin II receptor blocker, AUC = area under the ROC curve, BMI = body mass index, BNP = brain natriuretic peptide, CAD = coronary artery disease, CaMK4 = calmodulin-dependent protein kinase 4, CGRP = calcitonin gene-related peptide, CI = confidence interval, CK-MB = creatine kinase MB, EF = ejection fraction, eGFR = estimated glomerular filtration rate, GRACE = global registry of acute coronary events, GRKs = G protein-coupled receptor kinases, HDL-C = high-density lipoprotein cholesterol, IMD = intermedin, LDL-C = low-density lipoprotein cholesterol, LR = likelihood ratio, MACEs = major adverse cardiovascular events, MYO = myoglobin, NSTE-ACS = non-ST-segment elevation acute coronary syndrome, NSTEMI = non-ST-segment elevation myocardial infarction, NT-proBNP = N-terminal pro-B-type brain natriuretic peptide, PlA2 = phospholipase A2, ROC = receiver operating characteristic, STEMI = ST-segment elevation myocardial infarction.
PL and LS contributed equally to the writing of this article and share primary authorship.
This study is supported by the Research Foundation of Aerospace Central Hospital (no. YN201316 to BW) and the National Natural Sciences Foundation of China (nos. 91339203, 81170082, and 81270407 to YQ).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Braunwald E. Unstable angina and non-ST elevation myocardial infarction. Am J Respir Crit Care Med 2012; 185:924–932. [DOI] [PubMed] [Google Scholar]
- 2.Grech ED, Ramsdale DR. Acute coronary syndrome: unstable angina and non-ST segment elevation myocardial infarction. BMJ 2003; 326:1259–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni X, Zhang J, Tang C, et al. Intermedin/adrenomedullin2: an autocrine/paracrine factor in vascular homeostasis and disease. Science China. Life Sci 2014; 57:781–789. [DOI] [PubMed] [Google Scholar]
- 4.Guo XJ, Schmitz JC, Kenney BC, et al. Intermedin is overexpressed in hepatocellular carcinoma and regulates cell proliferation and survival. Cancer Sci 2012; 103:1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Wang LJ, Xiao F, et al. Intermedin: a novel regulator for vascular remodeling and tumor vessel normalization by regulating vascular endothelial-cadherin and extracellular signal-regulated kinase. Arterioscl Throm Vas 2012; 32:2721–2730. [DOI] [PubMed] [Google Scholar]
- 6.Du XD, Cao Y, Xue P, et al. Protective effect of intermedin on myocardial cell in a rat model of severe acute pancreatitis. Cell Mol Biol Lett 2011; 16:462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Xu MJ, Teng X, et al. Intermedin inhibits vascular calcification by increasing the level of matrix gamma-carboxyglutamic acid protein. Cardiovasc Res 2010; 85:864–873. [DOI] [PubMed] [Google Scholar]
- 8.Dai XY, Cai Y, Mao DD, et al. Increased stability of phosphatase and tensin homolog by intermedin leading to scavenger receptor A inhibition of macrophages reduces atherosclerosis in apolipoprotein E-deficient mice. J Mol Cell Cardiol 2012; 53:509–520. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Q, Yuan Y, Wang X, et al. Upregulated expression of intermedin and its receptor in the myocardium and aorta in spontaneously hypertensive rats. Peptides 2009; 30:391–399. [DOI] [PubMed] [Google Scholar]
- 10.Lv ZB, Wu K, Chen XP, et al. Plasma intermedin levels in patients with acute myocardial infarction. Peptides 2013; 43:121–125. [DOI] [PubMed] [Google Scholar]
- 11.Qin YW, Teng X, He JQ, et al. Increased plasma levels of intermedin and brain natriuretic peptide associated with severity of coronary stenosis in acute coronary syndrome. Peptides 2013; 42:84–88. [DOI] [PubMed] [Google Scholar]
- 12.Manuela C, Laura S, Benedetta S, et al. Adrenomedullin and intermedin gene transcription is increased in leukocytes of patients with chronic heart failure at different stages of the disease. Peptides 2014; 55:13–16. [DOI] [PubMed] [Google Scholar]
- 13.Tang B, Zhong Z, Shen HW, et al. Intermedin as a prognostic factor for major adverse cardiovascular events in patients with ST-segment elevation acute myocardial infarction. Peptides 2014; 58:98–102. [DOI] [PubMed] [Google Scholar]
- 14.Omland T, Persson A, Ng L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation 2002; 106:2913–2918. [DOI] [PubMed] [Google Scholar]
- 15.O’Malley RG, Bonaca MP, Scirica BM, et al. Prognostic performance of multiple biomarkers in patients with non-ST-segment elevation acute coronary syndrome: analysis from the MERLIN-TIMI 36 trial (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes-Thrombolysis In Myocardial Infarction 36). J Am Coll Cardiol 2014; 63:1644–1653. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon OS, Khan SQ, Narayan HK, et al. Prognostic value of mid-regional pro-adrenomedullin levels taken on admission and discharge in non-ST-elevation myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) II study. J Am Coll Cardiol 2010; 56:125–133. [DOI] [PubMed] [Google Scholar]
- 17.Eggers KM, Lagerqvist B, Venge P, et al. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2009; 54:357–364. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53:766–772. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto R, Satoh F, Murakami O, et al. Expression of adrenomedullin2/intermedin in human brain, heart, and kidney. Peptides 2007; 28:1095–1103. [DOI] [PubMed] [Google Scholar]
- 20.Santulli G. Coronary heart disease risk factors and mortality. JAMA 2012; 307:1138. [DOI] [PubMed] [Google Scholar]
- 21.Roh J, Chang CL, Bhalla A, et al. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 2004; 279:7264–7274. [DOI] [PubMed] [Google Scholar]
- 22.Takei Y, Inoue K, Ogoshi M, et al. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. Febs Lett 2004; 556:53–58. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Kikuchi K, Maruyama Y, et al. Immunocytochemical localization of adrenomedullin 2/intermedin-like immunoreactivity in human hypothalamus, heart and kidney. Peptides 2006; 27:1383–1389. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y, Liu YJ, Gonda T, et al. Coronary vasodilatory response to a novel peptide, adrenomedullin 2. Clin Exp Pharmacol Physiol 2004; 31 Suppl 2:S49–S50. [DOI] [PubMed] [Google Scholar]
- 25.Bell D, Zhao YY, Mccoy FPG, et al. Differential effects of an anti-oxidant intervention on cardiomyocyte expression of adrenomedullin and intermedin and their receptor components in chronic nitric oxide deficiency. Cell Physiol Biochem 2007; 20:269–282. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Bell D, Smith LR, et al. Differential expression of components of the cardiomyocyte adrenomedullin/intermedin receptor system following blood pressure reduction in nitric oxide-deficient hypertension. J Pharmacol Exp Ther 2006; 316:1269–1281. [DOI] [PubMed] [Google Scholar]
- 27.Abdelrahman AM, Pang CC. Vasodilator mechanism of intermedin/adrenomedullin-2 in anesthetized rats. Proc West Pharmacol Soc 2007; 50:43–46. [PubMed] [Google Scholar]
- 28.Grossini E, Molinari C, Mary DA, et al. Intracoronary intermedin 1-47 augments cardiac perfusion and function in anesthetized pigs: role of calcitonin receptors and beta-adrenoreceptor-mediated nitric oxide release. J Appl Physiol 2009; 107:1037–1050. [DOI] [PubMed] [Google Scholar]
- 29.Aslam M, Pfeil U, Gunduz D, et al. Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin-induced barrier failure in endothelial cell monolayers. Br J Pharmacol 2012; 165:208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei P, Yang XJ, Fu Q, et al. Intermedin attenuates myocardial infarction through activation of autophagy in a rat model of ischemic heart failure via both cAMP and MAPK/ERK1/2 pathways. Int J Clin Exp Pathol 2015; 8:9836–9844. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Gu L, Chen X, et al. Intermedin ameliorates atherosclerosis in ApoE null mice by modifying lipid profiles. Peptides 2012; 37:189–193. [DOI] [PubMed] [Google Scholar]
- 32.Dai XY, Cai Y, Sun W, et al. Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA. Cardiovasc Res 2014; 101:297–305. [DOI] [PubMed] [Google Scholar]
- 33.Meng Q, Shi D, Feng J, et al. Hypercholesterolemia up-regulates the expression of intermedin and its receptor components in the aorta of rats via inducing the oxidative stress. Ann Clin Lab Sci 2016; 46:5–17. [PubMed] [Google Scholar]
- 34.Yuan Y, Wang X, Zeng Q, et al. Effects of continuous intermedin infusion on blood pressure and hemodynamic function in spontaneously hypertensive rats. J Geriatr Cardiol 2012; 9:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Wang X, Tong M, et al. Intermedin suppresses pressure overload cardiac hypertrophy through activation of autophagy. PLoS One 2013; 8:e64757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc 2012; 1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanni F, Santulli G, Izzo R, et al. The Pl (A1/A2) polymorphism of glycoprotein IIIa and cerebrovascular events in hypertension: increased risk of ischemic stroke in high-risk patients. J Hypertens 2007; 25:551–556. [DOI] [PubMed] [Google Scholar]
- 38.Galasso G, Santulli G, Piscione F, et al. The GPIIIA PlA2 polymorphism is associated with an increased risk of cardiovascular adverse events. BMC Cardiovasc Disord 2010; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santulli G, Trimarco B, Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev 2013; 20:5–12. [DOI] [PubMed] [Google Scholar]


