Supplemental Digital Content is available in the text
Abstract
To formulate therapy goals, we aimed to define the relationship between fecal calprotectin and health-related quality of life in inflammatory bowel diseases (IBDs).
This retrospective single-center cross-sectional study included ambulatory IBD patients who had completed standardized questionnaires comprising items of health-related quality of life (Short Inflammatory Bowel Disease Questionnaire) and clinical disease activity scores, and who had provided stool samples for calprotectin determination within 30 days of questionnaire completion. Correlation analyses were performed between the indicated parameters. Post hoc analysis was conducted, taking into account only data from patients with fecal calprotectin concentrations measured within 3 days of questionnaire completion.
One hundred ninety-seven patients with Crohn disease and 111 patients with ulcerative colitis were enrolled in the study. Lower fecal calprotectin concentrations were associated with better health-related quality of life. The correlations were weak, but stronger if only fecal calprotectin concentrations measured within 3 days of questionnaire completion were included (results for 3 days; Crohn disease: n = 86, rS = −0.419, P < 0.001; ulcerative colitis: n = 43, rS = −0.432, P = 0.004). In Crohn disease, a significant correlation between fecal calprotectin concentration and health-related quality of life was found in patients with colonic involvement (n = 59, rS = −0.470, P < 0.001), but not in patients with purely ileal disease (n = 27, rS = −0.268, P = 0.18). Correlations between fecal calprotectin concentrations and clinical disease activity were also only weak to moderate.
Owing to its moderate correlation with fecal calprotectin concentrations in IBD patients with colonic involvement, health-related quality of life should be used in combination with other markers for IBD management. This is even more important in isolated ileal Crohn disease, where no significant correlation between fecal calprotectin concentration and health-related quality of life was found. Especially for use in research studies, care should be taken to keep the time between clinical evaluation of IBD patients and the determination of fecal calprotectin concentrations as short as possible.
INTRODUCTION
Inflammatory bowel diseases (IBDs)–comprising Crohn disease (CD) and ulcerative colitis (UC) as the 2 main entities–are chronic progressive diseases that predominantly affect young people. Optimal IBD management requires well-defined therapy goals. Currently, one of the biggest challenges is discerning the outcome parameters best suited for an individual patient. At one end of the spectrum is the patient's health-related quality of life (HRQoL); at the other, the severity of mucosal inflammation. For the patient, improvement of HRQoL is the most urgent therapy goal. Reduced HRQoL in IBD has been shown to relate to higher rates of sick leave and decreased work participation,1,2,3,4 which may have far-reaching consequences for the patients’ private and professional lives. Recently, it has been increasingly recognized that patient-reported outcomes encompassing mental, physical, social, and emotional aspects should influence disease management decisions.1,5 There is clear evidence that clinical activity of IBD is negatively correlated with HRQoL.6,7 Moreover, a negative correlation between endoscopic disease activity and HRQoL in IBD patients has previously been shown.8–10
However, evidence-based recommendations for therapy goals in IBD are more sophisticated. Mucosal healing or even histologic remission are increasingly considered more appropriate therapy aims than clinical disease activity,11,12,13 as they coincide with better rates of long-term remission and thus better prognoses.14,15 Fecal calprotectin (FC) is a suitable tool to assess mucosal inflammation and healing, obviating the need for direct visualization by endoscopy.16 It correlates better with endoscopic findings than with clinical activity and is more suitable than C-reactive protein (CRP) to detect active IBD.17–20 Therefore, FC is increasingly used as a biomarker for IBD monitoring.17 Interestingly, a prospective study by Voiosu et al21—including 19 CD patients and 29 UC patients in clinical remission—revealed that FC concentrations >30 μg/g showed a 93% sensitivity and a 50% specificity for detecting endoscopically visible inflammatory changes of the mucosa, while a combined test using FC >30 μg/g and SIBDQ <60 achieved an 81.2% sensitivity and a 75% specificity for detecting active endoscopic disease.
Physicians at our IBD outpatient clinic are often confronted with cases of disparity between HRQoL and FC concentrations. Routine use of FC raises the question of how to proceed in these cases. Therefore, we sought to quantify the relationship between FC and HRQoL in IBD patients in an exploratory study without prespecified hypothesis. To our knowledge, no published studies exist that investigated this relationship as their primary goal. To compare our data with data from previous studies, secondary objectives were to analyze the relationships between FC, humoral biomarkers and clinical disease activity, and between clinical disease activity and HRQoL.
METHODS
Study Design and Ethical Considerations
This is a retrospective single-center cross-sectional analysis of data collected at the IBD outpatient clinic of the Heidelberg University Hospital, a tertiary care center in Southwest Germany. The study protocol was approved by the local institutional ethics review board (Ethikkommission der Medizinischen Fakultät Heidelberg, Alte Glockengießerei 11/1, D-69115 Heidelberg; protocol number: S-619/2014; date of approval: 5 January, 2015). It is in accordance with the Declaration of Helsinki.
Eligibility Criteria
All patients who presented at the IBD outpatient clinic of the Heidelberg University Hospital at least once between February 1, 2012, and February 28, 2015, were potentially eligible. The inclusion criteria were diagnosis of CD or UC according to ECCO criteria,22,23 age between 18 and 80 years, availability of at least 1 standardized IBD questionnaire with a fully completed HRQoL section, and availability of at least 1 FC result obtained within 30 days before or after the visit to the outpatient clinic. The length of this time span was determined empirically. It was a compromise between a sufficient number of eligible patients and an interval short enough to exclude excessive changes of disease activity between the 2 measuring points based on our clinical experience. The exclusion criteria were diagnosis of IBD unclassified; presence of ileo- or colostomy, or status-post subtotal or total colectomy; known infection of the gastrointestinal tract, including infection with cytomegalovirus at the time of presentation at the outpatient clinic, or the time of FC determination; and regular, not on-demand use of nonsteroidal anti-inflammatory drugs. As the effect of proton pump inhibitors on FC concentrations was suggested to be small in comparison with the effect exerted by IBD, we did not exclude patients on proton pump inhibitor therapy from the study.
Determination of FC and Humoral Biomarkers
The decision on whether a patient's FC would be measured was individually made by the treating physician. Most physicians prompted FC measurement on a regular basis for therapy control. Fecal samples were analyzed using a commercially available quantitative enzyme-linked immunosorbent assay (fCAL ELISA, Bühlmann Laboratories AG, Schönenbuch, Switzerland) with a working range of 30 μg/g to 1800 μg/g. As further sample dilution is not performed in our routine clinical practice, concentrations above this range were recorded as >1800 μg/g. According to Sipponen et al,24 who determined a positive predictive value of 94% for endoscopic activity in CD patients with a cutoff FC concentration at 200 μg/g, patients with FC concentrations <200 μg/g were classified as having inactive disease. CRP and leukocyte counts were used as humoral biomarkers and determined by routine methods on the day of questionnaire completion.
Data Collection
All standardized IBD questionnaires available from February 2012 to February 2015 were screened. The paper questionnaire in German language—containing demographic, clinical, and psychological items on 4 pages–was developed in our department. The questionnaires are meant to be given to all IBD patients at every visit to the IBD outpatient clinic before their doctors see them. They are handed out by the receptionists, and their completion is voluntary. However, they were not consistently distributed during phases of staff shortage, and it was not documented how many patients received the questionnaires. After recruitment of all sufficiently completed questionnaires, the hospital's electronic documentation system was used to determine whether current FC results were available for the identified cases. To avoid duplication of cases belonging to 1 patient, only the most recent record for which FC was available was included per random decision. Additional information like laboratory results, comorbidities, disease extent and disease behavior according to the Montreal classification,25 and the patient's medication at the time of the visit were extracted from the electronic documentation system. Data were arranged in a Microsoft Excel spreadsheet (version 2010, Microsoft Corporation, Redmond, WA). The total number of IBD patients who visited the outpatient clinic during the study period and the number of corresponding cases were calculated using the electronic diagnosis documentation system of the hospital.
Scores
All scores applied in the present study were calculated from items listed on the standardized IBD questionnaire. The Harvey-Bradshaw Index (HBI) was used to quantify clinical activity of CD.26 Per Vermeire et al,27 clinical remission was defined as HBI <5. Mild disease was defined as HBI 5 to 7, moderate as HBI 8 to 16, and severe as HBI >16. In UC patients, clinical disease activity was assessed by the Simple Clinical Colitis Activity Index (SCCAI).28 Remission was considered at SCCAI <3.29 Mild activity was defined as SCCAI 3 to 5, moderate as SCCAI 6 to 11, and severe as SCCAI >11. The Short Inflammatory Bowel Disease Questionnaire (SIBDQ), in its validated German version, was used to evaluate disease-specific HRQoL. It includes 10 items, and total scores range from 10 (bad) to 70 (good).30,31 The SIBDQ contains the 4 subscores bowel symptoms, systemic symptoms, emotional function, and social function. The presence of depressive symptoms was evaluated with the Patient Health Questionnaire-9 (PHQ-9) in its validated German version; scores of >9 indicate a risk of major depression.32,33 According to the World Health Organization (WHO) definition, body mass index (BMI) was classified as follows: <18.5 kg/m2 underweight, 18.5 to 24.99 kg/m2 normal weight, 25–29.99 kg/m2 preobesity, ≥30 kg/m2 obesity.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics version 22.0 software (IBM Corporation, Armonk, NY, USA) and SAS software (Release 9.4, SAS Institute, Inc, Cary, NC). Data from CD patients and UC patients were analyzed separately. For demographic and clinical parameters, medians and interquartile ranges (IQR) were indicated. As variables were not normally distributed, the nonparametric Mann-Whitney U test (for comparison of 2 groups) and Kruskal-Wallis test (for comparison of 3 or more groups) were performed to compare subgroups. Pairwise comparison was subsequently conducted by Mann-Whitney U test and Bonferroni correction. Categorical variables were compared using Fisher exact test.
Correlation analysis was performed by calculation of Spearman rank correlation coefficient (rS). FC concentrations >1800 μg/g were not specified. This problem was approached by translating FC concentrations >1800 μg/g to 1801 μg/g for correlation analyses. Calculations were performed both including and excluding these translated values. Furthermore, 2 categories of FC concentrations were defined, as described above,24 and the subgroups were compared by Mann-Whitney U test. Initially, all patients with FC concentrations determined within 30 days of completion of the IBD questionnaire (marked as “±30d”) were included. In a post hoc analysis, subgroups were formed using only patients in whom FC concentrations were measured within 3 days of questionnaire completion (marked as “±3d”).
Demographic and disease-specific parameters with a potential confounding influence on SIBDQ were analyzed using logistic regression analyses with a SIBDQ of >58 versus ≤58 as cutoff. The SIBDQ of 58 was chosen because a German healthy control group31 reached a mean SIBDQ of 60 with a standard deviation of 5.5 and confidence limits of 58.5 and 61.5. Variables were entered into multivariate logistic regression analysis when P values for univariate analysis were <0.02. Odds ratios with their 95% confidence intervals were given.
Receiver operating characteristic (ROC) curves were calculated to analyze the test performance characteristics of SIBDQ for predicting FC. Youden Index was used to determine optimal cutoff values.34 Sensitivity, specificity, positive and negative predictive values (PPV and NPV) were presented. Two-sided P values <0.05 were considered statistically significant.
RESULTS
Description of the Study Population
Eligible Patients
In total, 2277 IBD patients visited our outpatient clinic from February 1, 2012 to February 28, 2015, making up 5203 cases. Within this interval, 1579 IBD questionnaires were completed. The questionnaire return rate cannot be calculated as the number of patients who received the questionnaire was not documented. Based on these 1579 questionnaires, 1133 cases were excluded due to at least one of the following factors: no—or no current—FC value was available; the questionnaire was not fully completed; or the diagnosis of CD or CD did not apply according to ECCO criteria.22,23 Only 2 patients had to be excluded due to Clostridium difficile infection and 4 patients due to the presence of ostomy. Four hundred forty-six cases fulfilled the inclusion criteria. Considering only the most recent cases among patients who had completed several questionnaires, 308 individual cases of IBD patients were finally eligible—197 with CD and 111 with UC. Among these 308 patients, 200 completed only 1 survey, 83 completed 2 surveys, 20 completed 3 surveys, and 5 completed 4 surveys within the study interval. In all, 108 of 308 patients completed at least 2 IBD surveys within the indicated time frame.
Demographic and Clinical Characteristics of the Study Population
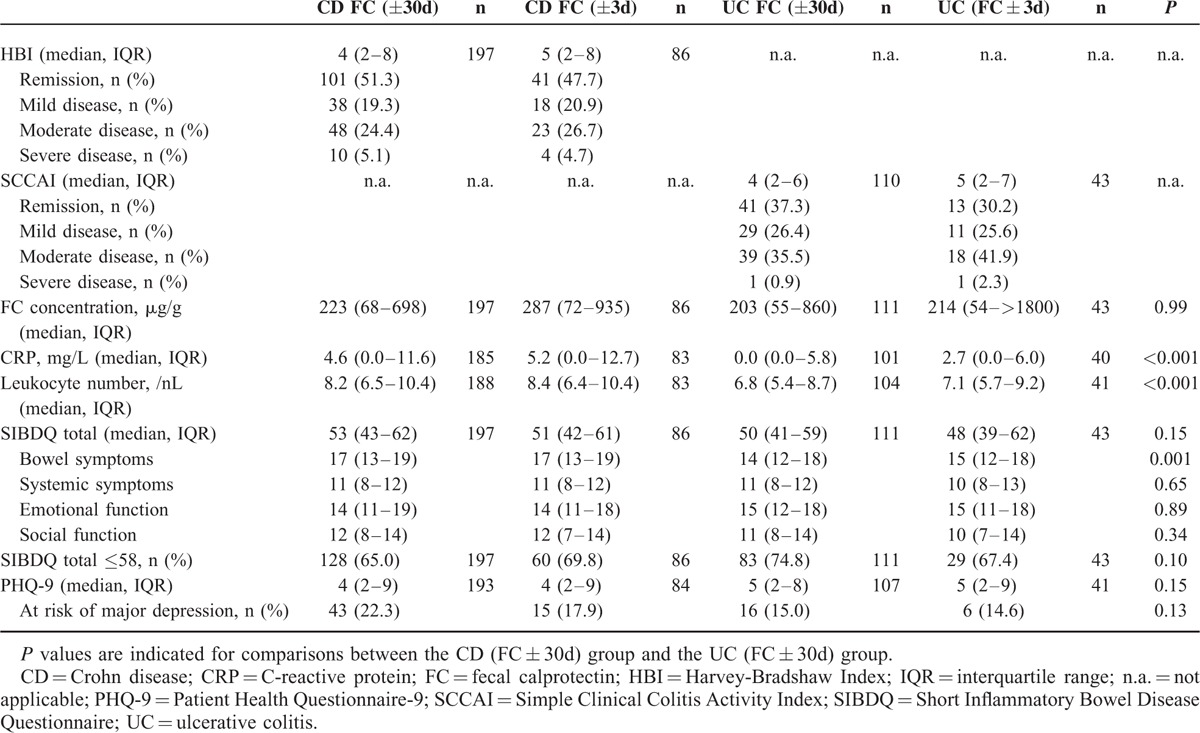
Baseline characteristics of the whole study population and of the subgroups whose FC concentrations had been determined within 3 days of questionnaire completion are detailed in Table 1. No relevant differences were detected between the FC (±30d) groups and the FC (±3d) subgroups.
TABLE 1.
Demographic and Clinical Characteristics of the Whole Study Population (FC ± 30d) and the Subgroups of Patients Whose FC Concentrations were Measured Within 3 Days of Questionnaire Completion (FC ± 3d)

Parameters of Disease Activity and HRQoL in the Study Population
Results of clinical and biochemical disease activity markers and HRQoL in the study population are shown in Table 2. In all, parameters were comparable between FC (±30d) groups and FC (±3d) subgroups.
TABLE 2.
Parameters of Disease Activity and Health-Related Quality of Life in the Whole Study Population (FC ± 30d) and the Subgroups of Patients Whose FC Concentrations Were Measured Within 3 Days of Questionnaire Completion (FC ± 3d)
Time Spans Between Questionnaire Completion and FC Determination
Median time spans between questionnaire completion and FC determination did not differ significantly between the CD group (4 days, IQR 2–8) and the UC group (5 days, IQR 2–8; P = 0.50). Supplemental digital content 1 (Figure) shows the distribution of time spans in the 2 disease groups.
Correlation Analyses
Relationship Between FC Concentrations and HRQoL in the Whole Study Population
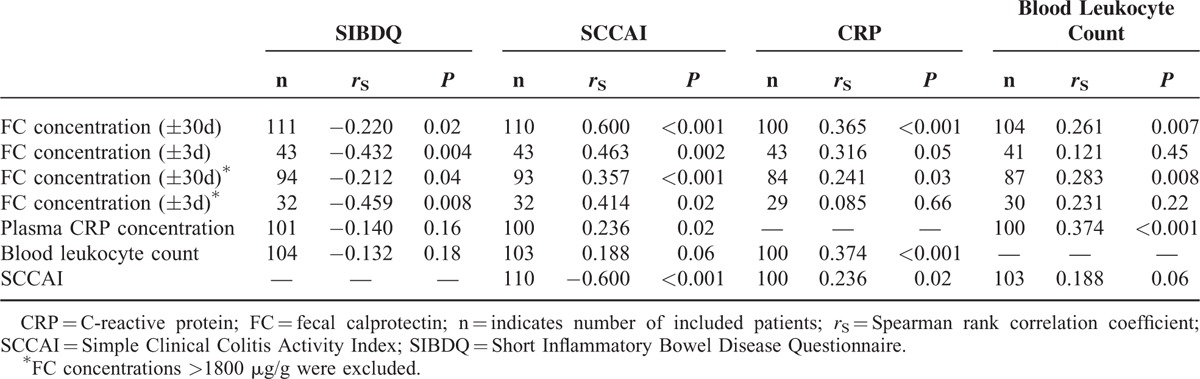
For both CD and UC, only very weak negative correlations were found between FC concentrations and SIBDQ scores, including all FC values determined within 30 days of questionnaire completion (Tables 3 and 4; Supplemental digital content 2, Figure). Excluding patients with FC values >1800 μg/g yielded nonsignificant results for CD, whereas for UC, results were comparable with those including all patients (Tables 3 and 4).
TABLE 3.
Results of Correlation Analyses Between Biomarkers, Clinical Disease Activity, and Health-Related Quality of Life in Crohn Disease
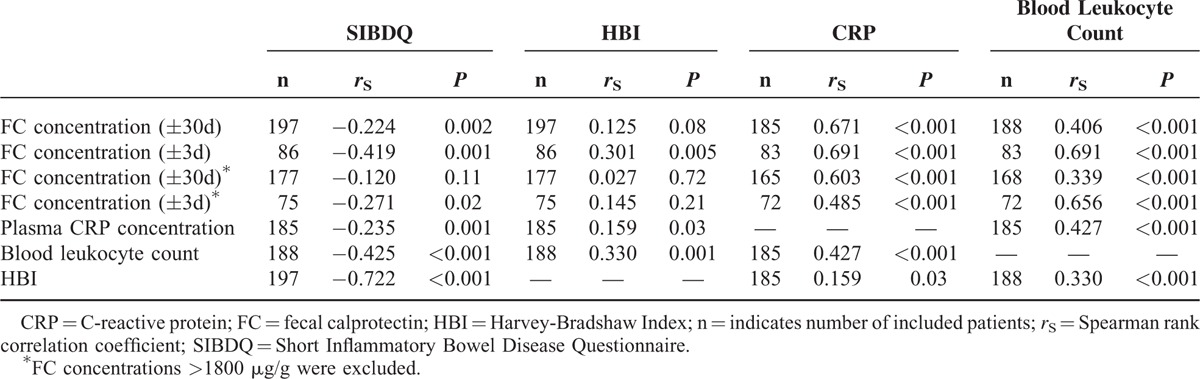
TABLE 4.
Results of Correlation Analyses Between Biomarkers, Clinical Disease Activity, and Health-Related Quality of Life in Ulcerative Colitis
Relationship Between FC Concentrations and HRQoL in the FC (±3d) Subgroups
Repeating the analyses using only FC values collected within 3 days of questionnaire completion resulted in stronger correlations between FC concentrations and SIBDQ scores in both CD and UC (Tables 3 and 4; Supplemental digital content 2, Figure). After excluding FC values >1800 μg/g, the correlations remained significant. Based on these findings, further analyses were performed considering only FC values measured within 3 days of questionnaire completion, and FC values >1800 μg/g were included, unless otherwise indicated. Supplemental digital content 3 (Table) illustrates how correlation coefficients changed depending on the time frame between questionnaire completion and FC measurement.
In CD, the median SIBDQ score was significantly higher in the group with FC (±3d) concentrations <200 μg/g (n = 35, 58 [IQR 49–64]) than in the group with FC concentrations ≥200 μg/g (n = 51, 47 [IQR 37– 55]) (P < 0.001; Figure 1A). Correspondingly, UC patients with FC (± 3d) concentrations <200 μg/g (n = 20) had a significantly higher median SIBDQ score (56, IQR 46–62) than those with FC concentrations ≥200 μg/g (n = 23, 40 [IQR 36–57]) (P = 0.02; Figure 1B).
FIGURE 1.
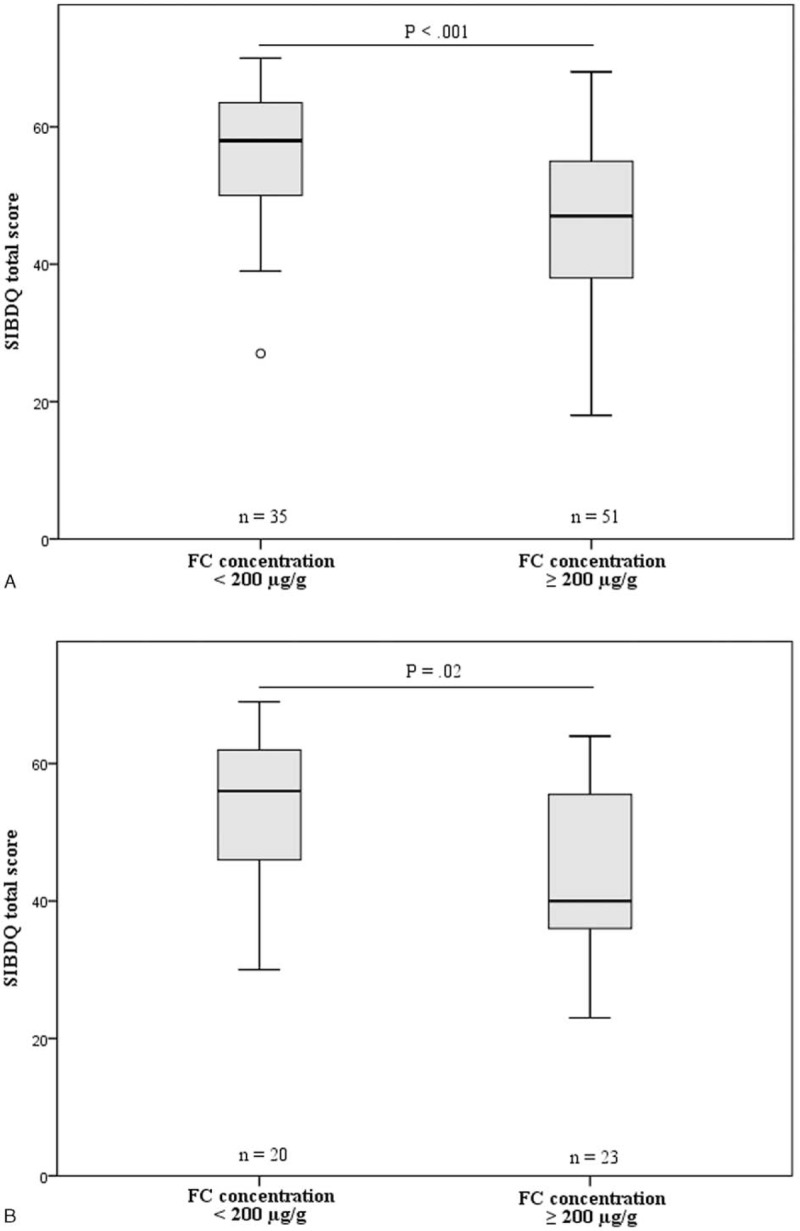
Boxplot diagrams illustrating the differences in SIBDQ scores between patient subgroups categorized according to their FC concentrations, representing inactive (FC < 200 μg/g) versus active disease (FC ≥ 200 μg/g). FC concentrations were determined within 3 days of questionnaire completion. A, Results for CD; B, Results for UC. The circle in Figure 1A represents an outlier. CD = Crohn disease; FC = fecal calprotectin; UC = ulcerative colitis.
Among SIBDQ subscores, FC concentrations (±3d) of CD patients correlated best with the subscore of systemic symptoms, while FC concentrations (±3d) of UC patients correlated only with the subscores of bowel symptoms and social function (Supplemental digital content 4, Table).
In CD patients with isolated involvement of the ileum (Montreal L1, ±L4), no significant correlation was found between FC (±3d) concentrations and SIBDQ scores (n = 27, rS = −0.268, P = 0.18), whereas that correlation was highly significant among patients with colonic or ileocolonic disease (Montreal L2 or L3, ±L4) (n = 59, rS = −0.470, P < 0.001). Also, in the CD group, disease location was related to FC concentrations: patients with involvement of the ileum and colon (Montreal L3) displayed significantly higher FC (±30d) concentrations than those with ileal (L1) involvement (patients with additional involvement of the upper gastrointestinal tract included; L1 [±L4]: n = 67, 118 μg/g, IQR 58–405 vs L3 [±L4]: n = 98, 285 μg/g, IQR 77–874; P = 0.02; overall P = 0.04; Figure 2). In contrast, disease extent of UC according to Montreal did not relevantly influence the strength of the correlation between FC concentrations and SIBDQ scores (Montreal E1+E2: n = 22, rS = −0.478, P = 0.02 vs Montreal E3: n = 21, rS = −0.448, P = 0.04).
FIGURE 2.
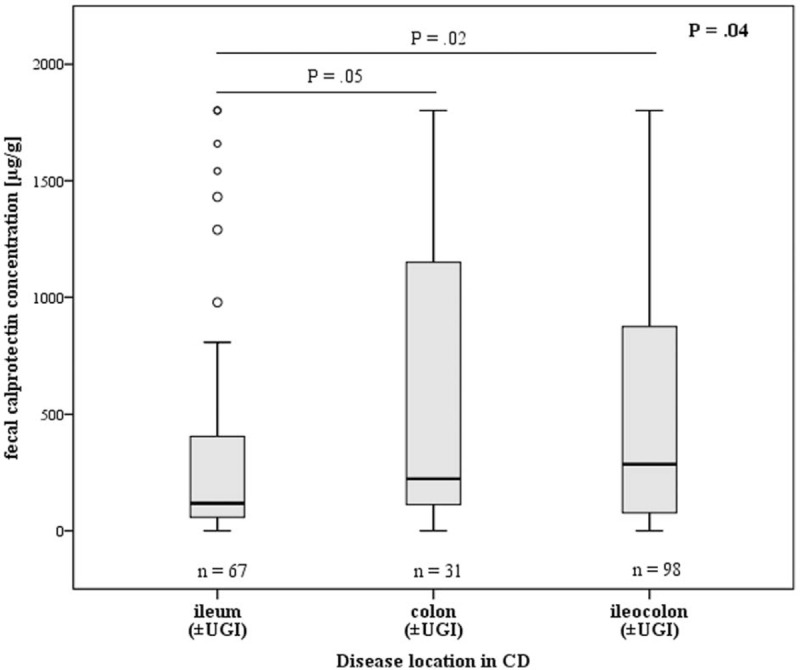
Fecal calprotectin concentrations determined within 30 days of the presentation at the outpatient clinic in relation to disease location in CD patients (n = 196, 1 patient suffered from isolated involvement of the upper gastrointestinal tract; patients with additional involvement of the UGI were included). Overall P was 0.04. After Bonferroni correction, no significant differences were found between ileum and colon (P = 0.05). Outliers are indicated as circles. CD = Crohn disease; UGI = upper gastrointestinal tract.
Relationships Between FC Concentrations, Humoral Biomarkers, and Clinical Disease Activity
Results of all correlation analyses listed in this paragraph are presented in Tables 3 and 4. In CD and UC, there were weak to moderate correlations between FC (±3d) concentrations and clinical disease activity. CD patients in clinical remission (n = 41) had significantly lower FC (±3d) concentrations than those with active disease (n = 45) (168 μg/g, IQR 57–448 vs 355 μg/g, IQR 151–1261; P = 0.01). Accordingly, UC patients in clinical remission (n = 13) displayed more than 12-fold lower FC (±3d) concentrations than those with active disease (n = 30) (54 μg/g, IQR 34–568 vs 666 μg/g, IQR 152–>1800; P = 0.02). In both diseases, CRP concentrations correlated with clinical disease activity worse than FC (±3d) concentrations, while there was a weak correlation between leukocyte counts and clinical disease activity only in CD. CRP concentrations correlated better with FC (±3d) concentrations in CD than in UC. Blood leukocyte counts were associated with FC (±3d) concentrations only in CD.
Relationship Between Clinical Disease Activity, HRQoL, and PHQ-9
A strong negative correlation was found between SIBDQ and HBI in CD patients (Table 3). The correlation between clinical disease activity and SIBDQ was slightly weaker in UC than in CD (Table 4). In addition, PHQ-9 was strongly correlated with clinical disease activity and SIBDQ in CD (n = 193, rS = 0.627, P < 0.001; n = 193, rS = −0.832, P < 0.001) and UC (n = 106, rS = 0.407, P < 0.001; n = 107, rS = −0.754, P < 0.001).
ROC Curves Analyses
To further assess how well SIBDQ detects inactive disease according to FC concentrations, ROC curve analyses were performed with inactive disease (FC < 200 μg/g) as the outcome parameter. Including all patients whose FC concentrations had been measured within 30 days of questionnaire completion, we found an AUC of 0.634 for CD and 0.618 for UC, indicating poor ability of SIBDQ to discriminate between inactive disease and active disease according to FC. When only FC values measured within 3 days of SIBDQ determination were considered, AUCs increased to 0.744 for CD and 0.712 for UC. In CD, AUC was 0.668 in patients with isolated ileal involvement versus 0.773 in patients with (ileo-)colonic involvement, both regardless of potential additional involvement of the UGI tract. Sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) were calculated for optimal SIBDQ cutoff scores to detect inactive disease within the FC (±3d) subpopulations. In CD, SIBDQ scores >51 showed a 71.4% sensitivity and a 68.6% specificity for detecting inactive disease (FC < 200 μg/g) (PPV: 61.0%; NPV: 77.8%), while in UC, SIBDQ scores >42 achieved a sensitivity of 90.0% and a specificity of 56.5% for detecting inactive disease (PPV: 64.3%; NPV: 86.7%). In comparison, for the SIBDQ cutoff score of 58, we obtained the following results for CD: sensitivity 48.6%, specificity 82.4%, PPV 65.4%, NPV 70.0%. For UC, we found sensitivity 45.0%, specificity 78.3%, PPV 64.3%, and NPV 62.1%.
Logistic Regression Analysis
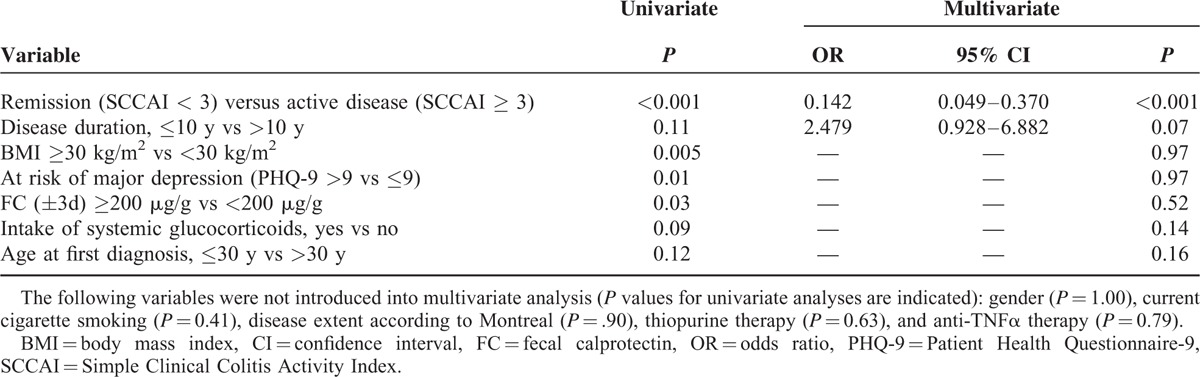
To identify parameters with a potentially confounding impact on HRQoL in CD and UC, we analyzed all variables listed in Tables 5 and 6. SIBDQ ≤ 58 was used as the dependent outcome variable. Clinical disease activity (HBI ≥ 5), intake of systemic glucocorticoids, FC ≥ 200 μg/g vs <200 μg/g, and BMI ≥ 25 kg/m2 were all found to be independent risk factors of poorer HRQoL in CD (Table 5). In UC, only clinically active disease (SCCAI ≥ 3) and a disease duration of 10 years or less were independent predictors of poorer HRQoL (Table 6). Comorbidities were not included in regression analyses owing to their wide spectrum in our study population. Supplemental digital content 5 (Table) shows how potential confounders identified using multiple logistic regression analyses influenced the results of correlation analyses between FC and SIBDQ.
TABLE 5.
Final Models of Multivariate Logistic Regression Analyses of Variables Associated with SIBDQ ≤58 in 197 Patients with Crohn Disease
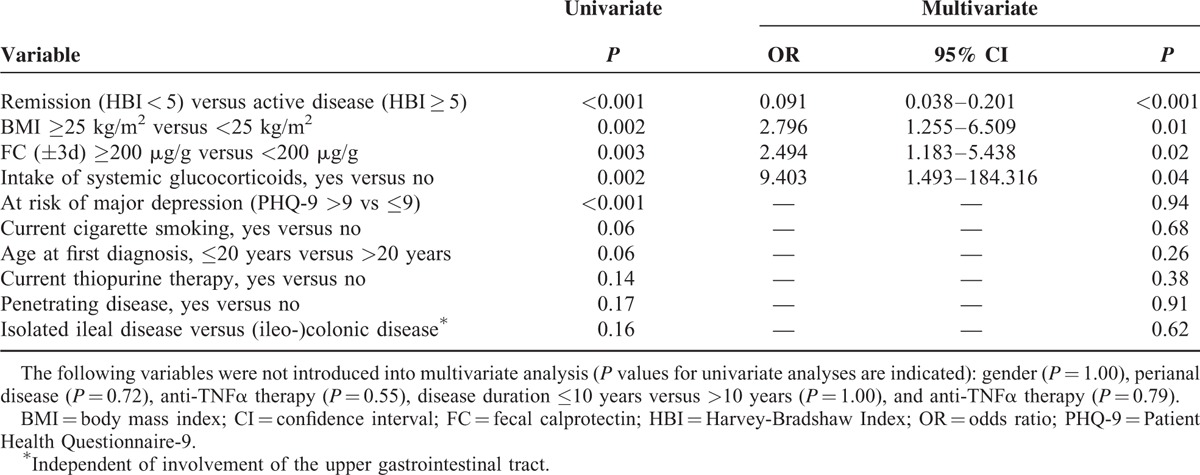
TABLE 6.
Final Models of Multivariate Logistic Regression Analyses of Variables Associated with SIBDQ ≤58 in 111 Patients with Ulcerative Colitis
DISCUSSION
Key Findings and Comparison With Published Literature
The major finding of the current study—according to its primary goal—is that there are moderate negative correlations between FC concentrations and HRQoL in UC and in CD with colonic involvement, but not in isolated ileal CD.
We identified one other study in the published literature in which the relationship between FC concentrations and HRQoL in IBD was analyzed, but in that case it was only a secondary objective. In that study, D’Haens et al35 reported a correlation between FC and HRQoL (IBDQ) in UC of the same scale as in our study (n = 39, r = −0.470, P = 0.003), while they described no significant correlation in CD (n = 87, r = −0.186, P = .09). The correlation coefficient for the 28 CD patients with purely ileal disease in the study by D’Haens et al is not indicated, thus comparison with our own data is limited.
Several previous studies have demonstrated strong correlations between FC concentrations and endoscopic disease activity in IBD.17–20 Owing to that close association, our results regarding the relationship between FC and HRQoL may be compared with the results of correlation analyses between endoscopic disease activity (instead of FC concentrations) and HRQoL. For example, Zahn et al8 pointed out a moderate relationship between endoscopic activity index and IBDQ in 2 groups of UC patients (r = −0.511 and r = −0.634, respectively). In comparison, the correlation between FC concentration and SIBDQ in our study is only slightly weaker (r = −0.432). The fact that this comparison is indirect, and that IBDQ was used in 1 study while SIBDQ was used in the other to determine HRQoL, makes this comparison somewhat weak.
Implications for Therapy Control
Translated into clinical practice, the key finding of our study implies that adhering solely to HRQoL to adapt IBD-specific therapy cannot be justified owing to the relatively weak correlation between HRQoL and FC concentration. This is also underlined by the results of ROC curve analyses investigating the performance of SIBDQ to recognize inactive disease according to FC (FC concentration <200 μg/g). This analysis is of interest as in clinical practice, an important question is whether a patient reporting well-being might still have active disease. According to our results, SIBDQ has overall a poor ability in differentiating between active and inactive disease according to FC concentrations. One of the SIBDQ cutoff scores we examined was 58, indicating good HRQoL,31 as this may be of special interest in clinical practice. According to our results, the probability that a CD patient with SIBDQ >58 has inactive disease is 61.0%, and that he has active disease with a score ≤58 is 77.8%. For UC patients, the corresponding probabilities were 64.3% and 62.1%. In all, also including PPV and NPV results achieved by using optimal cutoff SIBDQ scores, it can be stated that particular care must be taken in clinical practice to not undertreat patients with good HRQoL. In these patients, it is thus also wise to use additional activity parameters for therapy management, and good HRQoL should not be the sole therapy goal.
In CD patients with isolated ileal involvement, representing a large part of CD patients (32.5% in our study), relying on HRQoL as the major guide for therapy control seems to be ill-advised, as HRQoL and FC concentrations do not correlate at all in this subgroup. However, it must be taken into account that FC is of limited value in the assessment of disease activity in purely ileal CD, as prior data have shown that the correlation between FC concentrations and endoscopic disease activity is worse in ileal than in ileocolonic or colonic involvement.36 Accordingly, FC concentrations were significantly lower in ileal disease than in ileocolonic disease in our CD group. Thus, the spectrum of noninvasive disease markers is generally more limited in ileal CD than in CD with colonic involvement. Unfortunately, we cannot make any statement on CD with isolated involvement of the upper gastrointestinal tract based on our data, because only a single patient fulfilling this criterion could be included. Overall, it appears reasonable to incorporate both HRQoL and FC concentrations into a score for therapy evaluation. Pursuant to this approach, a small prospective study performed by Voiosu et al21 revealed that combining FC concentrations and SIBDQ into a score resulted in a better specificity in predicting active endoscopic disease than FC concentrations alone.
Secondary Findings
The correlation between FC and clinical disease activity was stronger in UC than in CD, but in both diseases, correlation coefficients were in the same scale as those for correlations between FC and HRQoL. In the literature, we found comparable, weak-to-moderate correlation results between FC and clinical disease activity and slightly higher correlation coefficients in UC than in CD.24,35,37,38 This implies that using either HRQoL or a classic disease activity score for treatment adaptation seems to be of minor clinical relevance, which may not be surprising given the overlapping of the scores. Correspondingly, the highest correlation coefficients that we found in our study were between SIBDQ and clinical disease activity scores.
Although it was not the primary aim of our study, of note is our finding of a closer association of HRQoL with the 3-day FC results than with the 30-day FC results in both CD and UC. Analysis of correlation coefficients depending on the length of the interval between determination of SIBDQ scores and FC concentrations showed that correlations were already weaker when a 7-day interval was used instead of a 3-day interval. This implies that for research assessments—and possibly for clinical use as well—a time span between clinical evaluation and FC determination of 30 days is too long, and that the time span should be as short as possible.
Multivariate regression analysis was performed to identify demographic and clinical factors with an impact on HRQoL and FC concentrations. It revealed that in CD—but not in UC—poorer HRQoL (SIBDQ ≤ 58) was independently associated with FC concentrations >200 μg/g, active disease according to clinical disease activity indices, BMI > 25 kg/m2, and the intake of systemic glucocorticoids. It is of note that 36.9% of our CD group and 42.3% of our UC group were overweight or obese. Obesity has traditionally been considered rare in IBD.39 In 2002, Blain et al38 reported an obesity rate of only 3% in 2065 CD patients. In more recent studies, the authors described considerably higher overweight and obesity rates among IBD patients, for example, one of 56% in a Scottish study40—corresponding to the local overweight and obesity rate—and one of 23.6% in a US pediatric IBD population.41 While some authors describe a relationship between obesity and more severe disease especially in CD,42,43 there are also data suggesting that obesity may be a marker of less severe disease.44–46 In our study, overweight and obesity influenced the risk of poorer HRQoL independently of clinical and biochemical disease activity. This is in line with previous, non-IBD-related studies, which described poorer HRQoL in overweight and obese patients.47–50 Given rising rates of obesity among IBD patients,46 overweight and obesity deserve more attention among physicians treating IBD patients. On that note, overweight and preobesity present a potential confounder concerning our correlation results between FC and SIBDQ. Interestingly, subgroup analysis revealed clearly stronger correlations in CD patients with BMI ≥ 25 than those with BMI < 25.
That the intake of systemic glucocorticoids had a negative impact on HRQoL independent of biochemical and clinical disease activity in CD might be attributed to side effects. Yet, as concomitant medications were not considered in the analyses, this suspicion must be expressed with caution. In UC, a disease duration of 10 years or less was also a predictor of poorer HRQoL; this effect is interesting, as it was independent of clinical and biochemical disease activity. We speculate that it might be explained by an increasing adaptation of IBD patients to their disease over time. Not surprisingly, in both CD and UC, clinical disease activity was the most important predictor of poorer SIBDQ. It has to be noted, however, that SIBDQ scores are a priori related to clinical activity scores, and even more to PHQ-9 scores, as they are all calculated from questionnaire items that partly overlap between the scores. Thus they are strongly correlated with one another, and major depressive symptoms according to PHQ-9 were not identified as an independent risk factor of poorer SIBDQ. To summarize, the data obtained by regression analyses indicate potential confounders of our correlation results, but should not be overrated, especially due to small patient numbers in some subgroups. Thus, mainly in the UC group, correlations probably did not reach significance due to small patient numbers in several cases.
Limitations of the Study
The most important limitation of this study is its retrospective design. Of 2277 IBD patients visiting our outpatient clinic within the study interval, we could only include 308. The reasons for this were mainly that patients did not receive a questionnaire, which they did not fill or incompletely filled in the questionnaire, and that FC concentration was not measured. Thus our study implies a relatively high risk of selection bias, because certain groups of patients—such as particularly compliant patients or patients with lower disease activity—may have achieved a higher questionnaire return rate than the remaining patients. We could not control for this confounder, as it was not documented in clinical routine how many and which patients did not return their questionnaires. Further, patient reception was tasked with distributing questionnaires to all patients at our IBD outpatient clinic, but the questionnaires were not consistently distributed during phases of staff shortage. Even though most physicians at our clinic order FC measurement on a routine basis, some of them may have investigated FC concentrations only in cases of unclear disease activity. At the same time, some patients did not submit stool samples. Both factors cannot be retrospectively controlled. The majority of our study patients had relatively low FC concentrations, which might be explained by a tendency of healthier patients to provide samples. It implies a bias in the direction of patients with lower disease activity and is of concern for generalizability of results to patients with the full spectrum of disease activity. It must be taken into account that our results are a priori not readily transferable to IBD patients at large, as the data were collected in a tertiary referral center where usually more patients with complex disease courses are seen than in practices, for example. Therefore, it remains to be determined if our results are reproducible at other institutions.
Before data collection, we had randomly defined that only the most recent cases of patients visiting the clinic multiple times during the study period were to be included. Using this approach, we may unintentionally have selected better-treated, healthier patients. However, a post hoc analysis using Wilcoxon test, which was performed for this reason, did not yield a significant difference between FC concentrations of patients at their first and their most recent visits, respectively (data not shown).
Initially, we deemed it reasonable and realistic to include all FC concentrations measured within 30 days to the visit at the outpatient clinic into the study. However, performing correlation analyses between thus acquired FC concentrations and HRQoL revealed correlation coefficients so small that we considered whether the chosen inclusion criterion might have been too broad. We decided to perform post hoc analyses including all FC values determined within 3 days of questionnaire completion, as we assumed that during that short interval, no relevant changes of HRQoL would occur. This approach resulted in relatively small patient numbers to be used for correlation analyses.
Another methodical shortcoming of our study is that FC concentrations were measured only up to a limit of 1800 μg/g. As correlation analysis was hampered by the lack of exact FC concentrations >1800 μg/g, we used the Mann-Whitney U test in addition to compare SIBDQ scores between patients with FC values <200 μg/g and patients with FC values ≥200 μg/g, corresponding to inactive and active disease according to previously published data.24 We would have preferred a categorization of FC concentrations into >2 categories, but decided against it as we did not find reliable cutoff values for FC to differentiate between moderate and severe disease in the literature.
A major limitation of the study is that QoL scales are greatly influenced by sociodemographic and psychological factors.51 These represent confounders whose impact on our study results could not be sufficiently assessed, as especially sociodemographic factors were not reliably documented. As expected, PHQ-9, indicating the risk of depression, correlated strongly with HRQoL in our study population.
While being aware of the availability of more reliable scores—like the Crohn Disease Activity Index (CDAI)52—we used the HBI to determine clinical CD activity, as we prioritized simplicity over highest possible reliability for our routine purposes. It would certainly have been an important validation step to ensure that FC concentrations correlate well with endoscopic and histologic disease activity in our study. Unfortunately, this evaluation could not be performed owing to scarcity of endoscopic data.
CONCLUSIONS
Our study reveals a significant, but only weak to moderate correlation between FC concentrations and HRQoL in both CD and UC. In CD, this relationship was found in patients with colonic involvement, but not in patients with isolated ileal disease. Correlations were stronger when only FC concentrations measured within 3 days of HRQoL determination were considered as compared with FC concentrations obtained within 30 days of HRQoL determination. The strength of the correlation between FC concentrations and HRQoL was comparable to that between FC concentrations and clinical disease activity. Overall, these results suggest that IBD management on the sole basis of HRQoL or clinical disease activity is not recommendable, especially in purely ileal CD. In clinical practice and in trials, HRQoL and clinical disease activity should not be the only outcome parameters, but rather incorporated into activity scores combining subjective with objective parameters. Also, in research studies and in clinical practice, it should be ensured that time spans between clinical evaluation of IBD patients and the determination of FC concentrations are as short as possible.
Supplementary Material
Acknowledgments
We thank C.W. Gauss for proofreading. Parts of this article appear in the doctoral thesis of Thomas Geiß.
Footnotes
Abbreviations: AUC = area under the curve, CD = Crohn disease, CRP = C-reactive protein, FC = fecal calprotectin, HBI = Harvey-Bradshaw Index, HRQoL = health-related quality of life, IBD = inflammatory bowel disease, NSAID = nonsteroidal anti-inflammatory drug, PHQ-9 = Patient Health Questionnaire-9, ROC = receiver-operating characteristic, SCCAI = Simple Clinical Colitis Activity Index, SIBDQ = Short Inflammatory Bowel Disease Questionnaire, UC = ulcerative colitis, UGI = upper gastrointestinal.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Huppertz-Hauss G, Høivik ML, Langholz E, et al. Health-related quality of life in inflammatory bowel disease in a European-wide population-based cohort 10 years after diagnosis. Inflamm Bowel Dis 2015; 21:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Høivik ML, Bernklev T, Solberg IC, et al. Patients with Crohn's disease experience reduced general health and vitality in the chronic stage: ten-year results from the IBSEN study. J Crohns Colitis 2012; 6:441–453. [DOI] [PubMed] [Google Scholar]
- 3.Bernklev T, Jahnsen J, Henriksen M, et al. Relationship between sick leave, unemployment, disability, and health-related quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis 2006; 12:402–412. [DOI] [PubMed] [Google Scholar]
- 4.Høivik ML, Moum B, Solberg IC, et al. Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: results from the IBSEN study. Inflamm Bowel Dis 2012; 18:1540–1549. [DOI] [PubMed] [Google Scholar]
- 5.Bodger K, Ormerod C, Shackcloth D, et al. IBD Control Collaborative. Development and validation of a rapid, generic measure of disease control from the patient's perspective: the IBD-control questionnaire. Gut 2014; 63:1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis 2008; 14:554–565. [DOI] [PubMed] [Google Scholar]
- 7.van der Have M, van der Aalst KS, Kaptein AA, et al. Determinants of health-related quality of life in Crohn's disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8:93–106. [DOI] [PubMed] [Google Scholar]
- 8.Zahn A, Hinz U, Karner M, et al. Health-related quality of life correlates with clinical and endoscopic activity indexes but not with demographic features in patients with ulcerative colitis. Inflamm Bowel Dis 2006; 12:1058–1067. [DOI] [PubMed] [Google Scholar]
- 9.Theede K, Kiszka-Kanowitz M, Nordgaard-Lassen I, et al. The impact of endoscopic inflammation and mucosal healing on health related quality of life in ulcerative colitis patients. J Crohns Colitis 2015; 9:625–632. [DOI] [PubMed] [Google Scholar]
- 10.Casellas F, Barreiro de Acosta M, Iglesias M, et al. Mucosal healing restores normal health and quality of life in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2012; 24:762–769. [DOI] [PubMed] [Google Scholar]
- 11.Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014; 24:367–378. [DOI] [PubMed] [Google Scholar]
- 12.Flynn A, Kane S. Mucosal healing in Crohn's disease and ulcerative colitis: what does it tell us? Curr Opin Gastroenterol 2011; 27:342–345. [DOI] [PubMed] [Google Scholar]
- 13.Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalization over 6 years of follow-up. Gut 2016; 65:408–414. [DOI] [PubMed] [Google Scholar]
- 14.De Cruz P, Kamm MA, Prideaux L, et al. Mucosal healing in Crohn's disease: a systematic review. Inflamm Bowel Dis 2013; 19:429–444. [DOI] [PubMed] [Google Scholar]
- 15.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61:1619–1635. [DOI] [PubMed] [Google Scholar]
- 16.Theede K, Holck S, Ibsen P, et al. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing of ulcerative colitis. Clin Gastroenterol Hepatol 2015; 13:1929–1936. [DOI] [PubMed] [Google Scholar]
- 17.Burri E, Beglinger C. Faecal calprotectin—a useful tool in the management of inflammatory bowel disease. Swiss Med Wkly 2012; 142:w13557. [DOI] [PubMed] [Google Scholar]
- 18.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010; 105:162–169. [DOI] [PubMed] [Google Scholar]
- 19.Ricanek P, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol 2011; 46:1081–1091. [DOI] [PubMed] [Google Scholar]
- 20.Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103:162–169. [DOI] [PubMed] [Google Scholar]
- 21.Voiosu T, Benguş A, Dinu R, et al. Rapid fecal calprotectin level assessment and the SIBDQ score can accurately detect active mucosal inflammation in IBD patients in clinical remission: a prospective study. J Gastrointestin Liv Dis 2014; 23:273–278. [DOI] [PubMed] [Google Scholar]
- 22.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010; 4:7–27. [DOI] [PubMed] [Google Scholar]
- 23.Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis PART 1: Definitions and diagnosis. J Crohns Colitis 2012; 6:965–990. [DOI] [PubMed] [Google Scholar]
- 24.Sipponen T, Savilahti E, Kolho KL, et al. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis 2008; 14:40–46. [DOI] [PubMed] [Google Scholar]
- 25.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980; 1:514. [DOI] [PubMed] [Google Scholar]
- 27.Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol 2010; 8:357–363. [DOI] [PubMed] [Google Scholar]
- 28.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998; 43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005; 54:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol 1996; 91:1571–1578. [PubMed] [Google Scholar]
- 31.Rose M, Fliege H, Hildebrandt M, et al. Validation of the new German translation version of the “Short Inflammatory Bowel Disease Questionnaire” (SIBDQ). Z Gastroenterol 2000; 38:277–285. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Löwe B, Spitzer RL, Zipfel S, Herzog W. Gesundheitsfragebogen für Patienten (PHQ-D). Manual und Testunterlagen. Pfizer, Karlsruhe; 2002. [Google Scholar]
- 34.Youden W J. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 35.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 36.Roseth AG, Aadland E, Grzyb K. Normalization of fecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol 2004; 39:1017–1020. [DOI] [PubMed] [Google Scholar]
- 37.Nancey S, Boschetti G, Moussata D, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2013; 19:1043–1052. [DOI] [PubMed] [Google Scholar]
- 38.Kopylov U, Rosenfeld G, Bressler B, et al. Clinical utility of faecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis 2014; 20:742–756. [DOI] [PubMed] [Google Scholar]
- 39.Blain A, Cattan S, Beaugerie L, et al. Crohn's disease clinical disease course and severity in obese patients. Clin Nutr 2002; 21:51–57. [DOI] [PubMed] [Google Scholar]
- 40.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts 2009; 2:370–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2011; 17:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2015; 21:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moran GW, Dubeau MF, Kaplan GG, et al. The increasing weight of Crohn's disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis 2013; 19:2949–2956. [DOI] [PubMed] [Google Scholar]
- 44.Chan SS, Luben R, Olsen A, et al. Body mass index and the risk for Crohn's disease and ulcerative colitis: data from a European Prospective Cohort Study (The IBD in EPIC Study). Am J Gastroenterol 2013; 108:575–582. [DOI] [PubMed] [Google Scholar]
- 45.Flores A, Burstein E, Cipher DJ, et al. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci 2015; 60:2436–2445. [DOI] [PubMed] [Google Scholar]
- 46.Nic Suibhne T, Raftery TC, McMahon O, et al. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis 2013; 7:e241–e248. [DOI] [PubMed] [Google Scholar]
- 47.Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf) 2005; 27:156–164. [DOI] [PubMed] [Google Scholar]
- 48.Soltoft F, Hammer M, Kragh N. The association of body mass index and health-related quality of life in the general population: data from the 2003 Health Survey of England. Qual Life Res 2009; 18:1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serrano-Aguilar P, Munoz-Navarro SR, Ramallo-Farina Y, et al. Obesity and health related quality of life in the general adult population of the Canary Islands. Qual Life Res 2009; 18:171–177. [DOI] [PubMed] [Google Scholar]
- 50.Mond JM, Baune BT. Overweight, medical comorbidity and health-related quality of life in a community sample of women and men. Obesity (Silver Spring) 2009; 17:1627–1634. [DOI] [PubMed] [Google Scholar]
- 51.Faust AH, Halpern LF, Danoff-Burg S, et al. Psychosocial factors contributing to inflammatory bowel disease activity and health-related quality of life. Gastroenterol Hepatol (NY) 2012; 8:173–181. [PMC free article] [PubMed] [Google Scholar]
- 52.Best WR. Predicting the Crohn's disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis 2006; 12:304–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


