Abstract
The aim of this study was to determine the clinical outcomes of long-term anticoagulation therapy in patients with symptomatic spontaneous isolated dissection of the superior mesenteric artery (SIDSMA) and to evaluate whether conservative treatment with anticoagulation therapy is a safe and effective treatment modality for these patients.
In this single center, observational cohort study, data from a prospectively recruiting symptomatic SIDSMA registry, including demographics, risk factors of interest, clinical characteristics and outcomes, and initial and follow-up computed tomography angiography (CTA) findings, were analyzed retrospectively.
During an 8-year period, a total of 52 consecutive patients who underwent conservative treatment with the use of long-term anticoagulation were included in this study. Clinical symptoms resolved within 11 days in all except 4 patients (7.7%); 3 received endovascular treatment for persistent symptoms and 1 received surgical repair. The mean duration of anticoagulation therapy was 9 (range: 3–60) months. A follow-up CTA showed complete remodeling in 20 (41.7%) patients, and the mean diameter and the incidence of false lumen thrombosis were also decreased significantly. There was no anticoagulation therapy-related mortality or morbidity except 2 (4.2%) minor bleeding complications, and no symptomatic recurrence or aggravation of the dissection occurred during the mean follow-up period of 47.5 (range: 10–97) months.
The present study showed that long-term anticoagulation therapy could result in a high rate of complete remodeling during the natural course of symptomatic SIDSMA. Conservative treatment with long-term anticoagulation therapy could be an optimal treatment strategy for symptomatic SIDSMA.
INTRODUCTION
Symptomatic spontaneous isolated dissection of the superior mesenteric artery (SIDSMA) without associated aortic dissection is rare, although the frequency of reports of asymptomatic dissection has increased with technical advances in multidetector computed tomography angiography (CTA), improved CTA resolution, and the increased popularity of CTAs.1–6 Because of its rarity, the incidence and natural history of symptomatic SIDSMA are not completely characterized. Considering the most reported studies involved in Asian population in the literature, there may be racial/ethnic disparities in the pathophysiology and prevalence of symptomatic SIDSMA. However, the exact pathophysiology remains the matter of some controversies. A variety of treatment options have been described for symptomatic SIDSMA, including conservative treatment with or without antithrombotic therapy, endovascular stenting, and surgical repair, but there is currently no consensus on the optimal treatment.5,6 Previously, we reported the preliminary results of conservative treatment with anticoagulation therapy in small number of patients with SIDSMA.4
In this study, we aimed to determine the long-term clinical outcomes of anticoagulation therapy during the natural course of symptomatic SIDSMA and to evaluate whether conservative treatment with long-term anticoagulation therapy is a safe and effective treatment modality for these patients.
SUBJECTS AND METHODS
Study Design and Patient Population
This single-center, retrospective observational study was based on data from a prospectively recruiting symptomatic SIDSMA registry. The study protocol was approved by the hospital's institutional review board, and all patients provided written informed consent. Between January 2007 and December 2014, a total of 52 consecutive patients with symptomatic SIDSMA who received conservative treatment were included in this study. Patients with asymptomatic SMA dissection (50 patients), SMA dissection associated with aortic or other visceral arteries, trauma- or operation-related SMA dissection, vasculitis, and patients with clinically overt bowel infarction and necrosis necessitating urgent laparotomy were excluded from this study. Demographics, risk factors of interest, and other data, including clinical characteristics, outcomes, and initial and follow-up contrast-enhanced CTA findings, were recorded prospectively for all consecutive patients in an Excel database (Microsoft Corp., Redmond, Washington) and analyzed retrospectively.
Diagnostic Work-Up and Categorization
Initial diagnosis of SIDSMA was made by contrast-enhanced CTA in all patients with acute-onset abdominal pain. According to the initial CTA findings, the external diameter of SMA measured on maximally dilated portion of the dissected segment and the presence of false lumen thrombosis were recorded, and the morphologic characteristics of the dissection were typed according to a modified classification based on Sakamoto and Zerbib classification7,8: type I, patent type with an entry and re-entry; type II, “cul-de-sac” type without re-entry; type III, with a thrombosed false lumen with an ulcer-like projection; type IV, with a completely thrombosed false lumen without an ulcer-like projection; type V, with combined dissection and stenosis of the SMA; and type VI, with partial or complete occlusion.
Treatment
Treatment strategy was determined according to patients’ symptoms and signs, as well as initial CTA findings. Conservative treatment consisted of strict blood pressure control (within normal range of systolic blood pressure), bowel rest, intravenous fluid therapy, nutritional support, and close observation, with the use of anticoagulation. Patients were released from fasting on resolution of abdominal pain. Full anticoagulation with intravenous heparin followed by oral warfarin therapy was carried out in all patients immediately after the diagnosis of SIDSMA, and anticoagulation was generally continued for 6 months to achieve a target international normalized ratio of 2.0 to 3.0. Oral warfarin therapy was started after patients were released from fasting on complete resolution of all symptoms. In patients who experienced persistent or aggravated abdominal pain while on anticoagulation for 7 days or longer or in patients with signs and symptoms suggestive of bowel ischemia, endovascular or surgical intervention was performed based on the follow-up CTA findings.
Follow-Up
Follow-up CTA was done within 6 months in patients who showed improvements in clinical symptoms, or sooner in patients with persistent or aggravated symptoms. Based on the initial and follow-up CTA findings, we analyzed the morphologic changes of the dissection, and clinical outcomes were categorized by the radiologist, unaware of the patients’ general health status, in the official CTA report as complete remodeling, incomplete remodeling, and no change. Complete remodeling was defined as complete resolution of the SMA trunk dissection: complete recovery of the true lumen and no false lumen, no residual stenosis or occlusion. Incomplete remodeling was defined as a decrease in the extent of the dissection and/or reduced false lumen thrombosis, but with residual stenosis or intramural thrombus. No change was defined as no morphologic change of the dissection without progression.
The initial treatment strategy for patients with complete remodeling was discontinuation of anticoagulation therapy without any further CTA follow-up, whereas in patients without complete remodeling, anticoagulation therapy was individualized based on the compliance and concomitant disease of each patient, and the patients underwent a CTA follow-up annually. However, recently, we changed the treatment strategy for patients without complete remodeling by changing the duration of oral anticoagulation treatment to 6 months and the timings of the follow-up CTA to 6 and 12 months. If there was no progression of the dissection on the 12-month follow-up CTA, patients were no longer invited to attend the outpatient clinic. In this study, we evaluated the morphologic changes of the dissection based on last follow-up CTA findings, and a follow-up telephone interview was conducted to evaluate long-term clinical outcomes in all patients.
Statistical Analysis
Categorical data were reported as counts and percentages, while continuous data were reported as means and standard deviations. Paired t test was used to compare the differences in paired nominal data and McNemar test was used to compare the differences in paired categorical data. A logistic regression analysis was used to evaluate factors associated with the morphologic improvement of the dissected SMA. The variables that were significant (P ≤ 0.3) in univariate analysis were incorporated into a multivariate logistic regression model. Multivariate analysis using a backward elimination was done to determine independent significance of the variables. All statistical analyses were performed using statistical software SPSS (version 18.0; SPSS, Chicago, IL), with a P ≤ 0.05 indicating statistically significance.
RESULTS
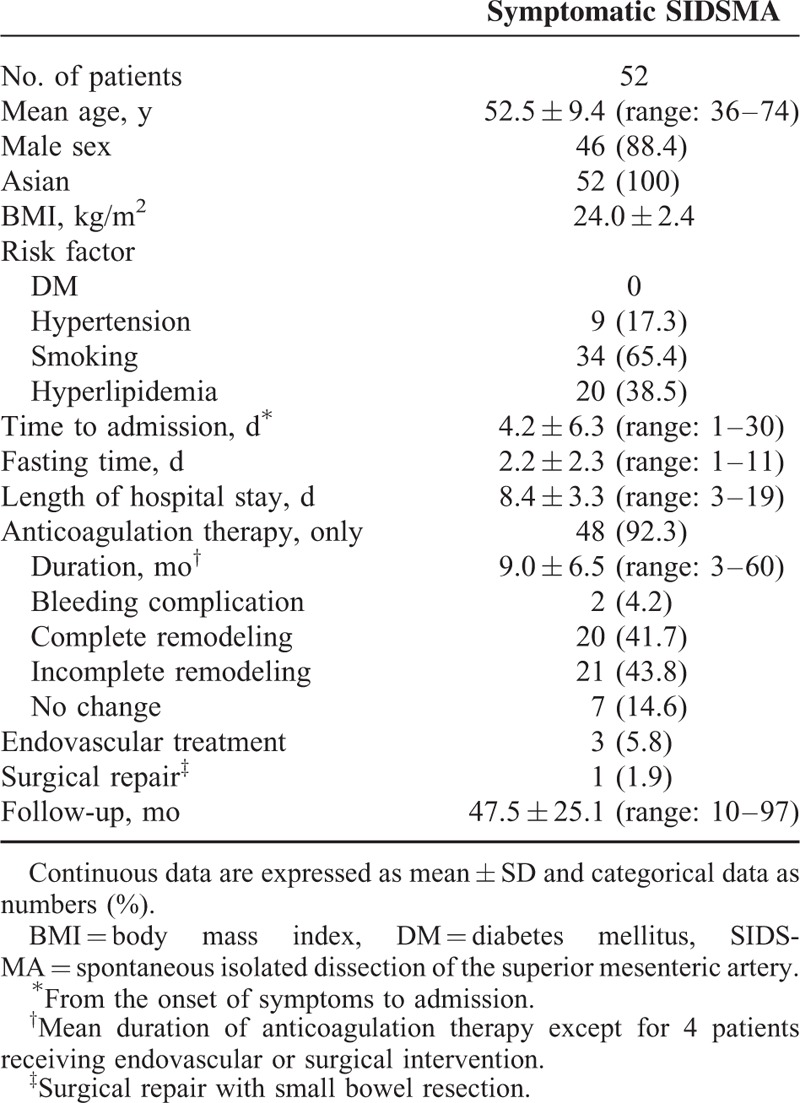
Demographics, risk factors of interest, and other data, including clinical characteristics and outcomes, are summarized in Table 1. All patients except for 6 were male (88.4%, 46/52) with a mean age of 53 (range: 36–74). Relevant comorbidities included smoking (65.4%), hyperlipidemia (38.5%), and hypertension (17.3%). All patients had acute-onset abdominal pain at presentation. The mean interval from the onset of symptoms to admission was 4 (range: 1–30) days, and a raised white blood cell count was noted in 14 (26.9%) patients. Conservative treatment with anticoagulation therapy was initially tried in all 52 patients. The mean fasting time was 2 (range: 1–11) days, and clinical symptoms resolved within 11 days in all except 4 (7.7%) patients; 3 received endovascular stenting for persistent symptoms and 1 underwent surgical repair of the dissection with small bowel resection (about 15 cm) for a small bowel infarction. The mean length of hospital stay in all 52 patients was 8 (range: 3–19) days, and the mean duration of anticoagulation therapy in the 48 patients who received conservative treatment was 9 (range: 3–60) months. There was no anticoagulation therapy-related mortality or morbidity except for 2 (4.2%) minor bleeding complications (1 thigh hematoma and 1 hematuria), which were treated by conservative care.
TABLE 1.
Demographics, Clinical Characteristics, and Outcomes
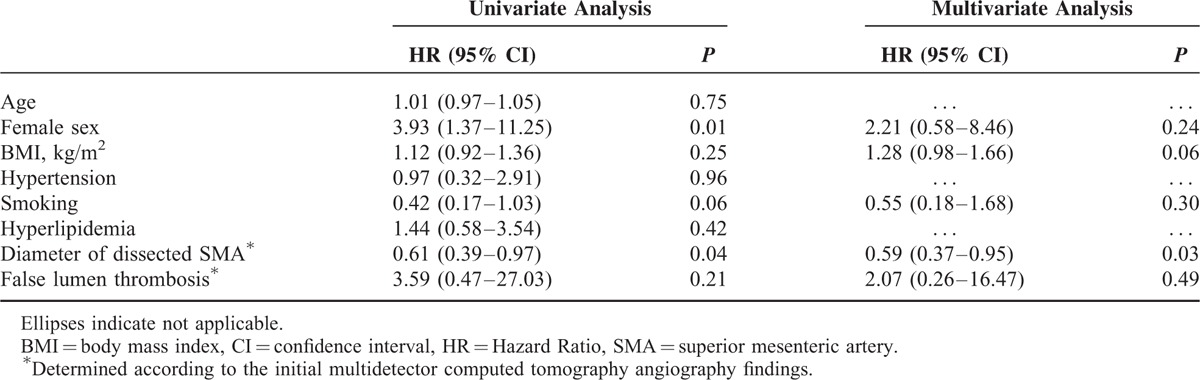
Initial and follow-up CTA findings in the 48 patients who received conservative treatment with anticoagulation therapy are summarized in Table 2. The mean follow-up time used for the CTA evaluation was 15.5 ± 12.2 (range: 3–60) months. The morphologic characteristics of the symptomatic SIDSMA were categorized into the following groups according to their initial CTA findings: type I in 2 (4.2%), type II in 4 (8.3%), type III in 17 (35.4%), type IV in 24 (50.0%), type V in 0 (0%), and type VI in 1 (2.1%) patient. The most common types were III and IV. Celiac artery and inferior mesenteric artery were patent in all patients. A comparison of the initial and follow-up CTAs showed a significant decrease in the mean diameter of the dissected SMA from 10.7 mm (range: 8.0–12.9) to 8.6 mm (range: 5.7–12.6) (P < .01), and a significant decrease in the incidence of false lumen thrombosis from 87.5% (42/48) to 6.3% (3/48) (P < 0.01). Follow-up CTA showed complete remodeling of the dissected SMA in 20 (41.7%) patients (Figure 1), incomplete remodeling in 21 (43.8%), and no change in 7 (14.6%); complete remodeling was achieved within a mean time of 11.7 months (range: 4–30). We found that most complete remodeling (14 patients, 70.0%) on follow-up CTA occurred within 12 months. According to the type of dissection, the rate of complete remodeling was 25.0% (1/4) in type II, 41.2% (7/17) in type III, and 50.0% (12/24) in type IV; the rate of no change was 100% (2/2) in type I, 50.0% (2/4) in type II, and 12.5% (3/24) in type IV. With the use of long-term anticoagulation, morphologic improvement was achieved in 41 (85.4%) patients, and no patient showed dissection progression on follow-up CTA. The initial diameter of the dissected SMA was the only factor significantly associated with morphologic improvement by univariate (P = 0.04) and multivariate (P = 0.03) analyses (Table 3). There was no symptomatic recurrence or aggravation of the dissection during the mean clinical follow-up period of 47.5 ± 25.1 months (range: 10–97).
TABLE 2.
Initial and Follow-Up CTA Findings in Patients Who Received Anticoagulation Therapy
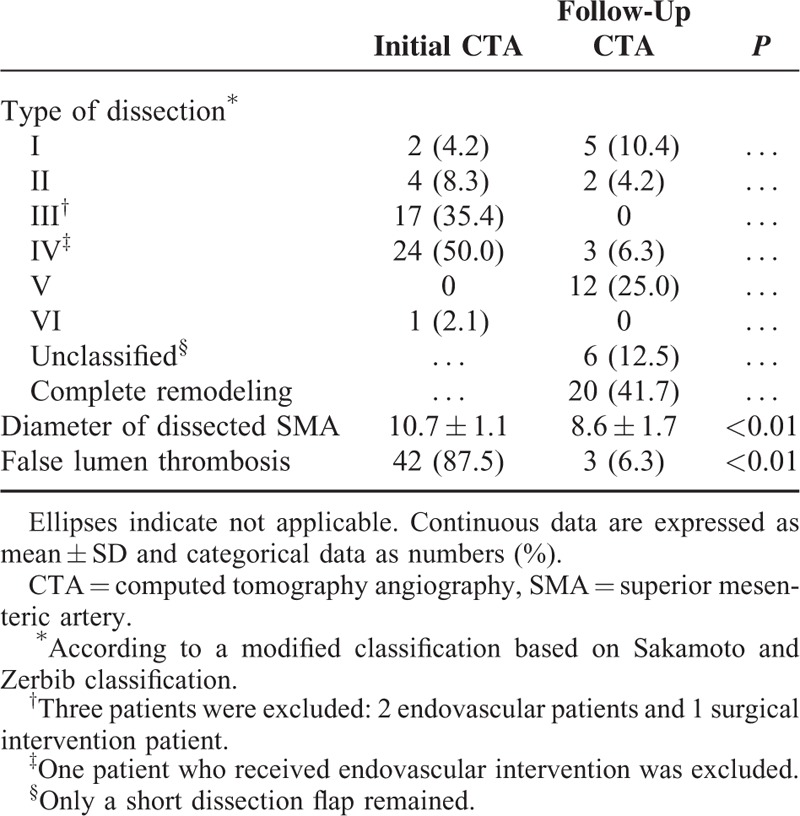
FIGURE 1.
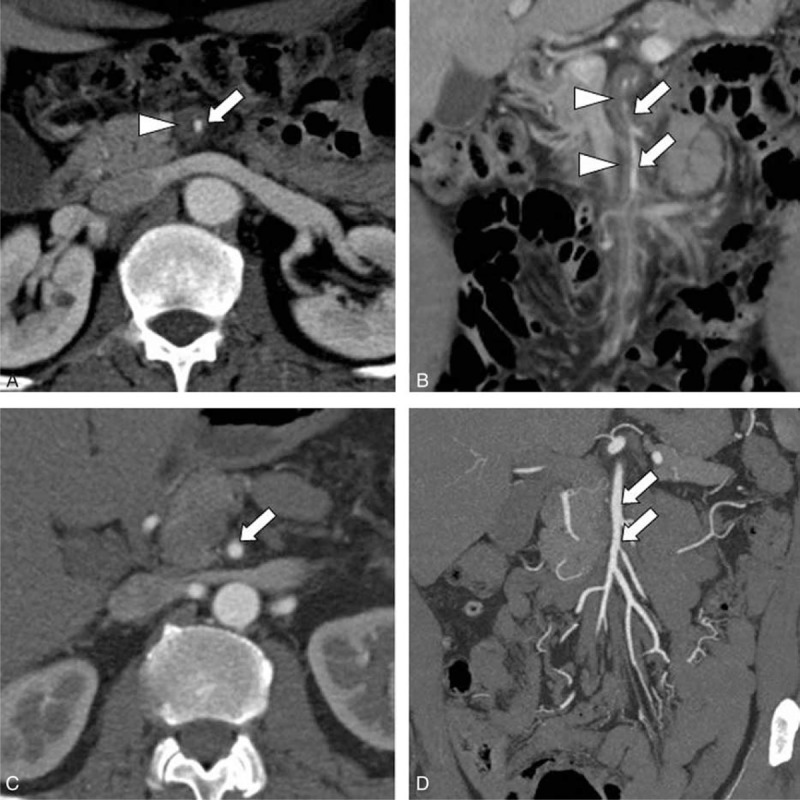
Serial contrast-enhancedCTA images of a patient (Sakamoto and Zerbib classification type IV) treated with anticoagulation therapy. A, B, Enhanced axial and coronal reconstruction CTA images show a narrowed true lumen of the superior mesenteric artery (arrows) with a thrombi-filled false lumen (arrowheads). C, D, After 6 months of anticoagulation therapy, follow-up-enhanced axial and coronal reconstruction CTA images demonstrate complete recovery of the true lumen (arrows) of the superior mesenteric artery. CTA = computed tomography angiography.
TABLE 3.
Factors Associated With the Morphologic Improvement
DISCUSSION
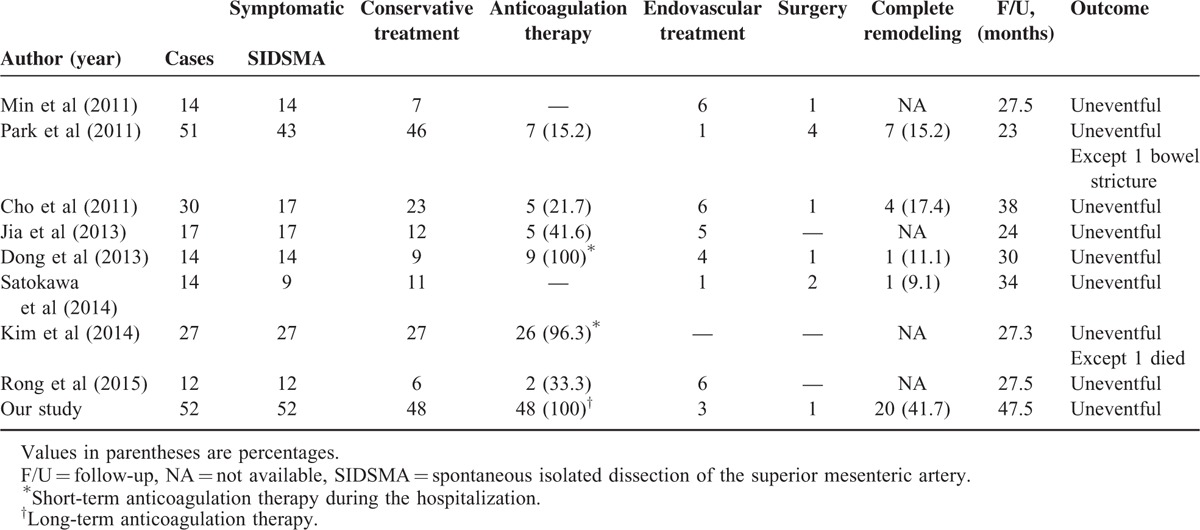
Although the exact pathophysiology and optimal treatment for SIDSMA remain the matter of some controversies, the primary objective of treatment is to limit dissection extension, to preserve the blood flow distally through the true lumen, and to prevent the rupture of the false lumen.9,10 In terms of the aforementioned primary objectives, many studies have reported excellent outcomes of conservative treatment with the use of short-term anticoagulation (during the hospitalization) or no anticoagulation therapy in patients with asymptomatic and symptomatic SIDSMA (Table 4).1–3,5,6,10–12 However, they observed a relatively lower rate of complete remodeling and a higher rate of endovascular or surgical intervention compared with the rates observed in the present study. Although the clinical significance of the morphologic changes of the dissection remains obscure, a high rate of complete remodeling might be of great benefit for these patients from a long-term perspective.
TABLE 4.
Summary of Current Studies of Treatment of Asymptomatic and Symptomatic SIDSMA (including our results)
The prognosis of “symptomatic SIDSMA” may not be as poor as was once believed. In contrast to dissection in larger vessels that have a rapid blood flow, such as the abdominal aorta, the risk of thrombus formation is significant in dissections of smaller vessels, such as the internal carotid artery and the SMA.13–16 Currently, there is no randomized controlled trial to determine whether, for patients with carotid artery dissection, antiplatelet or anticoagulation therapy is superior to control, or whether anticoagulation is superior to antiplatelet therapy; however, a systematic review pointed toward the superiority of anticoagulation over antiplatelet or control therapy across all relevant observation case series.15 In patients with an acute spontaneous internal carotid artery dissection, anticoagulation with intravenous heparin followed by oral warfarin for 6 months is generally recommended to prevent thromboembolic complications and to enhance healing process of the dissection, regardless of the symptom type. The rationale behind this approach is the high rate of recanalization that occurs within the first 3 months after the dissection and the observation that, after the discontinuation of anticoagulation, symptoms occasionally recur within 3 to 6 months after the onset of dissection, but rarely after 6 months.16 The disease pattern of acute symptomatic SIDSMA appears to resemble that of acute spontaneous internal carotid artery dissection. Therefore, we based the treatment strategy for symptomatic SIDSMA in this study on that recommended for acute spontaneous internal carotid artery dissection, and we achieved a higher rate of complete remodeling in patients with symptomatic SIDSMA than those reported in other studies using short-term anticoagulation or no anticoagulation therapy.5,6 In the literature, a recent study with a relatively large cohort described the natural course and clinical outcomes of patients with SIDSMA; of 46 asymptomatic and symptomatic patients who underwent conservative treatment with (7 patients, 15.2%) or without (39 patients, 84.8%) anticoagulation therapy, the authors observed complete remodeling of the dissection in 7 patients (15.2%) (Table 4).5 Based on these observations, we assumed that long-term anticoagulation therapy in our treatment strategy would induce a higher rate of complete remodeling during the natural course of symptomatic SIDSMA, although the retrospective nature and the small sample size of our study made it particularly challenging to reach definitive conclusions about the safety and efficacy of the treatment strategy. In patients with symptomatic SIDSMA, use of anticoagulation therapy appears to contribute to reduce the burden of acute intramural thrombus and to prevent dissection progression, thereby resulting in enhanced healing process of the dissection. However, the healing process of the dissection is time-consuming, and the duration of anticoagulation therapy may be an important factor for achieving a higher rate of complete remodeling based on our observation.
Recently, some authors proposed endovascular stenting as a primary treatment option for symptomatic patients with severe stenosis or patients with failed conservative treatment.1,17,18 However, the long-term durability of stents in patients with SIDSMA has not been confirmed, and there are some reports that SMA stents are associated with a higher risk of stent thrombosis or late restenosis than stents in other arteries in patients with atherosclerotic disease.5,6,12,19 Considering the higher success rate of conservative treatment and the substantial risk of SMA stent thrombosis or late restenosis in patients with symptomatic SIDSMA, endovascular treatment should be reserved for patients with persistent symptoms of bowel ischemia or signs of imminent rupture despite conservative treatment.19–21
Several limitations should be noted. Although we prospectively collected data based on our treatment strategy, our analysis was retrospective which did not allow for direct, randomized comparisons of the treatment outcomes with other therapeutic strategies. Patients were selected for the study based on CTA findings. Therefore, patients who presented with SMA occlusion and bowel infarction necessitating urgent laparotomy, or who died of bowel infarction related to SIDSMA, would not have been included in this study because they would never have been subjected to a CTA. Finally, considering that most studies involved in Asian population in the literature review, there may be racial/ethnic disparities in the pathophysiology and prevalence of symptomatic SIDSMA. However, our current findings were obtained at a single center, resulting in a small sample size of Asian population that will limit the overall relevance of our results.
In conclusion, despite the aforementioned potential limitations, the present study showed that long-term anticoagulation therapy can result in a high rate of complete remodeling during the natural course of symptomatic SIDSMA, and conservative treatment with the use of long-term anticoagulants may be the optimal treatment strategy for achieving complete healing of the dissection if there is no evidence of bowel infarction or bleeding. Future prospective trials with larger cohorts will lead to a better understanding of the safety and efficacy of long-term anticoagulation therapy in patients with symptomatic SIDSMA.
Footnotes
Abbreviations: CTA = computed tomography angiography, SIDSMA = spontaneous isolated dissection of the superior mesenteric artery.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Min SI, Yoon KC, Min SK, et al. Current strategy for the treatment of symptomatic spontaneous isolated dissection of superior mesenteric artery. J Vasc Surg 2011; 54:461–466. [DOI] [PubMed] [Google Scholar]
- 2.Satokawa H, Takase S, Seto Y, et al. Management strategy of isolated spontaneous dissection of the superior mesenteric artery. Ann Vasc Dis 2014; 7:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia ZZ, Zhao JW, Tian F, et al. Initial and middle-term results of treatment for symptomatic spontaneous isolated dissection of superior mesenteric artery. Eur J Vasc Endovasc Surg 2013; 45:502–508. [DOI] [PubMed] [Google Scholar]
- 4.Cho YP, Ko GY, Kim HK, et al. Conservative management of symptomatic spontaneous isolated dissection of the superior mesenteric artery. Br J Surg 2009; 96:720–723. [DOI] [PubMed] [Google Scholar]
- 5.Park YJ, Park KB, Kim DI, et al. Natural history of spontaneous isolated superior mesenteric artery dissection derived from follow-up after conservative treatment. J Vasc Surg 2011; 54:1727–1733. [DOI] [PubMed] [Google Scholar]
- 6.Kim HK, Jung HK, Cho J, et al. Clinical and radiologic course of symptomatic spontaneous isolated dissection of the superior mesenteric artery treated with conservative management. J Vasc Surg 2014; 59:465–472. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto I, Ogawa Y, Sueyoshi E, et al. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol 2007; 64:103–110. [DOI] [PubMed] [Google Scholar]
- 8.Zerbib P, Perot C, Lambert M, et al. Management of isolated spontaneous dissection of superior mesenteric artery. Langenbecks Arch Surg 2010; 395:437–443. [DOI] [PubMed] [Google Scholar]
- 9.Yun WS, Kim YW, Park KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg 2009; 37:572–577. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z, Fu W, Chen B, et al. Treatment of symptomatic isolated dissection of superior mesenteric artery. J Vasc Surg 2013; 57:69S–76S. [DOI] [PubMed] [Google Scholar]
- 11.Cho BS, Lee MS, Lee MK, et al. Treatment guidelines for isolated dissection of the superior mesenteric artery based on follow-up CT findings. Eur J Vasc Endovasc Surg 2011; 41:780–785. [DOI] [PubMed] [Google Scholar]
- 12.Rong JJ, Qian AM, Sang HF, et al. Immediate and middle term outcome of symptomatic spontaneous isolated dissection of the superior mesenteric artery. Abdom Imaging 2015; 40:151–158. [DOI] [PubMed] [Google Scholar]
- 13.Karacagil S, Hårdemark HG, Bergqvist D. Spontaneous internal carotid artery dissection. Review. Int Angiol 1996; 15:291–294. [PubMed] [Google Scholar]
- 14.Nagai T, Torishima R, Uchida A, et al. Spontaneous dissection of the superior mesenteric artery in four cases treated with anticoagulation therapy. Intern Med 2004; 43:473–478. [DOI] [PubMed] [Google Scholar]
- 15.Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev 2003; CD000255. [DOI] [PubMed] [Google Scholar]
- 16.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906. [DOI] [PubMed] [Google Scholar]
- 17.Gobble RM, Brill ER, Rockman CB, et al. Endovascular treatment of spontaneous dissections of the superior mesenteric artery. J Vasc Surg 2009; 50:1326–1332. [DOI] [PubMed] [Google Scholar]
- 18.Chu SY, Hsu MY, Chen CM, et al. Endovascular repair of spontaneous isolated dissection of the superior mesenteric artery. Clin Radiol 2012; 67:32–37. [DOI] [PubMed] [Google Scholar]
- 19.AbuRahma AF, Stone PA, Bates MC, et al. Angioplasty/stenting of the superior mesenteric artery and celiac trunk: early and late outcomes. J Endovasc Ther 2003; 10:1046–1053. [DOI] [PubMed] [Google Scholar]
- 20.Leung DA, Schneider E, Kubik-Huch R, et al. Acute mesenteric ischemia caused by spontaneous isolated dissection of the superior mesenteric artery: treatment by percutaneous stent placement. Eur Radiol 2000; 10:1916–1919. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev U, Baril DT, Ellozy SH, et al. Management of aneurysms involving branches of the celiac and superior mesenteric arteries: a comparison of surgical and endovascular therapy. J Vasc Surg 2006; 44:718–724. [DOI] [PubMed] [Google Scholar]


