Abstract
This study aimed to compare the effectiveness of a specific functional movement–power training (FMPT) program, a functional movement training (FMT) program and no training in the improvement of balance strategies, and neuromuscular performance in children with developmental coordination disorder (DCD).
It was a randomized, single-blinded, parallel group controlled trial.
Methods: 161 children with DCD (age: 6–10 years) were randomly assigned to the FMPT, FMT, or control groups. The 2 intervention groups received FMPT or FMT twice a week for 3 months. Measurements were taken before, after, and 3 months after the end of the intervention period. The primary outcomes were the composite score and strategy scores on the sensory organization test as measured by a computerized dynamic posturography machine. Secondary outcomes included the knee muscle peak force and the time taken to reach the peak force.
The balance strategies adopted in sensory challenging environments of the FMPT participants showed greater improvement from baseline to posttest than those of the FMT participants (7.10 points; 95% confidence interval, 1.51–12.69; P = 0.008) and the control participants (7.59 points; 95% confidence interval, 1.81–13.38; P = 0.005). The FMPT participants also exhibited greater improvement from baseline to the posttest in the knee extensor peak force and time to peak force in the knee flexors.
The FMPT program was more effective than the conventional FMT program in the enhancement of balance strategies and neuromuscular performance in children with DCD.
INTRODUCTION
Developmental coordination disorder (DCD) is a common motor disorder with a prevalence of approximately 6% in typical children of primary school age. Children in whom DCD is diagnosed are characterized by marked impairment in various motor functions,1 among which poor balance ability is a major concern because it predisposes children to falls, affects their motor skill development2 and participation in activities.3–5 Evidence-based treatment strategies to improve balance performance must, therefore, be developed.
It is known that balance responses can be incorporated into hip and ankle strategies that maintain the body's anterior–posterior (AP) stability in a fixed stance.6,7 The hip strategy involves hip flexion and extension movements with opposing ankle joint dorsiflexion and plantar flexion. It is a relatively poor balance strategy because the center of gravity displacement is large and thus induces postural instability. A better balance strategy, the ankle strategy, involves maintaining standing balance while rotating the body as a rigid mass about the ankle joints.7,8 Recently, our research team discovered that children with DCD tend to use the hip strategy excessively when they are forced to rely on vestibular input to maintain standing balance,9 and slowed hamstring muscle force production, a neuromuscular deficit, could be one of the causes.10 However, the present treatment regimens for children with DCD primarily focus on the induction of neuroplastic changes in the central nervous system (CNS) to enhance functional balance performance by means of functional movement training (FMT) or a task-oriented approach.11–13 Less emphasis has been placed on the treatment of neuromuscular impairments, such as slowed hamstring muscle force production, that could also affect balance outcomes.11 We postulated that interventions for children with DCD should address both the CNS and peripheral neuromuscular deficits to maximize their effectiveness in the improvement of postural control and thus the minimization of health care costs.
Power or strength training has been found to be effective in increasing the speed of muscle force production and balance in young people via various neuromuscular mechanisms.14,15 One recent case report has suggested that strength training may improve gross motor function in a child with DCD.16 Muscle power training might therefore be an ideal adjunct therapy to FMT for the improvement of the overall balance performance in children with DCD. However, no experimental study has yet investigated this potentially beneficial treatment regimen. This study was performed to compare the effectiveness of power training with FMT, FMT alone, and no intervention in the improvement of balance strategies and performance in children with DCD. We hypothesized that power training with FMT would be more effective in improving neuromuscular and balance performance and balance strategies than FMT alone or no training.
METHODS
Study Design
This was a single-blind, stratified, randomized controlled clinical trial and was registered with ClinicalTrials.gov (NCT02393404). Ethical approval was obtained from the Human Research Ethics Committee of the University of Hong Kong. The study was explained to each participant and parent, and written informed consent was obtained.
Participants
Children with DCD were recruited from local child assessment centers, hospitals, schools, nongovernment organizations, and parent groups by means of poster and website advertising. The inclusion criteria were a diagnosis of DCD based on the Diagnostic and Statistical Manual of Mental Disorders IV;1 a gross motor composite score of 42 or less on the Bruininks-Oseretsky Test of Motor Proficiency;17 aged between 6 and 10 years; and no intellectual impairment. The exclusion criteria were a diagnosis of an emotional, neurological, or other movement disorder (comorbid attention deficit hyperactivity disorder, attention deficit disorder, dyslexia, and suspected autism spectrum disorder were allowed); significant congenital, musculoskeletal, or cardiopulmonary disorders that might affect motor performance; active treatment; disruptive behaviour; or an inability to follow instructions.
Screening and Randomization
Two physiotherapists screened the volunteers by telephone, and those who seemed to meet the criteria stated above underwent an in-person evaluation and baseline assessment. The eligible participants were stratified by sex and randomly assigned to either the functional movement–power training (FMPT) group, the FMT group, or the control group in a ratio of 1:1:1. The randomization procedure was carried out by an independent person and used random number table to generate allocation sequence and numbered, sealed, and opaque envelopes to ensure concealed allocation.
Interventions
The FMT group received task-specific training concurrent with electromyographic (EMG) biofeedback to remediate motor learning difficulties12 and enhance neuroplasticity and balance performance.18,19 The FMT training protocol, which was modified from the balance assessment items in the Movement Assessment Battery for Children,20 is presented in Table 1. In addition, a 2-leg balance exercise (item 1) was added, during which the participant stood on a platform and EMG biofeedback was applied to the participant's dominant leg (i.e., the leg used to kick a ball)21 with the NeuroTrac MyoPlus 4 machine (Verity Medical Ltd., Braishfield, UK). The activity of the rectus femoris and gluteus maximus muscles were monitored by visual feedback signals (vertical bargraphs)22 because these muscles are essential for the control of hip sway and also affect ankle movements.10 The participants were instructed to contract the agonistic hip muscle as fast as possible (increasing height of the corresponding EMG bargarph), when their balance was being perturbed in the AP direction and then to relax the muscle (decreasing height of the corresponding EMG bargarph) to avoid overbalancing. All participants practiced these balance strategies repeatedly for 10 minutes before they moved on to the other FMT exercises. Maximum voluntary isometric contraction of each muscle was documented using the same EMG machine prior to training. Participants were instructed not to contract their muscles strongly/greater than 70% maximum voluntary isometric contraction (denoted by a beep sound) during the intervention to avoid muscle fatigue. In addition, a short break was allowed if participants complained of muscle fatigue during training.
TABLE 1.
Functional Movement–Power Training and Functional Movement Training Protocols∗
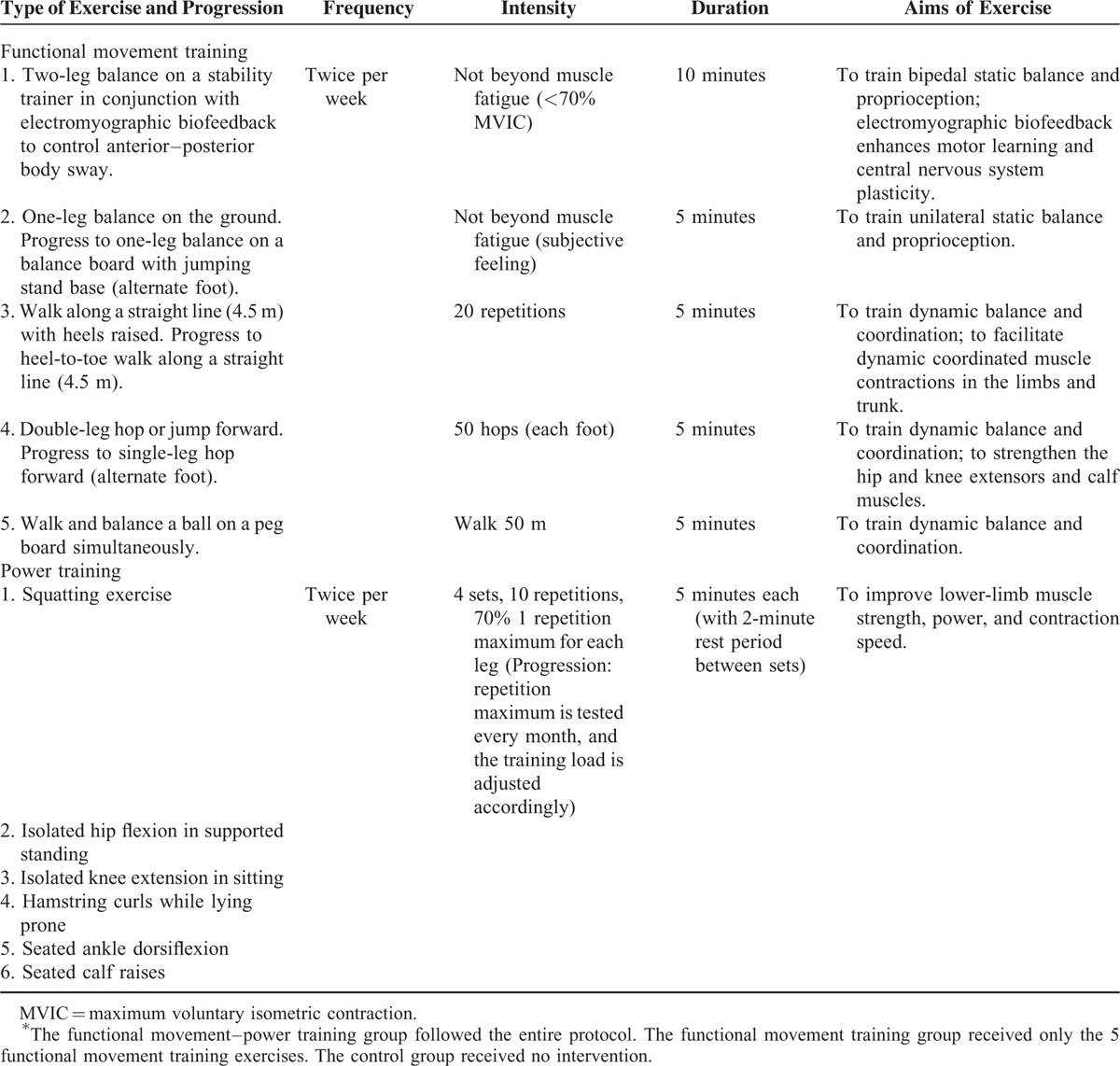
The participants in the FMPT group also received power/resistance training after the FMT. The power training exercises were intended to improve postural muscle strength and contraction speed in the legs. The training protocol, presented in Table 1, was derived from 2 resistance training programmes that are effective in increasing muscle contraction speed in young people.14,15 During the power training session, the participants contracted the gluteus maximus (squatting), iliopsoas (hip flexion), quadriceps (knee extension and squatting), hamstrings (hamstring curls), tibialis anterior (ankle dorsiflexion), and gastrocnemius and soleus (seated calf raises and squatting) muscles as quickly as possible against a load (70% of 1 repetition maximum). These leg muscles were selected because their strength and rate of strength development are important for control of the AP body sway while standing.23 The 1 repetition maximum, which is defined as the maximum load that can be lifted 1 time,24 was tested during the 1st session and every month throughout the intervention period. The training load (i.e., cuff weights) relative to the 1 repetition maximum was adjusted accordingly as a kind of progression.15 Each training session was supervised by a physiotherapist and conducted by a trained assistant. The children in the FMPT and FMT groups attended 2 training sessions per week (1.5 hours per session) at the University of Hong Kong Physical Activity Laboratory for 12 weeks.25 The control group received no training but continued their usual daily activities.
Primary Outcomes
Standing balance and balance strategies were assessed using the sensory organization test (SOT), which is a valid and reliable test in children.26,27 Each participant was instructed to stand barefoot on the force platform of a computerized dynamic posturography machine (Smart Equitest, NeuroCom International Inc., OR). The foot placement was standardized according to the participant's height. Each participant was then exposed to 6 sensory conditions of 3 trials each (18 trials in total). The sensory inputs (i.e., somatosensory, visual, and vestibular) available for each SOT condition have been fully described in our previous papers.3,9,28 The machine captured the trajectory of the participant's center of pressure during the 18 trials and generated an equilibrium score (ES) for each trial. The composite ES score (ranging from 0 to 100) from all 18 trials was used for analysis. It reflects the participant's general balance ability during standing.29
In addition to the ES score, the machine also detected the shear force in the AP direction and generated a strategy score (SS) that represents the amount of ankle and hip movement used in maintaining balance under the 6 sensory conditions. The SS was calculated according to the formula:
SS = [1 − (SHmax − SHmin)/25 lbs] × 100
where SHmax represents the greatest horizontal AP shear force detected by the force platform and SHmin is the smallest. Their difference was normalized to 25 lbs (111.25 N) of shear force, which is the average difference between the highest and lowest AP shear forces generated by a group of normal participants who used hip sway only to balance on a narrow beam.29 An SS of 100 indicates that the participant predominantly used the ankle to maintain standing balance, whereas an SS of 0 indicates that the participant mainly used the hip strategy. An independent SS (ranging from 0 to 100) was obtained for each test trial, and the average SS of all 18 trials was derived.29 The average SS for each sensory condition and the overall average SS were used for analysis.
Secondary Outcomes
The maximum isometric muscle strength (peak force) of the participants’ dominant knee flexors and extensors was measured using the Lafayette Manual Muscle Test System (Model 01165, Lafayette Instrument Company, Lafayette, LA) with standardized manual muscle testing procedures30 and dynamometer placements.31 Good to perfect reliability (intraclass correlation coefficient: 0.81–0.98) has been reported.32 Each participant completed 2 trials of manual muscle testing during which the peak force was generated for 2 seconds for each muscle group. The participants were instructed to voluntarily contract their muscles as hard and as quickly as possible. The average peak force of the 2 trials was used for analysis. The mean time to peak force, defined as the elapsed time from the start of the test until the maximum force is reached,31 was also documented for data analysis.
Test Procedures
Data collection was performed at the Balance Laboratory of the Hong Kong Polytechnic University by a physiotherapist and a trained assistant who were blinded to the group allocation. All participants were assessed before the start of the interventions (baseline), immediately after the interventions (posttest), and 3 months after the end of the interventions (follow-up test).
Statistical Analyses
On the basis of our pilot trial, we estimated that a sample of 45 participants per group would provide at least 80% power to detect a between-groups difference in a mean change from baseline to 3 months of 0.335 points in the primary outcomes, assuming a 25% attrition rate, at a 2-tailed alpha level of 5%. These predicted mean point differences equate to a medium to large effect size of 0.67.
All of the analyses were conducted on an intention-to-treat basis (last-observation-carried-forward method). The between-groups differences in demographic variables were assessed with 1-way analysis of variance for continuous data and with a Chi-square test for categorical data (i.e., sex, coexisting conditions, and routine medication). Baseline primary and secondary outcome variables were also assessed with 1-way analysis of variance to detect any significant between-groups differences before the interventions. Any changes in the primary and secondary outcomes following the intervention were quantified by subtracting the baseline scores from the postintervention scores. The differences from the baseline in each outcome measure were analyzed with mixed-model repeated-measures analysis of variance (between-subjects factor: group; and within-subject factor: time) followed by post-hoc tests. All P values were corrected using Bonferonni method to maintain the overall significance level at 5% (2-tailed). The results are presented as means with SDs or 95% confidence intervals (CIs). All of the statistical analyses were performed with SPSS 20.0 (IBM).
RESULTS
Study Population
From January to May 2014, 178 children were screened for eligibility; 161 qualified and underwent randomization: 53 to the FMPT group, 55 to the FMT group, and 53 to the no-training control group (Figure 1). The reasons for exclusion from the study were a formal diagnosis of autism spectrum disorder (n = 6), rehabilitation therapy outside the study (n = 5), and an inability to follow instructions (n = 4). Two participants declined to participate in the study without giving a reason.
FIGURE 1.
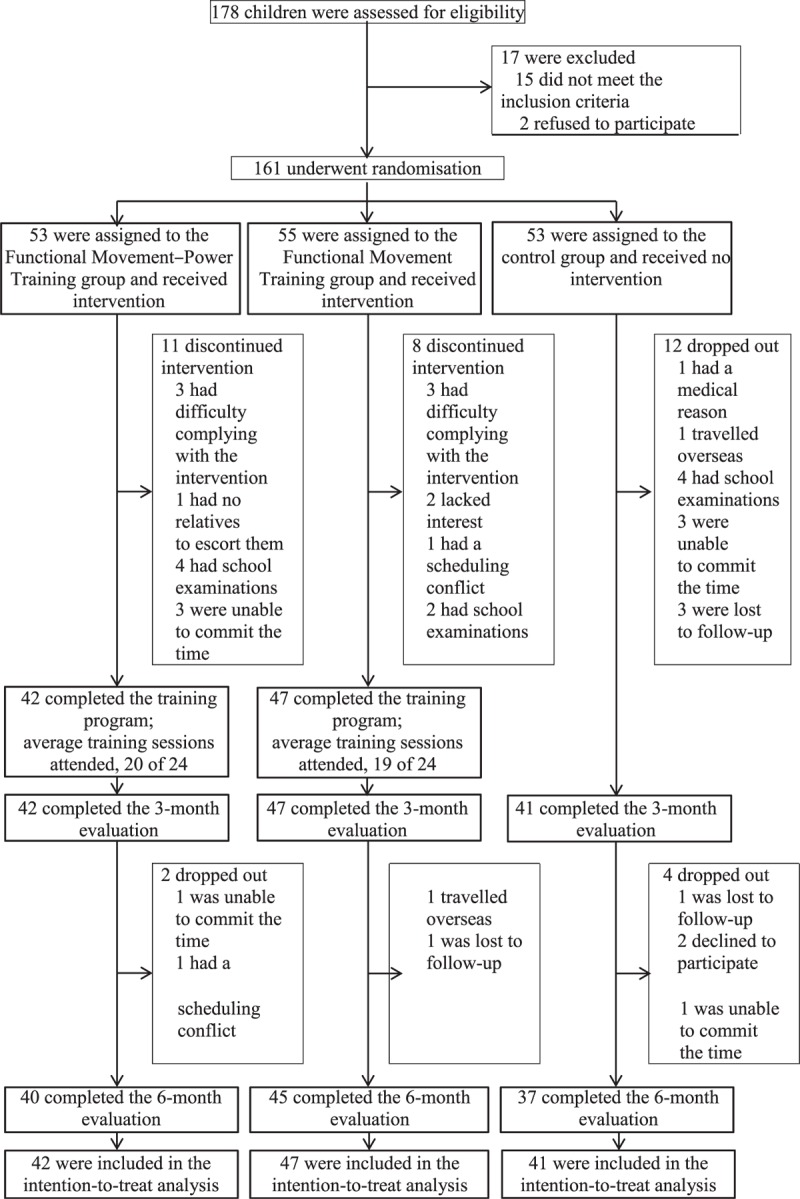
Participant flow chart.
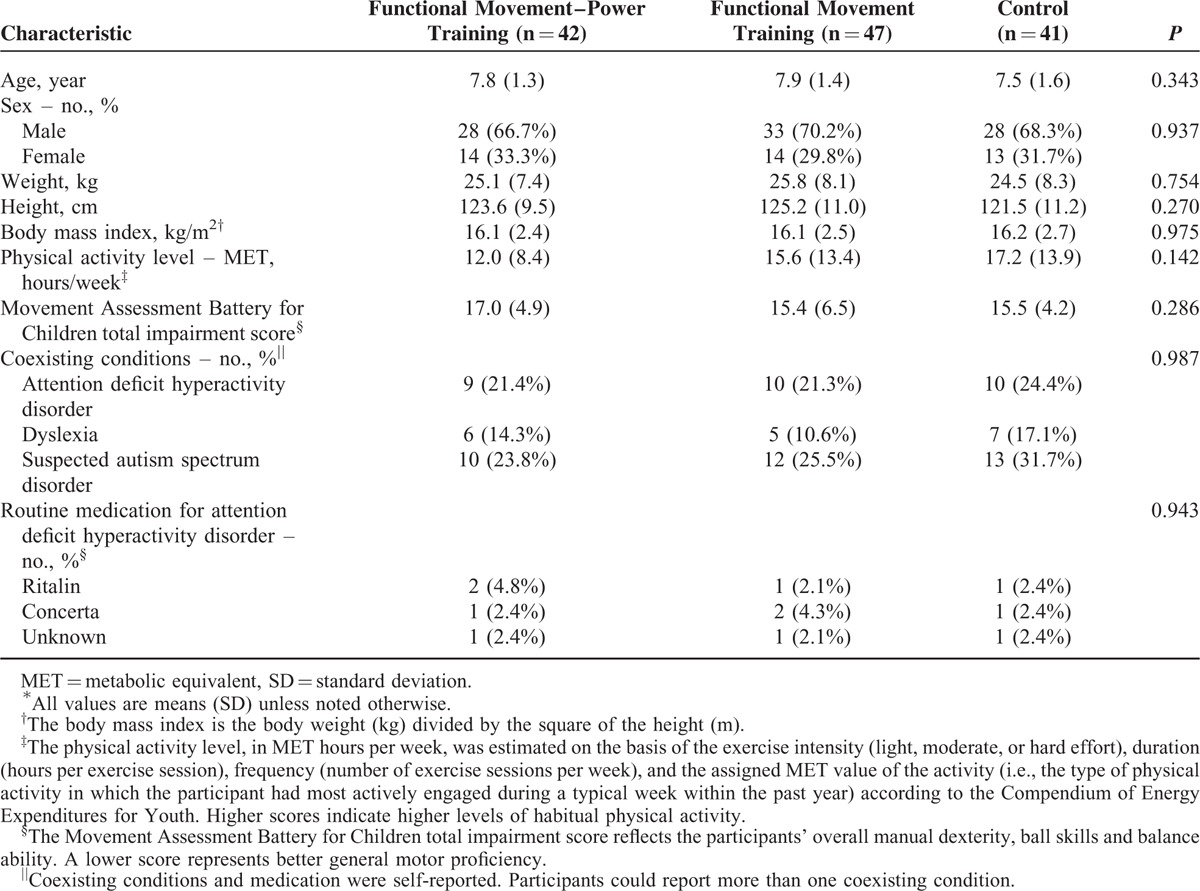
No statistically significant differences in any of the participant's baseline characteristics were found among the 3 groups (Table 2). A total of 130 children completed the interventions and the 3-month evaluation. Among which, 122 children (76%) completed the 6-month evaluation. No statistically significant differences were seen in the baseline demographic data between the participants who successfully completed the study and those who did not. Moreover, the attendance rates of the FMPT and FMT interventions were 83% and 79%, respectively (Figure 1). The attendance rates demonstrated no significant difference between the 2 intervention groups (P = 0.221). No changes in the participants’ medication used or physical activity level were noted overtime within the 3 groups.
TABLE 2.
Baseline Characteristics of the Participants∗
Primary Outcomes
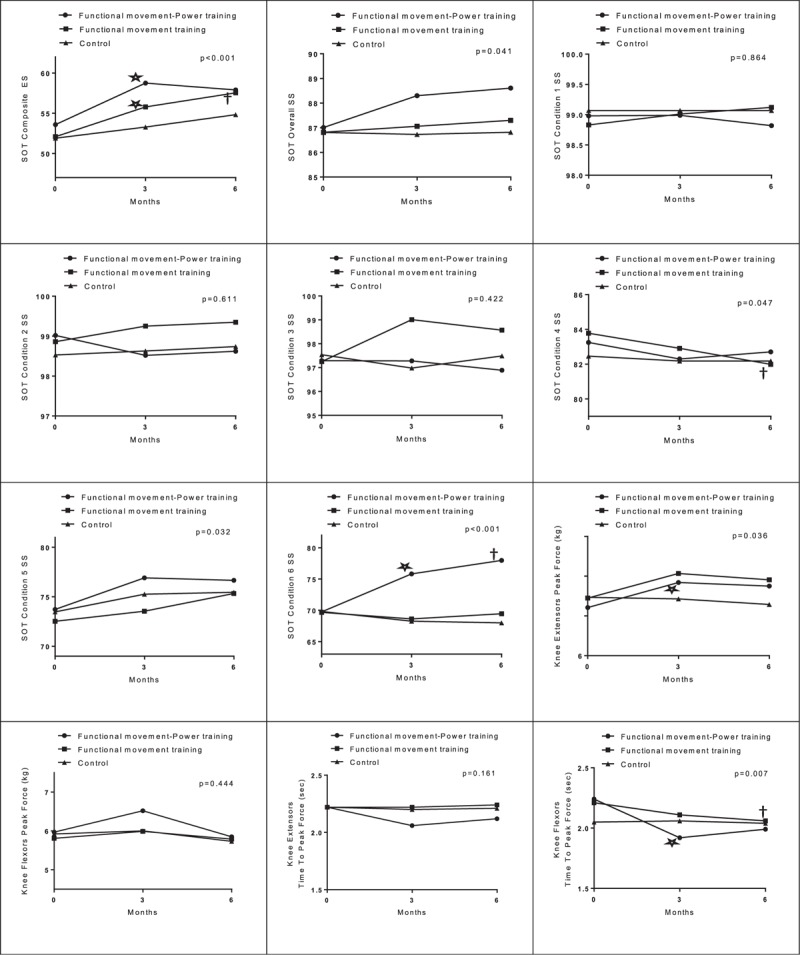
Tables 3, 4, and Figure 2 show the mean changes from baseline to 3 and 6 months in the 3 groups for all outcomes. At 3 months, only the FMPT group had a significant improvement in the SS in condition 6 (6.06 points; 95% CI −9.97 to −2.15; P = 0.003). The mean difference between the FMPT and FMT groups was 7.10 points (P = 0.008), whereas the difference between the FMPT and control groups was 7.59 points (P = 0.005). These between-groups differences in the SS in condition 6 gradually increased after the intervention period. At 6 months, the FMPT group had a significant improvement in the SS in condition 6 (8.21 points; 95% CI −12.27 to −4.15; P < 0.001). The between-groups differences were 8.43 points (P = 0.001) between the FMPT and FMT groups and 10.03 points (P < 0.001) between the FMPT and control groups. The overall SS and the SS for conditions 1 to 5 remained relatively stable in all 3 groups overtime.
TABLE 3.
Outcome Measures at Baseline, 3 and 6 months∗
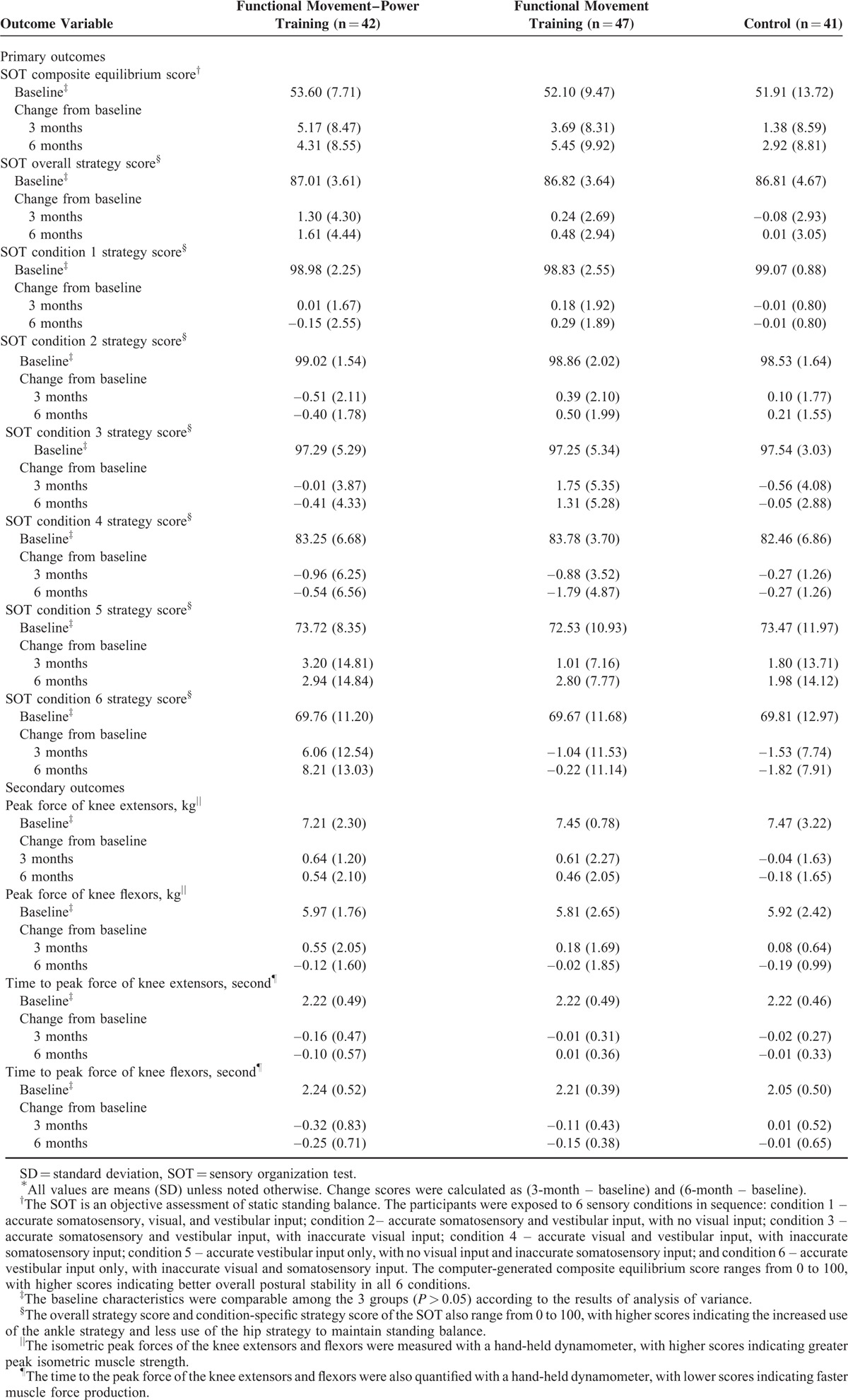
TABLE 4.
Between-Groups Differences in Mean Change From Baseline of All Outcome Measures∗
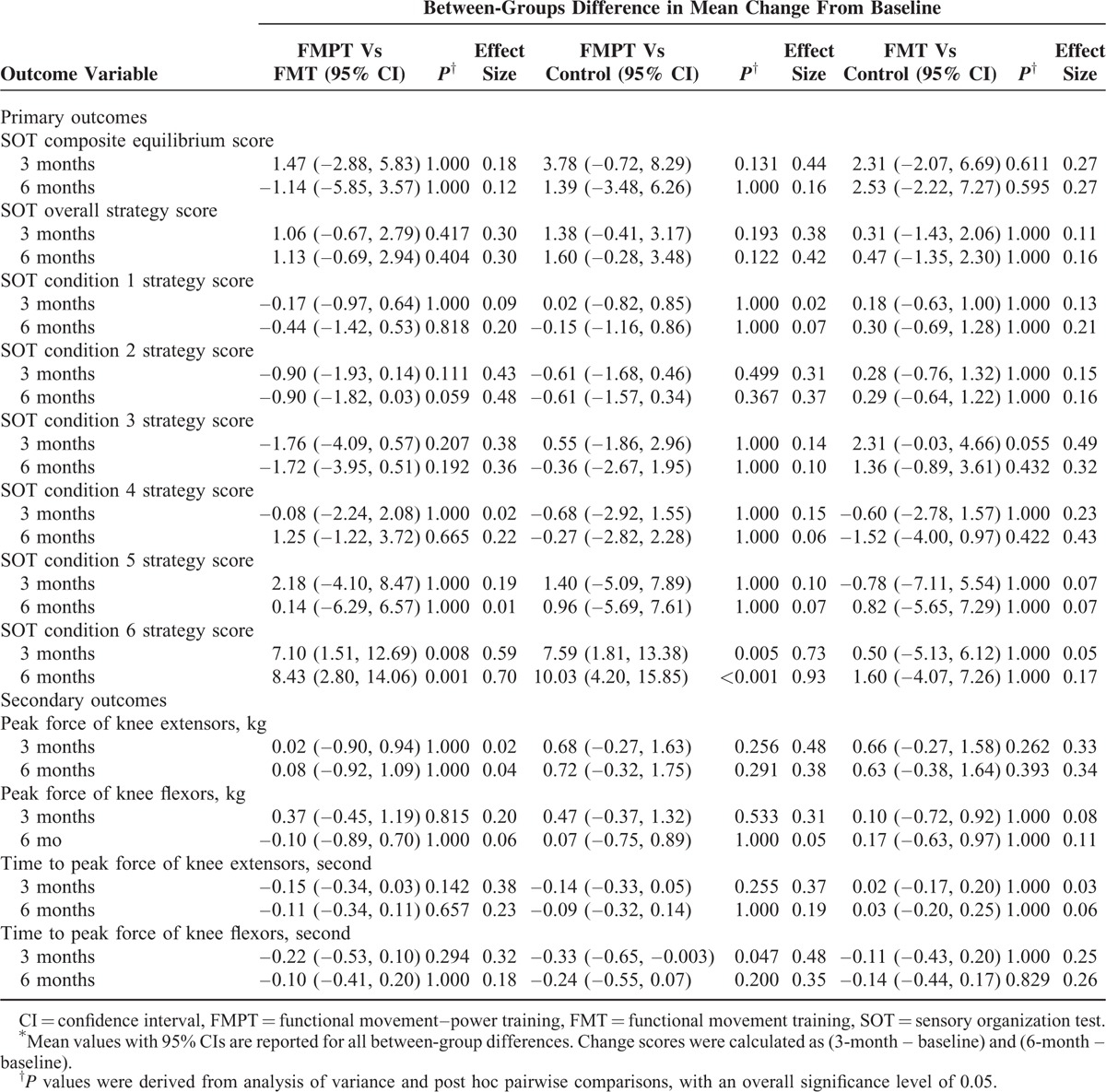
FIGURE 2.
Mean changes in all outcomes at 3 and 6 months.
Regarding balance performance, both the FMPT and FMT groups had greater improvement at 3 months than did the control group, as measured by the change in the composite ES. Specifically, from baseline to 3 months, the children in the FMPT and FMT groups had mean increases in the composite ES of 5.16 points (95% CI −7.81 to −2.53; P < 0.001) and 3.69 points (95% CI −6.13 to −1.25; P = 0.004), respectively. No significant change was observed in the control group. However, none of the between-groups differences were significant. The improvement in the composite ES in the FMT group was maintained at 6 months (mean change from baseline to 6 months, 5.44 points; 95% CI −8.36 to −2.54; P < 0.001) only, but the between-groups differences were not significant. A separate analysis was performed after removal of the dropouts (on-protocol analysis), and similar results were obtained (data not shown).
Secondary Outcomes
The changes from baseline to 3 months in the time to peak force of the knee flexors also favored the FMPT group (−0.32 seconds; 95% CI 0.06–0.58; P = 0.016) over the other 2 groups, and the difference between the FMPT and control groups was significant (−0.33 seconds; 95% CI, −0.65 to −0.003; P = 0.047). However, this improvement in the FMPT group was not maintained at 6 months. Instead, the change from baseline to 6 months in the time to peak force of the knee flexors was significant in the FMT group (−0.15 seconds; 95% CI 0.04–0.26; P = 0.009); however, no significant between-groups differences were found.
Improvement in the peak force of the knee extensors at 3 months was seen only in the FMPT group (0.63 kg; 95% CI −1.01 to −0.26; P = 0.001), but the mean between-groups differences were not significant. The improvement was not maintained at 6 months in the FMPT group (P = 0.105). No significant changes from baseline to 3 and 6 months were observed in the peak force of the knee flexors and the time to peak force of knee extensors in any of the groups (Figure 2).
Adverse Events
No adverse events were reported during the laboratory assessments. The training-related adverse events were mild: transient muscle soreness (2 subjects in both FMPT and FMT groups) and falls without injury (1 in the FMT group).
DISCUSSION
This study is the first to show that a 3-month program of twice-weekly FMPT was more effective than FMT alone or no training in improving balance strategies (i.e., a decrease in reliance on the hip strategy and an increase in reliance on the ankle strategy) in a sensorially challenging environment (e.g., only vestibular input was available in SOT condition 6) in children with DCD. The improvements were maintained for 3 months after the cessation of training. This finding supports our hypothesis that the balance strategies of children with DCD can be improved most by treating both their CNS and neuromuscular deficits. Theoretically, FMT can induce neuroplastic changes in the CNS (e.g., modification of Purkinje cell synapses in the cerebellum),11,12 and power training can increase the speed of muscle contraction (force production) via several neuromuscular mechanisms: earlier motor unit activation, enhanced maximal motor unit firing rate in the initial stages of activation,15 increased efferent neural drive to the agonist muscles,14 improved intermuscular and intramuscular coordination, and improved force control.33 In fact, we found that the children with DCD required a shorter time to reach the peak force in the knee flexors and had greater peak force in the knee extensors after FMPT training. These findings may explain the decreased use of the hip strategy (hip sway) in the participants in the FMPT group because their hamstrings and rectus femoris could contract strongly and in a timely fashion to control the hip flexion-extension movements when their balance was challenged.10
Although only the FMPT improved balance strategies, both the FMPT and FMT were effective in improving the overall standing balance performance in children with DCD. In addition, the improvement was maintained at 6 months in the FMT group, probably because FMT required the children to practice the balancing movements repeatedly with EMG biofeedback, which can effectively enhance CNS plasticity.11,25,34,35 In addition, the increased concomitant muscle force production speed of the knee flexors in the FMT group at 6 months may also have contributed to the improvement in balance performance. Further study is necessary to explore the relationships among balance performance, balance strategies, and muscle force production speed in children with DCD.
The present study demonstrated that integration of power training with FMT can improve the overall standing balance performance, balance strategies, and knee muscle strength in children with DCD. Our previous studies showed that intensive martial art (taekwondo [TKD]) training can also improve standing balance performance and knee muscle strength in the DCD population.9,36 So, which intervention is better? We postulated that FMPT may be a better choice than TKD given the fact that the power training component was specifically designed for strengthening lower extremity postural muscles, and children can practice FMPT at home easily without the use of martial art training equipment (TKD kick pad). In addition, we hypothesized that only FMPT can improve balance strategies of children with DCD, but not TKD training. It is because biomechanical analyses showed that TKD practitioners used a lot of hip strategy, rather than ankle strategy, to maintain postural stability during kicking.37 Certainly, further experimental study is necessary to compare the efficacy of these 2 interventions for improving balance performance and balance strategies in children with DCD.
The major limitation of this study was that the participants were not blinded to the group assignment, given the nature of exercise training. The participants who were assigned to the intervention groups may have had expectations about the benefits of exercise, which may have introduced some biases in the results.38 Another limitation was that the balance strategies were estimated from the horizontal AP shear forces detected by the force plate.29 Further studies could assess hip, knee, and ankle movements directly using kinematic measures such as electrogoniometry. Moreover, EMG biofeedback was used during FMT but the signals were not captured. Further studies could record the EMG signals during both FMT and FMPT and treat EMG muscle activity as an outcome measure to indicate the neuromuscular performance before and after trainings. Finally, this was a laboratory-based study. It is not certain whether the improved balance performance in children with DCD can be carried over to daily activities.
CONCLUSIONS
FMPT led to better results than conventional FMT in the improvement of balance strategies in a sensorially challenging environment, and the neuromuscular performance of children with DCD. FMPT appears to be effective as a stand-alone intervention designed to improve balance strategies, postural stability, and leg muscle performance in children with DCD.
Acknowledgements
The authors thank the Child Assessment Service (Department of Health, Hong Kong), Heep Hong Society, Hong Kong Christian Service (Infant Stimulation and Parent Effectiveness Training Service), TWGHs Hok Shan School, SKH St Matthew's Primary School, Aplichau Kaifong Primary School, Hennessy Road Government Primary School (am and pm), Tsung Tsin Primary School and Kindergarten, Watchdog Early Education Centre, and Caritas Nursery School (Tsui Lam) for enabling the recruitment of the participants.
Footnotes
Abbreviations: AP = anterior–posterior, CI = confidence interval, CNS = central nervous system, DCD = developmental coordination disorder, EMG = electromyographic, ES = equilibrium score, FMPT = functional movement–power training, FMT = functional movement training, SOT = sensory organization test, SS = strategy score, TKD = taekwondo.
Trial registration: ClinicalTrials.gov (NCT02393404)
This study was funded by the Research Grants Council of Hong Kong (ECS 27100614) and the University of Hong Kong Merit Award for RGC GRF/ECS Funded Projects.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Grove CR, Lazarus JAC. Impaired re-weighting of sensory feedback for maintenance of postural control in children with developmental coordination disorder. Hum Mov Sci 2007; 26:457–476. [DOI] [PubMed] [Google Scholar]
- 3.Fong SSM, Lee VYL, Pang MYC. Sensory organization of balance control in children with developmental coordination disorder. Res Dev Disabil 2011; 32:2376–2382. [DOI] [PubMed] [Google Scholar]
- 4.Fong SSM, Lee VYL, Chan NNC, et al. Motor ability and weight status are determinants of out-of-school activity participation for children with developmental coordination disorder. Res Dev Disabil 2011; 32:2614–2623. [DOI] [PubMed] [Google Scholar]
- 5.Tsang WWN, Guo X, Fong SSM, et al. Activity participation intensity is associated with skeletal development in pre-pubertal children with developmental coordination disorder. Res Dev Disabil 2012; 33:1898–1904. [DOI] [PubMed] [Google Scholar]
- 6.Cherng RJ, Hsu YW, Chen YJ, et al. Standing balance of children with developmental coordination disorder under altered sensory conditions. Hum Mov Sci 2007; 26:913–926. [DOI] [PubMed] [Google Scholar]
- 7.Nashner LM. Jacobson GP, Newman CW, Kartush JM. Computerized dynamic posturography. Handbook of Balance Function and Testing. St Louis, MO: Mosby Yearbook; 1997. 261–307. [Google Scholar]
- 8.Horak FB, Macpherson JM. Shepard JT, Rowell LG, Dempsey JA. Postural orientation and equilibrium. Handbook of Physiology, Section 7, Exercise: Regulation and Integration of Multiple Systems. New York, NY: Oxford University Press; 1996. 255–292. [Google Scholar]
- 9.Fong SSM, Tsang WWN, Ng GYF. Altered postural control strategies and sensory organization in children with developmental coordination disorder. Hum Mov Sci 2012; 31:1317–1327. [DOI] [PubMed] [Google Scholar]
- 10.Fong SSM, Ng SSM, Yiu BPHL. Slowed muscle force production and sensory organization deficits contribute to altered postural control strategies in children with developmental coordination disorder. Res Dev Disabil 2013; 34:3040–3048. [DOI] [PubMed] [Google Scholar]
- 11.Zwicker JG, Missiuna C, Harris SR, et al. Developmental coordination disorder: a review and update. Eur J Paediatr Neurol 2012; 16:573–581. [DOI] [PubMed] [Google Scholar]
- 12.Missiuna C, Rivard L, Bartlett D. Exploring assessment tools and the target of intervention for children with developmental coordination disorder. Phys Occup Ther Pediatr 2006; 26:71–89. [PubMed] [Google Scholar]
- 13.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res 2008; 51:S225–S239. [DOI] [PubMed] [Google Scholar]
- 14.Aagaard P, Simonsen EB, Andersen JL, et al. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 2002; 93:1318–1326. [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behavior contribute to the increase in contraction speed after dynamic training in humans. J Physiol 1998; 513:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman LB, Schilling DL. Implementation of a strength training program for a 5-year-old child with poor body awareness and developmental coordination disorder. Phys Ther 2007; 87:455–467. [DOI] [PubMed] [Google Scholar]
- 17.Bruininks RH. Bruininks–Oseretsky Test of Motor Proficiency: Examiner's Manual. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- 18.Jonsdottir J, Cattaneo D, Recalcati M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil Neural Repair 2010; 24:478–485. [DOI] [PubMed] [Google Scholar]
- 19.Zwicker JG, Missiuna C, Harris SR, et al. Developmental coordination disorder: a pilot diffusion tensor imaging study. Pediatr Neurol 2012; 46:162–167. [DOI] [PubMed] [Google Scholar]
- 20.Henderson SE, Sugden DA. Movement Assessment Battery for Children Manual. London, UK: The Psychological Corporation Ltd; 1992. [Google Scholar]
- 21.Fong SM, Ng GYF. The effects on sensorimotor performance and balance with Tai Chi training. Arch Phys Med Rehabil 2006; 87:82–87. [DOI] [PubMed] [Google Scholar]
- 22.Verity Medical Ltd. NeuroTrac Myo Plus 4 Operators Manual. Braishfield, UK: Verity Medical Ltd; 2010. [Google Scholar]
- 23.Robinovitch SN, Heller B, Lui A, et al. Effect of strength and speed of torque development on balance recovery with the ankle strategy. J Neurophysiol 2002; 88:613–620. [DOI] [PubMed] [Google Scholar]
- 24.Baechle TR, Earle RW. Essentials of Strength Training and Conditioning – National Strength and Conditioning Association. 3rd ed.Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 25.Schoemaker MM, Hijlkema MGJ, Kalverboer AF. Physiotherapy for clumsy children: an evaluation study. Dev Med Child Neurol 1994; 36:143–155. [DOI] [PubMed] [Google Scholar]
- 26.Fong SSM, Fu SN, Ng GYF. Taekwondo training speeds up the development of balance and sensory functions in young adolescents. J Sci Med Sport 2012; 15:64–68. [DOI] [PubMed] [Google Scholar]
- 27.Di Fabio R, Foudriat BA. Responsiveness and reliability of a pediatric strategy score for balance. Physiother Res Int 1996; 1:180–194. [DOI] [PubMed] [Google Scholar]
- 28.Fong SSM, Tsang WWN, Ng GYF. Taekwondo training improves sensory organization and balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2012; 33:85–95. [DOI] [PubMed] [Google Scholar]
- 29.NeuroCom. Balance Manager Systems: Instructions for Use. Clackamas, OR: NeuroCom International; 2008. [Google Scholar]
- 30.Kendall FP, McCreary EK, Provance PG. Muscle Testing and Function With Posture and Pain. 4th ed.Baltimore, MD: Williams & Wilkins; 1993. [Google Scholar]
- 31.Lafayette Instrument. Lafayette Manual Muscle Test System User Instructions. Lafayette, IN: Lafayette Instrument Company; 2012. [Google Scholar]
- 32.Taylor NF, Dodd KJ, Graham HK. Test-retest reliability of hand-held dynamometric strength testing in young people with cerebral palsy. Arch Phys Med Rehabil 2004; 85:77–80. [DOI] [PubMed] [Google Scholar]
- 33.Orr R, de Vos NJ, Singh NA, et al. Power training improves balance in healthy older adults. J Gerontol Med Sci 2006; 61:78–85. [DOI] [PubMed] [Google Scholar]
- 34.Schoemaker MM, Niemeijer AS, Reynders K, et al. Effectiveness of neuromotor task training for children with developmental coordination disorder: a pilot study. Neural Plast 2003; 10:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poole JL. Application of motor learning principles in occupational therapy. Am J Occup Ther 1991; 45:531–537. [DOI] [PubMed] [Google Scholar]
- 36.Fong SSM, Chung JWY, Chow LPY, et al. Differential effect of Taekwondo training on knee muscle strength and reactive and static balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2013; 34:1446–1455. [DOI] [PubMed] [Google Scholar]
- 37.Kim YK, Kim YH, Im SJ. Inter-joint coordination in producing kicking velocity of Taekwondo kicks. J Sports Sci Med 2011; 10:31–38. [PMC free article] [PubMed] [Google Scholar]
- 38.Crocetti MT, Amin DD, Scherer R. Assessment of risk of bias among pediatric randomized controlled trials. Pediatrics 2010; 126:298–305. [DOI] [PubMed] [Google Scholar]


