Abstract
BACKGROUND:
Transcutaneous bilirubin (TcB) meters are widely used for screening newborns for jaundice, with a total serum bilirubin (TSB) measurement indicated when the TcB value is classified as “positive” by using a decision rule. The goal of our study was to assess the clinical utility of 3 recommended TcB screening decision rules.
METHODS:
Paired TcB/TSB measurements were collected at 34 newborn nursery sites. At 27 sites (sample 1), newborns were routinely screened with a TcB measurement. For sample 2, sites that typically screen with TSB levels also obtained a TcB measurement for the study. Three decision rules to define a positive TcB measurement were evaluated: ≥75th percentile on the Bhutani nomogram, 70% of the phototherapy level, and within 3 mg/dL of the phototherapy threshold. The primary outcome was a TSB level at/above the phototherapy threshold. The rate of false-negative TcB screens and percentage of blood draws avoided were calculated for each decision rule.
RESULTS:
For sample 1, data were analyzed on 911 paired TcB-TSB measurements from a total of 8316 TcB measurements. False-negative rates were <10% with all decision rules; none identified all 31 newborns with a TSB level at/above the phototherapy threshold. The percentage of blood draws avoided ranged from 79.4% to 90.7%. In sample 2, each rule correctly identified all 8 newborns with TSB levels at/above the phototherapy threshold.
CONCLUSIONS:
Although all of the decision rules can be used effectively to screen newborns for jaundice, each will “miss” some infants with a TSB level at/above the phototherapy threshold.
What’s Known on This Subject:
Several decision rules for using transcutaneous bilirubin measurements to screen newborns for jaundice have been found to be effective in identifying newborns with significant jaundice while obviating the need for a total serum level in most infants.
What This Study Adds:
The data in the current study provide a comparison of the diagnostic utility of 3 specific, recommended decision rules for transcutaneous bilirubin screening in a large, diverse population of newborns from multiple newborn nurseries.
Systematic screening of healthy term and late-preterm newborns for jaundice with a transcutaneous bilirubin (TcB) measurement is a potentially effective strategy for identifying newborns with significant hyperbilirubinemia during their birth hospitalization.1 The use of a TcB meter for screening has several advantages, including being a point-of-care, noninvasive method for estimating bilirubin levels and providing nearly instantaneous results. In multiple studies, TcB measurements have been shown to provide reasonably accurate estimates of total serum bilirubin (TSB) levels.2–14
When using TcB measurement for screening, a blood draw for the gold standard TSB measurement is recommended for those infants whose TcB level is above some threshold value.1 The ideal screening test has a sensitivity and a specificity that are close to 100%. However, in many clinical settings, the worst error that can occur is that the result of the screening test is normal in a patient who has “disease” (ie, a false-negative test result).15 Therefore, threshold values for defining a “positive” or “abnormal” screening test result are adjusted to maximize the sensitivity at the expense of lowering specificity. In the context of TcB screening for jaundice in newborns, the goals for defining a positive test result are to identify all or nearly all newborns with a high TSB level, while obviating the need for a blood draw in most patients.1
Experts from the American Academy of Pediatrics (AAP) suggested 3 possible decision rules for TcB screening in a 2009 commentary.1 The listed decision rules included: (1) obtain a TSB level on a newborn with a TcB measurement that is ≥75th percentile on the Bhutani TSB nomogram; (2) obtain a TSB level when the TcB measurement is ≥70% of the recommended phototherapy threshold for a particular infant; and (3) obtain a TSB level when the TcB measurement is ≥13 mg/dL.5,6,16 This last rule is most applicable to newborns managed in outpatient settings, when bilirubin levels are typically peaking.4,17 Previously, Maisels18 proposed the pragmatic approach of drawing blood for a TSB level in a newborn whose TcB measurement is within 3 mg/dL of the phototherapy threshold.
We conducted a large, multisite study to compare the utility of different decision rules for TcB screening. The goal of the present study was to identify ≥1 robust decision rule that would provide a false-negative rate near 0% (corresponding to a sensitivity near 100%) in identifying newborns with a high TSB level, while eliminating the need for a blood draw in most infants.
Methods
The present study was conducted by the Better Outcomes through Research for Newborns (BORN) network. BORN is a national network of researchers and clinicians who provide care for healthy term and late-preterm neonates at academic and community medical centers. The network currently includes 383 members from 95 newborn nursery sites located in 35 states. The study was approved by the institutional review boards at each of the participating BORN nursery sites. Study data were collected on neonates born between January 2012 and May 2014.
Study Participants
Data were collected on 2 samples of newborns for the present study by using either the Bilichek (Philips, Monroeville, PA) or JM-103 (Draeger Medical, Telford, PA) brands of TcB meters. For the first sample, BORN members at nursery sites at which neonates were routinely screened for jaundice with TcB measurements retrospectively collected data on all newborns ≥35 weeks’ gestation who were admitted to the newborn nursery during two 2-week periods (sample 1). Data on all TcB and TSB measurements obtained in enrolled newborns <120 hours old were collected. TcB and TSB levels that were measured within 2 hours of each another in a study newborn were considered paired. We previously reported on the newborns included in this sample in a study designed to characterize discrepancies between paired TcB and TSB measurements.2
The decision to obtain a TSB level on a newborn included in sample 1 was made clinically and was likely influenced by the corresponding TcB level. Because of this method, it is possible that some neonates with high bilirubin levels were missed because the TcB level was falsely low and a blood sample for a TSB level not obtained. In assessing the utility of TcB measurement as a screening test for identifying infants with high TSB levels, our study design would thus tend to overestimate the sensitivity of TcB. To attempt to account for this possibility, we collected data on another sample of infants (sample 2). For sample 2, BORN nursery sites at which newborns are routinely screened for jaundice with a TSB level before discharge had members prospectively obtain a TcB measurement at the time of the blood draw during two 2-week periods. During these study periods, all infants born at ≥35 weeks’ gestation and admitted to the newborn nursery were enrolled, and data on paired TcB-TSB measurements obtained before the age of 120 hours were collected. At 1 nursery site at which data were collected for sample 2, a TcB measurement was made initially with a TSB level obtained only on those infants whose TcB level was ≥6.0 mg/dL.
For both samples, information regarding gestational age, race, and ethnicity was abstracted from the study newborn’s medical record. In addition, results of direct Coombs’ testing were recorded, if known. Finally, the medical record of each enrolled newborn was reviewed for identification of several “neurotoxicity risk factors,” as defined in the AAP neonatal hyperbilirubinemia guidelines. These risk factors included isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase deficiency, serum albumin levels <3.0 g/dL, acidosis, sepsis, and asphyxia.1,19 Infants with a positive direct Coombs’ test result were classified as having isoimmune hemolytic disease.
For the primary study outcome, each TSB measurement was classified as either at/above, or lower than, the phototherapy threshold based on criteria recommended in the AAP practice guideline.1,19 The phototherapy threshold was based on the TSB level, newborn age in hours, and whether the newborn was at low, medium, or high risk for neurotoxicity using the classification scheme included in the AAP guideline. Data on risk classification, TSB level, and age in hours were entered into BiliTool (bilitool.org), a web-based program that automates the assignment of risk towards the development of hyperbilirubinemia in newborns born at > 35 weeks gestation as recommended by the AAP, to classify each TSB measurement as at/above the phototherapy threshold or below the phototherapy threshold. Because phototherapy thresholds are not available in BiliTool for TSB levels obtained in an infant <12 hours of age, study TSB levels obtained before a newborn was 12 hours old were not classified.
A secondary outcome for the study was classifying TSB levels as “high risk,” defined as a TSB level that was ≥95th percentile on the Bhutani TSB nomogram.5 Using BiliTool, each TSB level was classified as high risk or not; TSB levels obtained in newborns who were <18 hours old were not categorized because Bhutani TSB nomogram data were not available on BiliTool for newborns in this age range.
Decision Rules
Three different classification schemas for TcB screening were assessed. For the first rule, TcB levels were plotted on the Bhutani TSB nomogram; levels ≥75th percentile were classified as a positive test (“≥75th percentile” decision rule).5 For the second decision rule, the corresponding TSB phototherapy threshold for each TcB measurement was determined by using the AAP practice guideline.1,19 This TSB phototherapy threshold was multiplied by 0.7; TcB levels above this value were classified as a positive test (“70% of phototherapy level” decision rule). The third decision rule was determined in a similar fashion except that a positive test result was defined as a TcB value greater than or equal to the phototherapy threshold minus 3.0 mg/dL (the “within 3 mg/dL” decision rule).
Analysis
Data from samples 1 and 2 were analyzed separately. For both samples, false-negative rates (false-negatives/[false-negatives + true-positives]), positive predictive values (PPVs),and negative predictive values (NPVs) for both the primary outcome (a TSB level at/above the phototherapy threshold) and the secondary outcome (a TSB level in the high-risk zone on the Bhutani nomogram) were assessed with each of the 3 TcB decision rules. In addition, the percentage of blood draws avoided, defined as ([true-negatives + false-negatives]/[total number of TcB measurements]), was calculated by using each decision rule in both samples. Finally, the number of blood draws needed to identify 1 child with a TSB level at/above the phototherapy threshold was calculated in both samples by dividing the number of positive screening results with each decision rule by the total number of “positive” TSB levels.
Because the false-negative rate and avoiding blood draws for TSB are not equally valued when assessing the utility of a decision rule for a diagnostic test used to assess newborn jaundice (ie, a low false-negative rate is more important than avoiding all unnecessary blood draws), the choice of the “best” rule is, ultimately, qualitative. However, we conducted pair-wise statistical analyses comparing false-negative rates, and percentage of blood draws avoided, between each of the 3 decision rules with each sample. These analyses were complicated by multiple measurements in the same newborn, low numbers of TSB measurements at/above the phototherapy threshold or in the high-risk zone, and a large number of TcB measurements in sample 1 without a corresponding TSB value. To account for these features, multilevel mixed effects logistic regression was used, allowing for random effects from individual newborns due to paired test modalities and multiple samples within individual patients. In each model, we regressed the decision rule on outcome and test modality, and then calculated the predicted probability of a positive decision for each test modality when the outcome was negative (false-positive rate) and the probability of a positive decision for each test modality when the outcome was positive (ie, sensitivity). The false-positive rates or sensitivities were then compared by using Wald tests. For sample 1, only TcB values that were linked to a TSB level were included in the analyses. For all statistical tests, P values <.05 were considered statistically significant.
Results
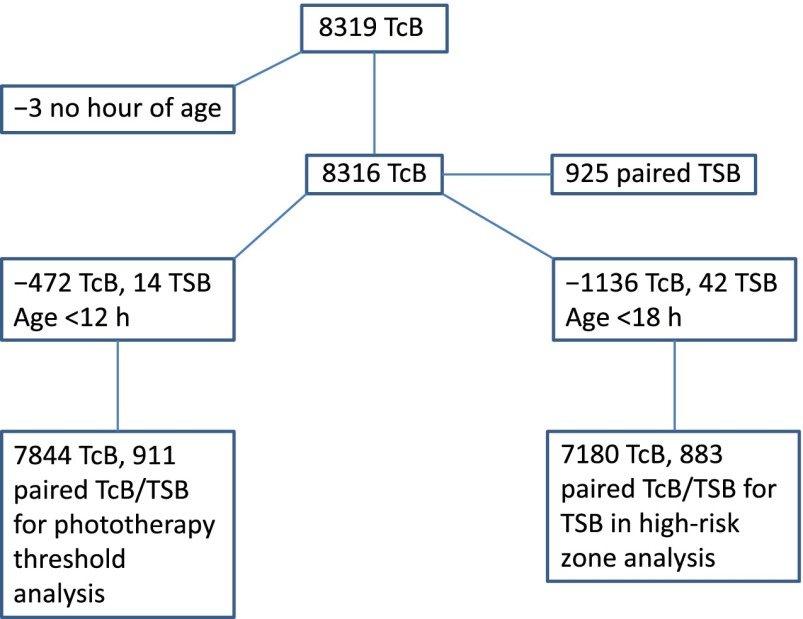
For sample 1, a total of 8319 TcB measurements were obtained on 4994 newborns and were linked to 925 TSB levels. As shown in Fig 1, all measurements were not included in the study analyses; ultimately, data on 911 paired TcB/TSB measurements in 759 newborns were assessed. For sample 2, there were 922 paired TcB/TSB measurements. However, 9 paired measurements were obtained in newborns <12 hours old; these levels were excluded from the analyses. The remaining 913 paired TcB/TSB levels were obtained in 857 newborns. The characteristics of infants included in samples 1 and 2 who contributed data on paired measurements are shown in Table 1.
FIGURE 1.
Flowchart of data collected and analyzed in sample 1.
TABLE 1.
Characteristics of Infants in Samples 1 and 2 Who Had ≥1 Paired TcB-TSB Measurement After 12 Hours of Age
| Characteristic | Sample 1 (n = 759) | Sample 2 (n = 857) |
|---|---|---|
| Race | ||
| American Indian | 6 (1.0)a | 4 (0.5) |
| African American | 154 (25.0) | 135 (18.2) |
| Asian | 48 (7.8) | 58 (7.8) |
| Pacific Islander | 3 (0.5) | 20 (2.7) |
| White | 396 (64.2) | 479 (64.7) |
| Multiple | 9 (1.5) | 26 (3.5) |
| Other | 1 (0.2) | 18 (2.4) |
| Hispanic ethnicity | 187 (24.6)b | 190 (28.0) |
| Gestation <38 wk | 134 (17.7) | 130 (15.2) |
| Direct Coombs’ test positive result | 63 (8.3) | 52 (6.6) |
| Other “neurotoxicity risk factor” | 3 (0.4) | 3 (0.4) |
| AAP risk classification* | ||
| Low risk | 569 (75.0) | 679 (79.2) |
| Medium risk | 180 (23.7) | 171 (20.0) |
| High risk | 10 (1.3) | 7 (0.8) |
Data are presented as n (%). *Risk classification based on criteria recommended in AAP guideline.
Race missing on 142 infants in sample 1 and 117 infants in sample 2.
Data missing on Hispanic ethnicity on 211 newborns in sample 1 and 378 in sample 2. If ethnicity information is missing, the newborn was classified as non-Hispanic.
For sample 1, the decision to obtain a TSB level was made by the clinicians caring for the newborns included in the sample. The rate of obtaining a TSB level according to classification of the TcB screening results (positive or negative) for each of the 3 decision rules is shown in Table 2. Although the rates of obtaining a TSB level with a negative TcB screen were <10% for all of the decision rules, the rates of obtaining a TSB level with a positive screen ranged from 48.0% to 58.1%. For the 929 TcB levels that were categorized as “positive” with all 3 decision rules, a TSB level was obtained in 548 cases (59.0%). Overall, the mean ± SD TSB level in sample 1 was 9.2 ± 2.5 mg/dL with a range of 1.8 to 16.6 mg/dL.
TABLE 2.
Rate of TSB Measurement by TcB Screening Results in the 3 Decision Rules Assessed in Sample 1
| Decision Rule | TcB Screen Positive, (%) | TcB Screen Negative, (%) |
|---|---|---|
| 70% phototherapy level | 611/1200 (50.9) | 300/6644 (4.5) |
| ≥75th percentile | 708/1476 (48.0) | 175/5704 (3.1) |
| Within 3 mg/dL | 423/728 (58.1) | 488/7116 (6.9) |
Among the 911 TSB levels that were classified in sample 1, a total of 31 (3.4% [95% confidence interval (CI), 2.3–4.8]) were at/above the phototherapy threshold as recommended by the AAP.1,19 The false-negative rates, PPV, and NPVs for both outcomes (TSB at/above the phototherapy threshold or in the high-risk zone on the Bhutani nomogram) with each of the decision rules are shown in Table 3. None of the decision rules identified all newborns with a TSB level above the phototherapy threshold; the false-negative rates for the 3 decision rules ranged from 3.2% to 9.7%. Because only 31 TSB measurements were above the phototherapy threshold, the 95% CIs around the estimated false-negative rates were wide. There were 2 newborns with TSB levels above the phototherapy threshold who were misclassified by using the ≥75th percentile decision rule. These neonates were 43 and 45 hours old at the time of measurement, respectively, and had TSB values of 14.9 and 15.0 mg/dL; both had a TcB measurement at the time of the TSB determination of 9.0 mg/dL. The threshold values for a positive screen using the ≥75th percentile decision rule at 43 and 45 hours of life are 10.1 mg/dL and 10.4 mg/dL.5 Both newborns were born at <38 weeks’ gestation and were classified as medium risk for neurotoxicity; the phototherapy thresholds for medium-risk newborns at 43 and 45 hours of life are 12.5 and 12.8 mg/dL.1,19 Thus, these infants were correctly categorized by using the 70% of phototherapy level decision rule but not with the within 3 mg/dL rule. In addition, 1 newborn, also classified as medium risk, who was 17 hours old at the time of TSB measurement, had a TcB level of 4.9 mg/dL and a TSB value of 11.8 mg/dL. The phototherapy threshold for a neonate at medium risk for neurotoxicity at 17 hours of life is 8.6 mg/dL.1,19 This newborn was therefore misclassified by using both the 70% of phototherapy level and the within 3 mg/dL rules; the newborn was not classified by using the ≥75th percentile decision rule because the infant was <18 hours old.
TABLE 3.
False-Negative Rates With Use of Different Decision Rules in Samples 1 and 2 for the Outcomes of a TSB Value Above the Phototherapy Threshold or a TSB in the High-Risk Zone
| Decision Rule | Outcome | Sample False-Negative Rate, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|
| Sample 1 | ||||
| ≥75th percentile | Phototherapy threshold (n = 883)a | 6.9 (0.8–22.8) | 3.8 (2.5–5.5) | 98.9 (95.9–99.9) |
| 70% of phototherapy level | Phototherapy threshold (n = 911) | 3.2 (0.08–16.7) | 4.9 (3.3–6.9) | 99.7 (98.2–100.0) |
| Within 3 mg/dL | Phototherapy threshold (n = 911) | 9.7 (2.0–25.8) | 6.6 (4.4–9.4) | 99.4 (98.2–99.9) |
| ≥75th percentile | High-risk zone (n = 883) | 5.9 (2.9–10.6) | 22.5 (19.4–25.7) | 94.3 (89.7–97.2) |
| 70% of phototherapy level | High-risk zone (n = 883) | 12.4 (7.9–18.4) | 25.0 (21.6–28.7) | 92.8 (89.2–95.5) |
| Within 3 mg/dL | High-risk zone (n = 883) | 25.4 (19.1–32.7) | 42.0 (37.2–47.0) | 91.1 (88.1–93.5) |
| Sample 2 | ||||
| ≥75th percentile | Phototherapy threshold (n = 905) | 0 (0–36.9) | 2.4 (1.0–4.8) | 100 (99.4–100) |
| 70% of phototherapy level | Phototherapy threshold (n = 913) | 0 (0–36.9) | 3.7 (1.6–7.2) | 100 (99.5–100) |
| Within 3 mg/dL | Phototherapy threshold (n = 913) | 0 (0–36.9) | 5.4 (2.4–10.4) | 100 (99.5–100) |
| ≥75th percentile | High-risk zone (n = 905) | 8.1 (1.7–21.9) | 11.4 (8.1–15.6) | 99.5 (98.6–99.9) |
| 70% of phototherapy level | High-risk zone (n = 905) | 10.8 (3.0–25.4) | 15.6 (11.0–21.3) | 99.4 (98.5–99.8) |
| Within 3 mg/dL | High-risk zone (n = 905) | 27.0 (13.8–44.1) | 18.8 (12.8–26.3) | 98.7 (97.6–99.4) |
Number of TSB measurements included in the analysis.
There were 169 TSB measurements in sample 1 that were in the high-risk zone on the Bhutani nomogram (19.1% [95% CI, 16.6–21.9]). The percentages of blood draws avoided with each of the decision rules assessed in sample 1 are shown in Table 4, and they ranged from 79.4% for the ≥75th percentile decision rule to 90.7% for the within 3 mg/dL decision rule.
TABLE 4.
Percentages of Blood Draws Avoided for a TSB Level Because the TcB Screen Was Negative Using Different Decision Rules in Samples 1 and 2
| Decision Rule | Sample 1 Blood Draws Avoided, % (95% CI) | Sample 2 Blood Draws Avoided, % (95% CI) |
|---|---|---|
| ≥75th percentile | 79.4 (78.5–80.4%) | 67.2 (64.0–70.2%) |
| 70% of phototherapy level | 84.7 (83.9–85.5%) | 76.5 (73.6–79.2%) |
| Within 3 mg/dL | 90.7 (90.1–91.4%) | 83.8 (81.2–86.1%) |
For sample 2, data were collected on 913 eligible paired TcB-TSB measurements; unlike sample 1, each TcB value was linked to a TSB level. The mean ± SD TSB value was 6.5 ± 2.3 mg/dL, with a range of 0.5 to 17.2 mg/dL. Of the 913 TSB levels that were collected from newborns who were at least 12 hours old, 8 (0.9%) were at/above the AAP-recommended phototherapy threshold; all of these values were correctly identified by using each of the decision rules (ie, the false-negative rates were all 0%). The percentages of blood draws avoided applying each of the decision rules to sample 2 are shown in Table 4.
In sample 1, based on the number of “positive” TcB screens using the ≥75th percentile, the 70% of phototherapy level, and the within 3 mg/dL decision rules, and the number of corresponding TSB levels at/above the phototherapy threshold, 26, 20, and 15 blood draws, respectively, would be needed to identify 1 TSB level at/above the phototherapy threshold. In sample 2, using the same criteria, 37, 27, and 19 blood draws would be required. There were no TSB levels in either sample 1 or 2 that were at/above the threshold for exchange transfusion as recommended by the AAP.19
When comparing the 3 decision rules statistically, the false-positive rate was significantly higher using the ≥75th percentile decision rule than either of the other 2 rules in both sample 1 and sample 2 and with both outcomes (P values ranging from <.0001 to .007). For the outcome of a TSB level above the phototherapy threshold, there was no significant difference in false-negative rates between any of the decision rules when used with sample 1 (P = .31, P = .30, and P = .62, respectively, for the comparisons of the ≥75th percentile vs 70% of phototherapy level, ≥75th percentile vs within 3 mg/dL, and within 3 mg/dL vs 70% of phototherapy level rules). However, for the secondary outcome (identifying a newborn with a TSB level in the high-risk zone on the Bhutani nomogram), in sample 1, the false-negative rate using the ≥75th percentile decision rule was significantly lower than with the 70% of phototherapy level decision rule (P = .009) or the within 3 mg/dL decision rule (P = .0008). There were no statistically significant differences in false-negative rates in sample 2.
Discussion
The results of the present study suggest that, with the use of specific decision rules, TcB measurement can be used effectively to screen newborns for jaundice during their birth hospitalization. Use of all 3 of the decision rules yielded false-negative rates <10% for the outcome of a TSB above the phototherapy threshold among those in sample 1 while eliminating the need for a blood draw for the TSB level after ∼80% to 90% of TcB measurements. Although there were no significant differences in false-negative rates, use of the ≥75th percentile decision rule would lead to a blood draw for a TSB level significantly more often than the other rules evaluated.
In addition to newborns with TSB levels above the phototherapy threshold, it may be important to identify those with levels in the high-risk zone on the Bhutani nomogram, even if the level does not require immediate treatment, for the purposes of planning appropriate outpatient follow-up.1,19,20 Although the ≥75th percentile decision rule led to more unnecessary blood draws than the other decision rules, it correctly identified more newborns with a TSB level in the high-risk zone than the other 2 rules.
Because of the modest numbers of TSB levels above the phototherapy threshold in both samples, it is difficult to compare the utility of the different decision rules evaluated in this study. However, our results suggest that the 70% of phototherapy level decision rule may be the most useful in a variety of clinical settings during the birth hospitalization in that it eliminates the need for more blood draws because of a false-positive screen than the ≥75th percentile rule while providing false-negative rates that are, at least, comparable to those of the other decision rules. Use of the within 3 mg/dL rule might eliminate the need for the most blood draws of any of the evaluated decision rules, and its false-negative rate was not statistically different from that of the 70% of phototherapy level rule, suggesting that it may also be used effectively. Overall, perhaps the most striking finding of this study is that none of the 3 decision rules identified all newborns who had a TSB level above the phototherapy threshold or all newborns with a TSB level in the high-risk zone on the Bhutani nomogram. Thus, in a newborn whose jaundice appears on visual assessment to be more significant than indicated by a TcB measurement, a TSB level may still be warranted.
With all of the decision rules, the PPV of a positive TcB screen was relatively low for each outcome. Thus, use of any of the tested decision rules would lead to an unnecessary blood draw for a TSB level in a large proportion of newborns, as demonstrated by our finding that, depending on the decision rule used, 15 to 37 blood draws ordered because of a positive TcB screen would be required to identify a single newborn with a TSB level for which phototherapy is recommended. One of the more interesting findings of our study was that, even when the TcB screen was positive when all 3 decision rules were applied, a TSB level was only obtained 59% of the time. This result could suggest that no specific TcB screening decision rule was consistently used across the BORN sites. Alternatively, BORN clinicians could have used criteria for phototherapy initiation different from those recommended by the AAP. Previous research suggests that clinicians inconsistently apply the AAP guidelines for starting phototherapy.21 It is possible that in our study, many clinicians elected to observe newborns with a TcB level which indicated that the TSB level could be at/above the recommended phototherapy threshold, rather than immediately obtaining a confirmatory blood level. It is also important to note that a TSB level was obtained in conjunction with 11.1% of the TcB levels measured for clinical purposes in sample 1. Application of either the ≥75% percentile or 70% of phototherapy level decision rules would have led to a substantially higher rate of blood draws for a TSB level (ie, 21.6% and 15.3%, respectively), whereas use of the within 3 mg/dL decision rule would have resulted in a blood draw after 9.3% of TcB levels in sample 1.
Our findings are compatible with the results of previous research on the utility of TcB measurement as a screening tool for identifying newborns with significant jaundice. Bhutani et al5 assessed the utility of the >75th percentile decision rule among 419 newborns. There were 23 newborns with a TSB level in the high-risk zone; none of these infants had a negative TcB screen (ie, none had a TcB level <75th percentile on the Bhutani nomogram). However, the upper limit of the 95% CI around a point estimate of 0 with 23 measurements can be approximated at ∼15%, which includes the point estimates for false-negative rates for the ≥75th percentile and 70% of phototherapy level decision rules in both samples 1 and 2. In a Danish study, Ebbesen et al6 assessed the effectiveness of a decision rule by using the 70% of the phototherapy threshold (based on recommendations by the Danish Pediatric Society) as the cutoff value for defining a “positive” TcB screen in premature and term newborns. Among the group of 314 healthy term and late pre-term infants included in the study, only 3 had a TSB level above the phototherapy threshold; all of these infants had a positive TcB screen. In this sample, use of a 70% of phototherapy level decision rule would have obviated the need for a blood draw in 80.6%. Finally, the within 3 mg/dL decision rule is a common sense–based approach to TcB screening. To our knowledge, there has been no previously published validation of this decision rule.
Conclusions
At the onset of this study, our goal was to identify the “best” decision rule that could be applied across multiple settings. Unfortunately, the results do not unequivocally support the selection of 1 decision rule over the others. The results of the present study instead provide insights on the strengths and weaknesses of each of the 3 decision rules that can be used in guiding clinical decision-making.
Acknowledgments
This study was supported by the Academic Pediatric Association. The authors offer special appreciation to Beth King and Allison Hartle, research assistants. They also thank Chuan Zhou, PhD, for his biostatistical support.
Study sites and co-investigators were as follows: Maricopa Medical Center, David Brodkin; University of California San Francisco Children’s Hospital, Valerie Flaherman; University of California San Diego Hillcrest, Michelle Leff; Lucile Packard Children’s Hospital at Stanford, Janelle Aby; Kaiser Permanente Downey Medical Center, Anthony Burgos; Naval Medical Center San Diego, Carey A. Welsh; Denver Health Medical Center, Betsey Chambers; Yale–New Haven Children’s Hospital, Jaspreet Loyal; Tampa General Hospital affiliated with the University of South Florida, Maya Balakrishnan; Rush University Medical Center, Carrie Drazba; University of Chicago Medical Center, Larry Gray; Wishard Health Services/Eskenazi Health, Kinga Szucs; University of Louisville Hospital, Lawrence Wasser; University of Michigan Health System Women’s Hospital Birth Center, Jocelyn Schiller; University of Minnesota Hospital, Diane Madlon-Kay; SSM St Mary’s Health Center, Donna Halloran; Truman Medical Center Hospital Hill, Elizabeth Simpson; Morristown Memorial Hospital, Eberechi Nwaobasi-Iwuh; Flushing Hospital Medical Center, Lourdes Cohen; NYPH–Weill Cornell Medical Center, Jennifer DiPace; Women’s Hospital of Greensboro of the Moses Cone Health System, Kaye Gable; North Carolina Women’s Hospital (UNC-Chapel Hill), Carl Seashore; Gaston Memorial, Laura Sinai; Firelands Regional Medical Center, Tara Williams; Cincinnati Children’s Hospital Medical Center, Scott Wexeblatt; Oregon Health & Science University, Carrie Phillipi; Thomas Jefferson University Hospital, Esther Chung; Children’s Hospital at Erlanger, Andrea Goins; University Medical Center in Lubbock, TX, Kirsten Robinson; University of Virginia Health System, Ann Kellams; Virginia Commonwealth University Health System, Linda D. Meloy; Naval Medical Center Portsmouth, Matthew McLean; and University of Washington Medical Center, James A. Taylor.
Glossary
- AAP
American Academy of Pediatrics
- BORN
Better Outcomes through Research for Newborns
- CI
confidence interval
- NPV
negative predictive value
- PPV
positive predictive value
- TcB
transcutaneous bilirubin
- TSB
total serum bilirubin
Footnotes
Dr Taylor conceptualized and designed the study, collected data at 1 site, analyzed the study data, and drafted the initial manuscript; Drs Burgos, Simpson, Flaherman, and Chung assisted in the design of the study and the development of data collection forms; they each also collected study data at 1 site, assisted in the analysis and interpretation of study data, and critically reviewed the manuscript; Drs Goyal and Von Kohorn assisted in the design of the study and the development of data collection forms; they also assisted in interpretation of study data and critically reviewed the manuscript; Ms Dhepyasuwan assisted in the design of the study and the development of data collection forms, collated data from all study sites, assisted in analysis and interpretation of study data, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Taylor is the co-owner of BiliCam, LLC, a company developing a noninvasive method for measuring bilirubin levels in newborns. He currently derives no income from the company. Dr Burgos owns stock in BiliTool, Inc, including co-ownership of technological assets, with no regular income derived. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Academic Pediatric Association. Dr Flaherman is also supported by the National Institutes of Health (K23 HD059818) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124(4):1193–1198 [DOI] [PubMed] [Google Scholar]
- 2.Taylor JA, Burgos AE, Flaherman V, et al. Between transcutaneous and serum bilirubin measurements. Pediatrics 2015;135(2):224–231 [DOI] [PMC free article] [PubMed]
- 3.Engle WD, Jackson GL, Sendelbach D, Manning D, Frawley WH. Assessment of a transcutaneous device in the evaluation of neonatal hyperbilirubinemia in a primarily Hispanic population. Pediatrics. 2002;110(1 pt 1):61–67 [DOI] [PubMed] [Google Scholar]
- 4.Engle WD, Jackson GL, Stehel EK, Sendelbach DM, Manning MD. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. J Perinatol. 2005;25(7):486–490 [DOI] [PubMed] [Google Scholar]
- 5.Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106(2). Available at: www.pediatrics.org/cgi/content/full/106/2/E17 [DOI] [PubMed] [Google Scholar]
- 6.Ebbesen F, Rasmussen LM, Wimberley PD. A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr. 2002;91(2):203–211 [DOI] [PubMed] [Google Scholar]
- 7.Samanta S, Tan M, Kissack C, Nayak S, Chittick R, Yoxall CW. The value of Bilicheck as a screening tool for neonatal jaundice in term and near-term babies. Acta Paediatr. 2004;93(11):1486–1490 [DOI] [PubMed] [Google Scholar]
- 8.Maisels MJ, Ostrea EM Jr, Touch S, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics. 2004;113(6):1628–1635 [DOI] [PubMed] [Google Scholar]
- 9.Sanpavat S, Nuchprayoon I. Noninvasive transcutaneous bilirubin as a screening test to identify the need for serum bilirubin assessment. J Med Assoc Thai. 2004;87(10):1193–1198 [PubMed] [Google Scholar]
- 10.Sanpavat S, Nuchprayoon I. Comparison of two transcutaneous bilirubinometers—Minolta AirShields Jaundice Meter JM103 and Spectrx Bilicheck–in Thai neonates. Southeast Asian J Trop Med Public Health. 2005;36(6):1533–1537 [PubMed] [Google Scholar]
- 11.Rubaltelli FF, Gourley GR, Loskamp N, et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics. 2001;107(6):1264–1271 [DOI] [PubMed] [Google Scholar]
- 12.Boo NY, Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. J Paediatr Child Health. 2007;43(4):297–302 [DOI] [PubMed] [Google Scholar]
- 13.Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113(6):1636–1641 [DOI] [PubMed] [Google Scholar]
- 14.Grohmann K, Roser M, Rolinski B, et al. Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics. 2006;117(4):1174–1183 [DOI] [PubMed] [Google Scholar]
- 15.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277(6):488–494 [PubMed] [Google Scholar]
- 16.Maisels MJ. Transcutaneous bilirubin measurement: does it work in the real world? Pediatrics. 2015;135(2):364–366 [DOI] [PubMed] [Google Scholar]
- 17.Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103(1):6–14 [DOI] [PubMed] [Google Scholar]
- 18.Maisels MJ. Transcutaneous bilirubinometry. NeoReviews. 2006;7(5):e217–e225 [Google Scholar]
- 19.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia . Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316 [DOI] [PubMed] [Google Scholar]
- 20.Bhutani VK, Stark AR, Lazzeroni LC, et al; Initial Clinical Testing Evaluation and Risk Assessment for Universal Screening for Hyperbilirubinemia Study Group. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr 2013;162(3):477–482.e1 [DOI] [PubMed]
- 21.Kuzniewicz MW, Escobar GJ, Newman TB Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 2009;124:1031–1039 [DOI] [PMC free article] [PubMed]