Abstract
BACKGROUND:
Antidepressant effects on increased suicidality in children have raised public concern in recent years. Approved in 2002 for attention-deficit/hyperactivity disorder treatment, the selective noradrenalin-reuptake-inhibitor atomoxetine was initially investigated for the treatment of depression. In post-hoc analyses of clinical trial data, atomoxetine has been associated with an increased risk of suicidal ideation in children and adolescents. We analyzed whether the observed increased risk of suicidal ideation in clinical trials translates into an increased risk of suicidal events in pediatric patients treated with atomoxetine compared with stimulants in 26 Medicaid programs.
METHODS:
Employing a retrospective cohort design, we used propensity score–adjusted Cox proportional hazard models to evaluate the risk of suicide and suicide attempt in pediatric patients initiating treatment with atomoxetine compared with stimulants from 2002 to 2006.
RESULTS:
The first-line treatment cohort included 279 315 patients. During the first year of follow-up, the adjusted hazard ratio for current atomoxetine use compared with current stimulant use was 0.95 (95% CI 0.47–1.92, P = .88). The second-line treatment cohort included 220 215 patients. During the first year of follow-up, the adjusted hazard ratio for current atomoxetine use compared with current stimulant use was 0.71 (95% CI 0.30–1.67, P = .43).
CONCLUSIONS:
First- and second-line treatment of youths age 5 to 18 with atomoxetine compared with stimulants was not significantly associated with an increased risk of suicidal events. The low incidence of suicide and suicide attempt resulted in wide confidence intervals and did not allow stratified analysis of high-risk groups or assessment of suicidal risk associated with long-term use of atomoxetine.
What’s Known on This Subject:
Antidepressant and atomoxetine effects on increased suicidality in children have raised public concern in recent years resulting in boxed warnings. However, this association is based on clinical trial data.
What This Study Adds:
This study analyzed if the observed increased risk of suicidal ideation in clinical trials translates into an increased risk of suicidal events in youths aged 5 to 18 treated with atomoxetine compared with central nervous system stimulants in 26 Medicaid programs.
Concern about an association between antidepressant use and increased suicidality peaked with the results of a meta-analysis in 2003, which concluded nearly twice the rate of suicidality in antidepressant users compared with placebo.1 These findings resulted in boxed warnings in the United States and Europe, indicating an increased risk of suicidal thinking and behavior in children and adolescents treated with antidepressant medications.2,3
Atomoxetine was approved in 2002 as a novel mechanism of action, nonstimulant and noncontrolled substance alternative for attention-deficit/hyperactivity disorder (ADHD) treatment. Although not approved by the Food and Drug Administration for depression, the selective norepinephrine reuptake inhibitor atomoxetine and was originally developed as an antidepressant.4,5 Approximately 1 year after the boxed warning for antidepressants in 2004, the Food and Drug Administration and the European Medicines Agency directed the manufacturer of atomoxetine to include a boxed warning regarding an increased risk of suicidal ideation in children and adolescents treated for ADHD.3,6 For clinical context, the EU summary of product characteristics for methylphenidate lists “suicidal tendencies” as a contraindication, whereas US labels do not.
The boxed warning decision for atomoxetine was based on a meta-analysis including 14 trials with 2208 patients (1357 atomoxetine/851 placebo). The analysis showed a statistically significant higher risk of suicidal ideation in the atomoxetine treatment arm with 5 cases paralleled by none in the placebo group. One patient attempted suicide during atomoxetine treatment compared with none on placebo. All cases of suicidal events occurred in children younger than 12 years and within 32 days of treatment initiation. Although the age range of study subjects was 6 to 17.9 years, the mean age was 10.5 years (SD ± 2.4), indicating a population predominantly comprised younger children.7 Similarly, the follow-up time ranged from 6 to 18 weeks but was skewed toward shorter follow-up periods. Two additional meta-analyses were published that were likewise compromised by sample size, resulting in limited inferences for rare events.8,9 Also, there is some evidence of increased rates of suicide in nontrial populations.10
In summary, available evidence lacks inferences for nonclinical trial populations, older adolescents, risk after 3 months of treatment, and, importantly, whether suicidal ideation indeed manifests in risk of suicide.7
ADHD is the most common mental health disorder in children and adolescents, with ∼2.7 million youths receiving pharmacotherapy for treatment of ADHD in the United States.11–13 Although central nervous system stimulants are the principal and most common pharmacotherapy, an estimated 15% of youths with ADHD received atomoxetine in 2003.12,14 The objective of this study was to evaluate whether atomoxetine is associated with an increased risk of suicide attempt and suicide in patients newly treated with atomoxetine when compared with use of stimulants.
Methods
Source Population
The study cohort was assembled from Medicaid Analytic eXtract (MAX) data, consisting of administrative health care claims, obtained from the 26 US states with the largest pediatric populations eligible for Medicaid fee-for-service benefits between 1999 and 2006. MAX data, made available by the Centers for Medicare and Medicaid Services, provide details on Medicaid eligibility, demographic information, diagnoses and procedures associated with in- and outpatient visits, as well as medications reimbursed by Medicaid.
In this cohort study, subjects entered the cohort at the first dispensed prescription (index date) for atomoxetine or stimulants. Employing a new user design, the index date had to be preceded by a minimum of 6 months of continuous Medicaid eligibility (baseline period) with at least 1 diagnosis of a mental health disorder commonly treated with atomoxetine or stimulants. Included disorders defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes included besides ADHD, adjustment reaction, conduct disorder or mixed emotional disturbances of childhood or adolescence, and unspecified emotional disturbance of childhood or adolescence (Supplemental Table 5).15 All subjects had to be at least 5 and not >18 years of age at the index date. Subjects were excluded if they had drug claims for pemoline or methamphetamine because of low utilization or monoamine oxidase inhibitors because they are contraindicated during treatment with stimulants. Finally, we excluded subjects with severe or terminal diseases that alter the baseline risk for suicidality and that were generally rare, including any diagnosis of HIV/AIDS, malignant neoplasm, organ transplant, dialysis dependency, pervasive developmental disorders, or severe or profound mental retardation (Supplemental Table 6).16
Although only stimulants were considered first-line therapy of ADHD during the study period, we observed about half of all atomoxetine initiations in pharmacotherapy-naive patients. Furthermore, we observed significant differences in the baseline characteristics of patients in which atomoxetine was introduced as first- versus second-line treatment. Therefore, we established 2 subcohorts to evaluate suicidal risk separately for first- and second-line atomoxetine treatment. The first subcohort included subjects initiating treatment for the first time with either atomoxetine or stimulant between 2003 and 2006 (first-line treatment cohort) following a minimum of 180 days of continuous Medicaid eligibility. For the second subcohort, we matched second-line atomoxetine initiators (who either switched to or added atomoxetine after initial treatment with stimulants) by the number of months since stimulant initiation, to patients exposed to stimulants at the same number of months since stimulant treatment initiation in a 1 to 3 ratio (second-line treatment cohort). The index date for the second-line treatment cohort was the date of matching and also required a 180-day baseline period of continuous Medicaid eligibility immediately before the matching date.
Subjects were followed until the end of Medicaid eligibility, their 19th birthday, death, a hospitalization of >30 days, or pregnancy, whichever occurred first.
Study End Points
The primary study end point was a composite including completed suicide and suicide attempt requiring hospitalization or an emergency department visit. To identify suicides, we linked subjects identified in MAX to the Social Security Agency Death Master File. All deaths obtained through this linkage or flagged in the MAX eligibility files were then verified with the National Death Index. We defined completed suicides based on the ICD, Tenth Revision (ICD-10) codes X60–X84 on the National Death Index death certificate.17
Suicide attempts were identified from billing records for emergency department visits or hospitalizations with ICD-9-CM codes for external cause of injury E950.x–E959.x involving deliberate self-harm.18 Previous research has shown adequate sensitivity and specificity >90% as well as positive predictive values >85% for these end points.19–22
Atomoxetine and Stimulant Exposure
Periods of atomoxetine or stimulant exposure, including any dose or dosage form of atomoxetine, methylphenidate, and mixed amphetamine salts, were defined on the basis of pharmacy dispensing claims. We defined begin of atomoxetine and stimulant exposure based on the filling date on pharmacy claims for respective prescriptions. The end date for each prescription fill was calculated from the recorded dispensed days’ supply plus a grace period of 25% to incorporate residual supply as a result of drug holidays (eg, days without school). Because many states restrict dispensing amounts of controlled substances, the majority (>85%) of prescription fills for stimulants but also for atomoxetine involved a 30-day supply.23
If active prescriptions for both medications were present, exposure was defined as current atomoxetine exposure and flagged as dual therapy. Periods after current use were defined as former use.
Covariates
We ascertained potential confounding variables from the 6-month baseline period preceding the index date, including age and calendar year, gender, race/ethnicity, state of residence, reasons for Medicaid eligibility (eg foster care), the number of hospitalizations for mental and nonmental diagnoses, and diagnoses of other psychiatric disorders such as substance use disorder, anxiety, bipolar disorder, schizophrenia, depression, or oppositional defiant disorder (Supplemental Table 7).24 Because Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria to measure ADHD severity are not reflected in ICD-9-CM coding, we only distinguished ADHD subtypes with regard to presence or absence of hyperactivity. We also incorporated measures of the total number of distinct psychiatric disorder diagnoses and psychotropic drug classes during baseline as an indicator of mental illness severity. Variables capturing exposure to other psychotropic drugs during baseline period included antidepressants, anticonvulsants, antipsychotics, anxiolytics, α-agonists, lithium, and opioid analgesics (Supplemental Table 8).
Finally, we captured any suicide attempt and in/outpatient visits involving suicidal ideation (ICD-9-CM V62.84, 300.9) during baseline.18
Data Analysis
For each subcohort, we used logistic regression models to calculate exposure propensity scores to estimate the likelihood to receive atomoxetine conditional on baseline covariates.25,26 The propensity score is a common method to control for confounding in observational research with the advantage to summarize numerous covariates as a single composite score, especially when the number of observed covariates was large and the number of observed outcomes was small.25–29
Participants were then weighted by the inverse of their propensity score to evaluate the level of balance achieved between exposed and unexposed groups across all baseline covariates.
We fitted 2 separate Cox proportional hazard models comparing new users of atomoxetine versus new use of stimulants (first-line user cohort) and patients starting atomoxetine treatment after initial stimulant treatment to patients who continued use of stimulants (second-line user cohort). Models were adjusted for the propensity score, as well as time-varying age and presence of dual therapy.
For computational efficiency, we segmented follow-up time after the index date into 15-day increments with exposure status determined based on the majority of days assigned to current or former use of atomoxetine or stimulants.
SAS9.2 (SAS Institute, Cary, NC) was used for data management and analyses. Matching was performed by using R Foundation software, Version 2.15.1 (Vienna, Austria).30,31
Results
First-Line Treatment
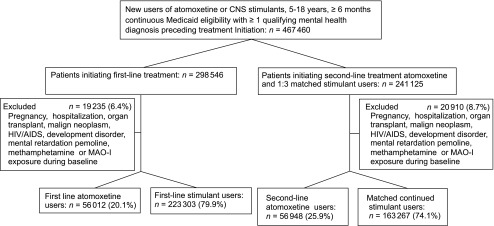
The cohort included 297 315 patients initiating ADHD treatment (first-line) with atomoxetine (56 012, 20.1%) or stimulants (223 303, 79.9%) and accrued 428 272 person-years of follow-up (Fig 1). Patients treated with stimulants contributed 190 026 person-years (44.3%) and 144 144 person-years (33.7%) of current and former stimulant exposure, whereas patients initiating atomoxetine treatment contributed 46 929 person-years (11.0%) and 47 173 person-years (11.0%) of current and former atomoxetine exposure, respectively. The most common reasons for censoring were end of Medicaid eligibility (94.9%) and hospitalization >30 days (3.5%). A total of 92 (0.03%) children and adolescents died during follow-up of causes other than the study end point. In general, covariates were similarly distributed among the 2 treatment groups with age and calendar year showing the greatest imbalance. Inverse weighting of subjects by their propensity score established balanced groups with <0.5% absolute difference (Table 1).
FIGURE 1.
Flowchart of study cohort. CNS, central nervous system; MAO-I, monoamine oxidase inhibitors.
TABLE 1.
Baseline Sociodemographic and Clinical Characteristics, First-Line Treatment Cohort
| Atomoxetine | Stimulant | |||||
|---|---|---|---|---|---|---|
| Unweighted | Inverse PS Weighteda | Unweighted | Inverse PS Weighteda | |||
| N (%) | 56 012 | (20.1) | 223 303 | (79.9) | — | |
| Mean index age, y (± SD) | 9.82 | (3.4) | 9.2 | 8.97 | (3.1) | 9.1 |
| Mean end age, y (± SD) | 11.58 | (3.5) | 10.7 | 10.49 | (3.3) | 10.7 |
| Mean follow-up, y (± SD) | 1.75 | (1.1) | 1.5 | 1.51 | (1.1) | 1.5 |
| Male gender, n (%) | 36 919 | (65.9) | (67.6) | 152 098 | (68.1) | (67.7) |
| Age, y, n (%) | ||||||
| 5 | 5489 | (9.8) | (14.8) | 35 759 | (16.0) | (14.8) |
| 6-8 | 22 113 | (39.5) | (42.6) | 97 591 | (43.7) | (42.8) |
| 9–11 | 13 746 | (24.5) | (22.6) | 49 584 | (22.2) | (22.7) |
| 12–14 | 9126 | (16.3) | (13.3) | 27 551 | (12.3) | (13.2) |
| 15–18 | 5538 | (9.9) | (6.7) | 12 818 | (5.7) | (6.6) |
| Race/ethnicity, n (%) | ||||||
| Caucasian | 39 911 | (71.3) | (60.8) | 129 691 | (58.1) | (60.7) |
| Black | 11 197 | (20.0) | (26.9) | 65 124 | (29.2) | (27.3) |
| Hispanic | 3583 | (6.4) | (9.4) | 22 219 | (10.0) | (9.2) |
| Other | 1418 | (2.5) | (2.9) | 6550 | (2.9) | (2.9) |
| Reason for Medicaid eligibility, n (%) | ||||||
| TANF | 5256 | (9.4) | (9.4) | 20 771 | (9.3) | (9.3) |
| Foster care | 4922 | (8.8) | (9.4) | 20 965 | (9.4) | (9.3) |
| SSI | 1711 | (3.1) | (2.2) | 4113 | (1.8) | (2.1) |
| Calendar year, n (%) | ||||||
| 2003 | 19 778 | (35.3) | (27.3) | 55 874 | (25.0) | (27.1) |
| 2004 | 19 378 | (34.6) | (28.2) | 58 939 | (26.4) | (28.1) |
| 2005 | 10 884 | (19.4) | (23.9) | 56 490 | (25.3) | (24.1) |
| 2006 | 5962 | (10.6) | (20.6) | 52 000 | (23.3) | (20.7) |
| Index diagnosis, n % | ||||||
| ADHD with hyperactivity | 34 543 | (61.7) | (66.9) | 153 696 | (68.8) | (67.3) |
| ADHD without hyperactivity | 15 653 | (27.9) | (24.4) | 51 680 | (23.1) | (24.1) |
| Adjustment reaction | 9392 | (16.8) | (15.2) | 32 141 | (14.4) | (14.9) |
| Disturbance of conduct | 6680 | (11.9) | (11.8) | 27 036 | (12.1) | (12.1) |
| Other or mixed emotional disturbances | 6619 | (11.8) | (10.7) | 23 460 | (10.5) | (10.8) |
| Unspecified emotional disturbance | 359 | (0.6) | (0.7) | 1769 | (0.8) | (0.8) |
| Other mental comorbidities, n % | ||||||
| Substance use disorder | 987 | (1.8) | (1.0) | 1758 | (0.8) | (1.0) |
| Anxiety | 3791 | (6.8) | (5.7) | 11 863 | (5.3) | (5.6) |
| Bipolar disorder | 1458 | (2.6) | (2.1) | 4497 | (2.0) | (2.1) |
| Schizophrenia | 121 | (0.2) | (0.2) | 297 | (0.1) | (0.2) |
| Depression | 5184 | (9.3) | (7.4) | 15 004 | (6.7) | (7.3) |
| Mild mental retardation | 71 | (0.1) | (0.1) | 331 | (0.1) | (0.1) |
| Tic disorder | 351 | (0.6) | (0.3) | 350 | (0.2) | (0.3) |
| Oppositional defiant disorder | 6160 | (11.0) | (9.8) | 21 358 | (9.6) | (9.9) |
| Psychosis | 341 | (0.6) | (0.5) | 997 | (0.4) | (0.5) |
| Other mental health diagnosis | 7562 | (13.5) | (14.0) | 32 618 | (14.6) | (14.4) |
| Distinct mental health disorders, n % | ||||||
| 1 | 30 632 | (54.7) | (57.4) | 128 456 | (57.5) | (57.0) |
| 2 | 14 626 | (26.1) | (25.2) | 56 719 | (25.4) | (25.5) |
| 3 | 6368 | (11.4) | (10.7) | 23 653 | (10.6) | (10.8) |
| ≥4 | 4386 | (7.8) | (6.7) | 14 475 | (6.5) | (6.8) |
| Other comorbidities, n % | ||||||
| Obesity | 585 | (1.0) | (1.1) | 2541 | (1.1) | (1.1) |
| Smoking | 150 | (0.3) | (0.2) | 257 | (0.1) | (0.2) |
| Suicidal ideation | 26 | (0.0464) | (0.05) | 125 | (0.0560) | (0.05) |
| Suicide attempt | 34 | (0.0607) | (0.04) | 78 | (0.0349) | (0.05) |
| Non–mental health hospitalization | ||||||
| 0 | 55 430 | (99.0) | (99.0) | 221 028 | (99.0) | (99.0) |
| 1 | 535 | (1.0) | (1.0) | 2086 | (0.9) | (0.9) |
| ≥2 | 47 | (0.1) | (0.1) | 189 | (0.1) | (0.1) |
| Mental health hospitalization | ||||||
| 0 | 55 064 | (98.3) | (98.7) | 220 315 | (98.7) | (98.6) |
| 1 | 802 | (1.4) | (1.2) | 2630 | (1.2) | (1.2) |
| ≥2 | 146 | (0.3) | (0.2) | 358 | (0.2) | (0.2) |
| Psychotropic drug use, n (%) | ||||||
| Antidepressant | 7829 | (14.0) | (10.9) | 22 146 | (9.9) | (10.8) |
| Antipsychotic | 4929 | (8.8) | (7.5) | 15 603 | (7.0) | (7.4) |
| Anticonvulsant | 3194 | (5.7) | (4.6) | 9448 | (4.2) | (4.5) |
| Anxiolyticb | 2034 | (3.6) | (3.5) | 7590 | (3.4) | (3.5) |
| Lithium | 194 | (0.3) | (0.3) | 580 | (0.3) | (0.3) |
| α-agonist | 2410 | (4.3) | (5.0) | 11 632 | (5.2) | (5.0) |
| Opioid analgesics | 3694 | (6.6) | (5.8) | 12 603 | (5.6) | (5.8) |
| No. of psychotropic drug classes, n (%) | ||||||
| 0 | 40 743 | (72.7) | (76.3) | 173 143 | (77.5) | (76.5) |
| 1 | 10 383 | (18.5) | (17.0) | 36 640 | (16.4) | (16.9) |
| 2 | 3462 | (6.2) | (4.8) | 9922 | (4.4) | (4.8) |
| 3 | 1154 | (2.1) | (1.5) | 2988 | (1.3) | (1.5) |
| ≥4 | 270 | (0.5) | (0.3) | 610 | (0.3) | (0.3) |
PS, propensity score; SSI, Supplemental Security Income; TANF, Temporary Assistance for Needy Families.
“Inverse PS-weighted” denotes sample distributions of baseline characteristics after propensity score weighting.
Including sedatives and hypnotics.
We observed a total of 140 suicidal events (suicide or suicide attempt). The majority (60%) of suicide attempts occurred in girls, and the majority of suicides (89%) was in boys. The average age at a suicidal event was 15.5 years (SD ± 2.7) and occurred after a mean of 1.12 years (median 0.93 years) after the index date.
We observed 50 suicidal events during current stimulant exposure (26.3 per 100 000 person-years), 47 during former stimulant use (32.6 per 100 000 person-years), 18 during current atomoxetine use (38.4 per 100 000 person-years) and 25 suicidal events during former atomoxetine use (53.0 per 100 000 person-years).
During the first year of follow-up, the unadjusted hazard ratio (HR) for current atomoxetine use compared with current stimulant use was 1.51 (95% confidence interval [CI] 0.77–2.95, P = .23) (Table 2). The fully adjusted HR for current atomoxetine use compared with current stimulant use was 0.95 (95% CI 0.47–1.92, P = .88). Varying follow-up times showed no appreciable effect on risk estimates.
TABLE 2.
HRs for Suicide and Suicide Attempt, First-Line Treatment Cohort
| Exposure | Events | Unadjusted | Adjusted for Time-Dependent Age, Dual Therapy and Propensity Score | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Full follow-up | |||||||
| Current CNS stimulant | 50 | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | 18 | 1.45 | 0.85–2.48 | .18 | 0.88 | 0.50–1.56 | .66 |
| Former atomoxetine | 25 | 2.19 | 1.33–3.60 | .002 | 0.88 | 0.53–1.46 | .62 |
| Former CNS stimulant | 47 | 1.35 | 0.89 to 2.04 | .16 | 0.87 | 0.56–1.33 | .520 |
| 24-mo follow-up | |||||||
| Current CNS stimulant | 43 | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | 16 | 1.45 | 0.82–2.57 | .21 | 0.94 | 0.52–1.72 | .85 |
| Former atomoxetine | 19 | 2.25 | 1.28–3.93 | .005 | 0.95 | 0.54–2.68 | .86 |
| Former CNS stimulant | 33 | 1.22 | 0.76–1.96 | .41 | 0.83 | 0.52–1.35 | .46 |
| 12-mo follow-up | 70 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 1.51 | 0.77–2.95 | .23 | 0.95 | 0.47–1.92 | .88 |
| Former atomoxetine | — | 2.42 | 1.15–5.11 | .02 | 1.11 | 0.52–2.37 | .79 |
| Former CNS stimulant | — | 1.24 | 0.67–2.30 | .50 | 0.95 | 0.51–1.77 | .87 |
| 6-mo follow-up | 47 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 1.63 | 0.77–3.42 | .19 | 1.07 | 0.50–2.28 | .86 |
| Former atomoxetine | — | 1.77 | 0.59–5.31 | .31 | 0.86 | 0.28–2.60 | .78 |
| Former CNS stimulant | — | 1.18 | 0.53–2.61 | .68 | 1.00 | 0.45–2.21 | .99 |
| 3-mo follow-up | 23 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 1.60 | 0.62–4.20 | .33 | 1.00 | 0.38–2.66 | .990 |
| Former atomoxetine | — | 1.93 | 0.24–15.90 | .54 | 0.89 | 0.11–7.43 | .92 |
| Former CNS stimulant | — | 0.96 | 0.20–4.65 | .96 | 0.83 | 0.17–4.07 | .810 |
CNS, central nervous system.
Second-Line Treatment
The second-line treatment cohort included after matching 220 215 patients who were initially treated with stimulants, contributing a combined 300 772 person-years of follow-up. Of those, 56 948 (25.9%) subsequently initiated atomoxetine contributing 37 948 person-years (12.6%) and 43 344 person-years (14.2%) of current and former atomoxetine exposure, respectively. A total of 163 267 patients (74.1%) still exposed to stimulants were matched to these second-line atomoxetine initiators by month since initial stimulant treatment start, contributing 142 015 person-years (47.2%) and 78 054 person-years (26.0%) of current and former stimulant exposure, respectively (Fig 1). The most common reason for censoring were end of Medicaid eligibility (94.2%) and hospitalization >30 days (3.9%). A total of 29 499 (12.2%) patients were excluded.
In general, covariates were similarly distributed among the 2 treatment groups except of the calendar year of their entry into the study. Inverse weighting of subjects by their propensity score established balanced groups with <0.5% absolute difference (Table 3).
TABLE 3.
Baseline Sociodemographic and Clinical Characteristics, Second-Line Treatment Cohort
| Atomoxetine | Stimulant | |||||
|---|---|---|---|---|---|---|
| Unweighted | Inverse PS Weighteda | Unweighted | Inverse PS Weighteda | |||
| N (%) | 56 948 | (25.9) | — | 163 267 | (74.1) | — |
| Mean age at initial treatment (± SD) | 8.31 | (2.6) | 8.46 | 8.58 | (2.8) | 8.53 |
| Mean atomoxetine start/match age (± SD) | 9.88 | (2.8) | 10.07 | 10.14 | (3.0) | 10.07 |
| Mean end age, y (± SD) | 11.82 | (3.0) | 11.71 | 11.55 | (3.2) | 11.58 |
| Mean follow-up, y (± SD) | 3.51 | (1.7) | 3.25 | 2.97 | (1.8) | 3.05 |
| Mean time to switch/match, y (± SD) | 1.57 | (1.3) | 1.56 | 1.56 | (1.3) | 1.55 |
| Mean follow-up after switch/match (± SD) | 1.94 | (1.1) | 1.65 | 1.41 | (1.1) | 1.50 |
| Male gender (%) | 40 657 | (71.4) | (70.4) | 114 426 | (70.1) | (70.4) |
| Age, y, n (%) | ||||||
| 5 | 2453 | (4.3) | (4.2) | 6396 | (3.9) | (4.1) |
| 6–8 | 22 697 | (39.9) | (37.4) | 60 744 | (37.2) | (37.9) |
| 9–11 | 19 108 | (33.6) | (33.5) | 54 866 | (33.6) | (33.5) |
| 12–14 | 9305 | (16.3) | (17.6) | 28 819 | (17.7) | (17.3) |
| 15–18 | 3385 | (5.9) | (7.4) | 12 442 | (7.6) | (7.2) |
| Race/ethnicity, n (%) | ||||||
| Caucasian | 39 564 | (69.5) | (61.5) | 95 877 | (58.7) | (61.5) |
| Black | 12 035 | (21.1) | (27.2) | 48 086 | (29.5) | (27.3) |
| Hispanic | 3494 | (6.1) | (7.8) | 13 590 | (8.3) | (7.8) |
| Other | 1855 | (3.3) | (3.4) | 5714 | (3.5) | (3.4) |
| Reason for Medicaid eligibility, n (%) | ||||||
| TANF | 5871 | (10.3) | (9.0) | 13 786 | (8.4) | (9.0) |
| Foster care | 6462 | (11.3) | (11.9) | 19 991 | (12.2) | (11.9) |
| SSI | 1304 | (2.3) | (2.0) | 3026 | (1.9) | (2.0) |
| Calendar year, n (%) | ||||||
| 2003 | 25 839 | (45.4) | (32.6) | 45 830 | (28.1) | (32.6) |
| 2004 | 17 022 | (29.9) | (23.9) | 35 602 | (21.8) | (23.9) |
| 2005 | 8487 | (14.9) | (20.6) | 37 238 | (22.8) | (20.8) |
| 2006 | 5600 | (9.8) | (22.8) | 44 597 | (27.3) | (22.8) |
| Index diagnosis, n % | ||||||
| ADHD with hyperactivity | 38 399 | (67.4) | (68.1) | 110 486 | (67.7) | (67.8) |
| ADHD without hyperactivity | 12 508 | (22.0) | (21.2) | 33 390 | (20.5) | (20.9) |
| Adjustment reaction | 6720 | (11.8) | (10.9) | 17 208 | (10.5) | (10.9) |
| Disturbance of conduct | 4678 | (8.2) | (7.5) | 11 514 | (7.1) | (7.4) |
| Other or mixed emotional disturbances | 6243 | (11.0) | (9.9) | 15 170 | (9.3) | (9.7) |
| Unspecified emotional disturbance | 327 | (0.6) | (0.5) | 813 | (0.5) | (0.5) |
| Other mental comorbidities, n % | ||||||
| Substance use disorder | 521 | (0.9) | (0.8) | 1098 | (0.7) | (0.7) |
| Anxiety | 3358 | (5.9) | (5.2) | 7811 | (4.8) | (5.1) |
| Bipolar disorder | 2194 | (3.9) | (3.1) | 4536 | (2.8) | (3.1) |
| Schizophrenia | 154 | (0.3) | (0.2) | 316 | (0.2) | (0.2) |
| Depression | 4440 | (7.8) | (6.9) | 10 508 | (6.4) | (6.8) |
| Mild mental retardation | 116 | (0.2) | (0.2) | 398 | (0.2) | (0.2) |
| Tic disorder | 429 | (0.8) | (0.4) | 378 | (0.2) | (0.4) |
| Oppositional defiant disorder | 5793 | (10.2) | (9.1) | 13 976 | (8.6) | (9.0) |
| Psychosis | 412 | (0.7) | (0.6) | 803 | (0.5) | (0.6) |
| Other mental health diagnosis | 8620 | (15.1) | (14.3) | 22 552 | (13.8) | (14.2) |
| Distinct mental health disorders, n (%) | ||||||
| 0 | 5722 | (10.0) | (10.0) | 16 540 | (10.1) | (10.1) |
| 1 | 26 680 | (46.8) | (50.7) | 84 545 | (51.8) | (50.6) |
| 2 | 13 481 | (23.7) | (22.9) | 36 628 | (22.4) | (22.8) |
| 3 | 6312 | (11.1) | (9.9) | 15 472 | (9.5) | (9.9) |
| ≥4 | 4753 | (8.3) | (6.9) | 10 082 | (6.2) | (6.8) |
| Other comorbidities, n % | ||||||
| Obesity | 415 | (0.7) | (0.8) | 1368 | (0.8) | (0.8) |
| Smoking | 56 | (0.1) | (0.1) | 126 | (0.1) | (0.1) |
| Suicidal ideation | 20 | (0.035) | (0.04) | 66 | (0.040) | (0.04) |
| Suicide attempt | 29 | (0.051) | (0.04) | 47 | (0.029) | (0.04) |
| Non–mental health hospitalization | ||||||
| 0 | 56 345 | (98.9) | (99.1) | 161 860 | (99.1) | (99.1) |
| 1 | 556 | (1.0) | (0.9) | 1286 | (0.8) | (0.8) |
| ≥2 | 47 | (0.1) | (0.1) | 121 | (0.1) | (0.1) |
| Mental health hospitalization | ||||||
| 0 | 55 831 | (98.0) | (98.8) | 161 637 | (99.0) | (98.8) |
| 1 | 930 | (1.6) | (1.0) | 1376 | (0.8) | (1.0) |
| ≥2 | 187 | (0.3) | (0.2) | 254 | (0.2) | (0.2) |
| Psychotropic drug use, n (%) | ||||||
| Antidepressant | 11 296 | (19.8) | (16.6) | 25 390 | (15.6) | (16.6) |
| Antipsychotic | 8343 | (14.7) | (13.2) | 20 847 | (12.8) | (13.2) |
| Anticonvulsant | 4627 | (8.1) | (6.9) | 10 659 | (6.5) | (6.9) |
| Anxiolytic | 2258 | (4.0) | (3.6) | 5606 | (3.4) | (3.5) |
| Lithium | 390 | (0.7) | (0.5) | 759 | (0.5) | (0.5) |
| α-agonist | 7568 | (13.3) | (11.7) | 17 861 | (10.9) | (11.5) |
| Opioid analgesics | 3469 | (6.1) | (5.8) | 9000 | (5.5) | (5.7) |
| No. of psychotropic drug classes, n (%) | ||||||
| 0 | 36 347 | (63.8) | (67.5) | 112 288 | (68.8) | (67.5) |
| 1 | 13 304 | (23.4) | (21.8) | 34 596 | (21.2) | (21.8) |
| 2 | 5203 | (9.1) | (7.9) | 12 172 | (7.5) | (7.9) |
| 3 | 1748 | (3.1) | (2.4) | 3589 | (2.2) | (2.4) |
| ≥4 | 346 | (0.6) | (0.4) | 622 | (0.4) | (0.4) |
PS, propensity score; SSI, Supplemental Security Income; TANF, Temporary Assistance for Needy Families.
“Inverse PS weighted” denotes sample distributions of baseline characteristics after propensity score weighting.
We observed 46 suicidal events during current stimulant treatment (32.4 per 100 000 person-years), 17 during former stimulant use (21.8 per 100 000 person-years), 11 during current atomoxetine use (29.0 per 100 000 person-years) and 16 suicidal events during former atomoxetine use (37.4 per 100 000 person-years) (Table 4). The majority of suicide attempts occurred in girls (60%), whereas the majority of suicides were in boys (73%). The average age at a suicidal event was 14.7 years (SD ± 2.4) and occurred after a mean of 0.98 years (median 0.6 years) after the index date.
TABLE 4.
HRs for Suicide and Suicide attempt, Second-Line Treatment Cohort
| Exposure | Suicidal Events | Unadjusted | Adjusted for Time-Dependent Age, Dual Therapy and Propensity Score | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Full follow-up | |||||||
| Current CNS stimulant | 46 | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | 11 | 0.87 | 0.45–1.69 | 0.68 | 0.65 | 0.31–1.36 | 0.25 |
| Former atomoxetine | 16 | 1.30 | 0.72–2.35 | 0.380 | 0.67 | 0.36–1.24 | 0.20 |
| Former CNS stimulant | 17 | 0.72 | 0.41–1.28 | 0.27 | 0.44 | 0.25–0.78 | 0.005 |
| 24-mo follow-up | 77 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 0.68 | 0.32–1.44 | 0.31 | 0.57 | 0.25–1.30 | 0.18 |
| Former atomoxetine | — | 1.32 | 0.68–2.56 | 0.410 | 0.70 | 0.35–1.39 | 0.31 |
| Former CNS stimulant | — | 0.77 | 0.42–1.40 | 0.39 | 0.47 | 0.25–0.86 | 0.02 |
| 12-mo follow-up | 55 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 0.88 | 0.40–1.91 | 0.74 | 0.71 | 0.30–1.67 | 0.43 |
| Former atomoxetine | — | 1.25 | 0.51–3.05 | 0.63 | 0.66 | 0.26–1.65 | 0.37 |
| Former CNS stimulant | — | 0.82 | 0.40–1.72 | 0.61 | 0.54 | 0.26–1.13 | 0.10 |
| 6-mo follow-up | 29 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 0.30 | 0.07–1.27 | 0.10 | 0.28 | 0.06–1.25 | 0.09 |
| Former atomoxetine | — | 0.55 | 0.07–4.27 | 0.57 | 0.31 | 0.04–2.50 | 0.27 |
| Former CNS stimulant | — | 0.96 | 0.34–2.73 | 0.94 | 0.70 | 0.25–1.99 | 0.50 |
| 3-mo follow-up | 20 | ||||||
| Current CNS stimulant | — | 1.00 | — | — | 1.00 | — | — |
| Current atomoxetine | — | 0.40 | 0.09–1.73 | 0.22 | 0.37 | 0.08–1.71 | 0.200 |
| Former atomoxetine | — | 1.32 | 0.16–10.67 | 0.80 | 0.74 | 0.09–6.22 | 0.78 |
| Former CNS stimulant | — | 0.85 | 0.17–4.13 | 0.84 | 0.63 | 0.13–3.08 | 0.570 |
CNS, central nervous system.
During the first year of follow-up, the unadjusted HR for current atomoxetine use compared with current stimulant use was 0.88 (95% CI 0.40–1.91, P = .74). When adjusted for the propensity score, age and dual therapy, the HR for current atomoxetine use was 0.71 (95% CI 0.30–1.67, P = .43 (Table 4). Varying follow-up times showed no appreciable effect on HRs.
Discussion
Our study did not observe a statistically or clinically meaningful increase in the risk of suicidal events (suicide or suicide attempt) associated with first- or second-line treatment of youth age 5 to 18 with atomoxetine compared with stimulants. All point estimates were close to 1, consistent over varying periods of follow-up time, and consistent among current and former use, indicating no excess immediate or residual suicidal risk. Adjustments for age, dual therapy, and propensity score usually decreased HRs, suggesting that atomoxetine was not channeled toward patients at lower suicide risk.
It is noteworthy that most of the study time was before the possible risk of suicidal ideation was communicated. Our drug utilization analyses (data not shown) as well as other published data show a steep increase in atomoxetine utilization early after approval in 2002 followed by a gradual decline starting in 2004.32 We also found that atomoxetine users were older, twice as likely to have substance use disorder, had more oppositional defiant disorder, and distinctly more depression than stimulant users, all significant risk factors for suicide (Table 1). Thus, restricting of our study cohort to the early years of atomoxetine use alleviate concerns that that atomoxetine may have been channeled toward patients less risk for suicide.
Finally, unadjusted HRs suggested that atomoxetine users were at higher risk for suicide or suicide attempt, an association that vanished if adjusted for our measured confounders. Any residual (unmeasured confounder) that could mask an elevated suicidal risk of atomoxetine would need to have the opposite association than the confounding effects of age, substance use disorder, oppositional defiant disorder, and depression.
Importantly, because CIs of all HRs were wide, our study cannot exclude an excess risk of atomoxetine smaller than 40% to 70% (depending on follow-up time). However, considering the baseline incidence rate (during stimulant use) of 30 suicidal events per 100 000 person-years, the resulting increase in the absolute risk would be small. Even if general concerns about bias are considered, the observed incidence rates provide assurance regarding a limited potential for clinically significant risk differences.
Current treatment recommendations emphasize the evidence that supports the efficacy of stimulants in the treatment of ADHD but indicate that atomoxetine and α-agonists may offer viable alternatives.33 Guidelines also point to the varying side effect profiles and make special note of treatment of adolescents in light of concerns about diversion and substance abuse associated with stimulants. Our real-world findings should be integrated in treatment decisions that weigh stimulant and atomoxetine effectiveness against their respective side effect profiles, especially considering the demonstrated risk for injury and potential self-harm associated with untreated ADHD itself.34,35
Interestingly, former use periods of both atomoxetine and stimulants showed consistent trends toward a reduced risk compared with current use periods. One possible explanation is residual confounding, which might be more pronounced in drug user to nonuser than in head-to-head comparisons. For example, patients who discontinue treatment altogether may have dissipating ADHD or comorbidity severity, resulting in reduced suicidal risk. Alternatively, ADHD treatment in adolescents and young adults could be associated with substance use problems, which in turn present a critical risk factor for suicide.
Major strengths of our study are its large population, its new-user design and balanced treatment groups resulting from propensity score adjustment.36–38 Our study included data from 4 years and 26 US state Medicaid programs. Although mostly Caucasian (60%), our study population allowed good representation of Hispanic, African American, and vulnerable pediatric populations with complex psychiatric needs. We established balanced treatment groups by restriction to patients with indication for stimulant or atomoxetine treatment and with comprehensive coverage of health care services, permitting a broad selection of covariates for propensity score adjustment. We further stratified our analysis to patients who initiated treatment with either stimulant or atomoxetine versus those who switched or added atomoxetine after stimulant treatment had been initiated. Because this second-line treatment group had a longer history of mental health problems and related treatment and an increased suicidal risk, stratification improved our ability to establish balance between treatment groups. Follow-up time was sufficient to cover the time to development of suicidal events as suggested by clinical trial data.
Our study is based on health care claims data, intended for reimbursement and not as electronic medical records. Therefore, codes used for billing practices might not accurately reflect clinical diagnoses, and pharmacy claims, representing dispensed prescriptions, do not necessarily imply drug utilization. However, Medicaid pharmacy data have been validated to define psychotropic drug exposure with a positive and negative predictive value >85%.39 Some important covariates influencing treatment decisions and/or suicidality risk such as disease severity, violence, or other stressful life events are not captured in administrative health data. The following sections discuss the effect of potential biases on our results.
First, our ability to detect suicidal events was dependent on clinician diagnoses, and reported incidence estimates might be underestimated. Consistency of our rates with previous estimates provides some assurance about the sensitivity of our method.24,40,41 Gender distribution and the distribution of methods to commit/attempt suicide were also consistent with previous research.24 Importantly, reduced sensitivity of our outcome ascertainment would have resulted in reduced statistical power but not systematically biased HRs.
Alternatively, publicized safety concerns might have resulted in increased clinician awareness, resulting in increased suicidal diagnoses in atomoxetine users. We minimized diagnostic bias by including only events with significant harm rather than investigating suicidal ideation and by excluding self-harm with ambiguous intent.16,42–44 Importantly, if such bias were present, it would alter risk estimates toward an increased risk during atomoxetine exposure, which we did not observe.
Second, it is conceivable that suicidal patients were channeled toward atomoxetine because of lesser concerns about substance abuse. We minimized confounding by using an active comparator and by requiring a mental health diagnosis that is consistent with indications for both atomoxetine and stimulants.26 Sensitivity analyses requiring at least 2 diagnoses to determine presence of mental disorders or excluding high-risk patients with history of suicidal ideation or suicide attempt showed no appreciable effects on HRs. Of note, because our confounding adjustment alleviated an initially increased risk of atomoxetine, it is unlikely that adjustment for residual confounders would have an opposite effect.
Finally, in addition to the low incidence of suicidal events, limited follow-up resulted in wide confidence of risk comparisons. Statistical power also limited our ability to stratify analyses to high-risk groups or long-term users of atomoxetine. However, the overall small suicidal event rates indicate a small absolute risk increase potential in typical clinical practice.
Conclusions
First- and second-line treatment of youth aged 5 to 18 with atomoxetine compared with stimulant treatment was not associated with an increased risk of suicide attempts requiring medical attention and suicides in current practice. Limited utilization periods of atomoxetine and low incidence of suicidal events resulted in limited statistical power, which did not allow stratified analysis of high-risk groups or assessment of suicidal risk associated with long-term use. However, the observed low suicidal event numbers indicate a small absolute risk increase potential in typical clinical practice.
Acknowledgments
This study was approved by the University of Florida Institutional Review and Privacy Boards (269-2012), National Death Index (2012-0044), and the Centers for Medicare and Medicaid Services Privacy Board (DUA 23778), including a waiver for the Health Insurance Portability and Accountability Act authorization. Data were obtained under data users agreements with the Centers for Medicare and Medicaid and the National Death Index and are not available for sharing.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- CI
confidence interval
- HR
hazard ratio
- ICD
International Classification of Diseases
- MAX
Medicaid Analytic eXtract
Footnotes
Dr Linden conceptualized as well as designed the study and conducted all data analyses; had full access to all the data in the study; takes responsibility for the integrity of the data and the accuracy of the data analysis; affirms that the manuscript is an honest, accurate, and transparent account of the study being reported and no important aspects of the study have been omitted; finally he interpreted the results and drafted the manuscript; Drs Bussing, Gerhard, Segal, and Shuster conceptualized and designed the study and interpreted the results and provided critical revisions of the manuscript for important intellectual content; Mr Kubilis conducted the data acquisition and management, conceptualized and designed the study, interpreted the results, and provided critical revisions of the manuscript for important intellectual content; Dr Winterstein organized the data acquisition, conceptualized and designed the study, participated in all data analyses, interpreted the results, and drafted the manuscript; all authors approved the final manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The Centers for Medicaid and Medicare Services granted free data reuse for doctoral research. This study was partially funded by a grant from the Agency for Healthcare Research and Quality (R01-HS0185606). Dr Linden’s current affiliation is Boehringer Ingelheim GmbH. Boehringer Ingelheim GmbH did not contribute any direct or indirect financing to this study and did not influence the content of the publication in any aspect at any time. At the time of study conduct, Dr Linden was in the Department of Pharmaceutical Outcomes and Policy at the University of Florida. Dr Linden was partially supported by the Mary Kay Owens Healthcare Innovation and the DuBow Family fellowships. Dr Bussing has received past research support from Otsuka and Pfizer and served as consultant to Pfizer; her contribution was not supported by any grant mechanism. Dr Gerhard’s contribution was partially supported by grant HS 106097 from the Agency for Healthcare Research and Quality (Centers for Education and Research on Therapeutics). Dr Shuster’s contribution was partially supported by grant 1UL1TR000064 from the National Center for Translational Sciences, National Institutes of Health. None of these entities had influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332–339 [DOI] [PubMed] [Google Scholar]
- 2.Center for Drug Evaluation and Research Public Health Advisory—Suicidality in children and adolescents being treated with antidepressant medications. 2004. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm161679.htm. Accessed February 2012
- 3.European Medicines Agency (EMEA) Report of the Committee for Medicinal Products for Human Use: Increased risk of suicidal thoughts and behavior in children treated with antidepressants resulting in updated warnings added to product information 2005. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SSRI_31/WC500013126.pdf. Accessed September 2012
- 4.Banaschewski T, Roessner V, Dittmann RW, Santosh PJ, Rothenberger A. Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry. 2004;13(suppl 1):I102–I116 [DOI] [PubMed] [Google Scholar]
- 5.Preti A. Tomoxetine (Eli Lilly & Co). Curr Opin Investig Drugs. 2002;3(2):272–277 [PubMed] [Google Scholar]
- 6.Center for Drug Evaluation and Research Public Health Advisory—Suicidal thinking in children and adolescents being treated with Strattera (atomoxetine). September 2005. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm152628.htm. Accessed July 2012
- 7.Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209–218 [DOI] [PubMed] [Google Scholar]
- 8.Bangs ME, Wietecha LA, Wang S, Buchanan AS, Kelsey DK. Meta-analysis of suicide-related behavior or ideation in child, adolescent, and adult patients treated with atomoxetine. J Child Adolesc Psychopharmacol. 2014;24(8):426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushe CJ, Savill NC. Suicide related events and attention deficit hyperactivity disorder treatments in children and adolescents: a meta-analysis of atomoxetine and methylphenidate comparator clinical trials. Child Adolesc Psychiatry Ment Health. 2013;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy S, Cranswick N, Potts L, Taylor E, Wong I Mortality associated with attention-deficit hyperactivity disorder (ADHD) drug treatment: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Safety 2009:32(11):1089–1096 [DOI] [PubMed] [Google Scholar]
- 11.Guevara J, Lozano P, Wickizer T. Utilization and cost of health care services for children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(1):171–108 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children --- United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59(44):1439–1443 [PubMed] [Google Scholar]
- 13.Bhatara VS, Aparasu RR. Pharmacotherapy with atomoxetine for US children and adolescents. Ann Clin Psychiatry. 2007;19(3):175–180 [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics . Clinical practice guideline: Ttreatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(104):1033–1044 [DOI] [PubMed] [Google Scholar]
- 15.Winterstein AG, Gerhard T, Kubilis P, et al. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ. 2012;345:e4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mościcki E. Epidemiology of completed and attempted suicide: toward a framework for prevention. Clin Neurosci Res. 2001;1(5):310–323 [Google Scholar]
- 17.World Health Organization International Statistical Classification of Diseases, 10th Revision (ICD-10) 1992. Available at: http://www.who.int/classifications/icd/en/. Accessed September 2012
- 18.Centers for Disease Control and Prevention International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) 1988. Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed September 2012
- 19.Iribarren C, Sidney S, Jacobs DR Jr, Weisner C. Hospitalization for suicide attempt and completed suicide: epidemiological features in a managed care population. Soc Psychiatry Psychiatr Epidemiol. 2000;35(7):288–296 [DOI] [PubMed] [Google Scholar]
- 20.Moyer LA, Boyle CA, Pollock DA. Validity of death certificates for injury-related causes of death. Am J Epidemiol. 1989;130(5):1024–1032 [DOI] [PubMed] [Google Scholar]
- 21.McKenzie K, Enraght-Moony EL, Walker SM, McClure RJ, Harrison JE. Accuracy of external cause-of-injury coding in hospital records. Inj Prev. 2009;15(1):60–64 [DOI] [PubMed] [Google Scholar]
- 22.Walkup J, Townsend L, Crystal S, Olfson M MINI-SENTINEL systematic evaluation of health outcome of interest definitions for studies using administrative data—suicide report. 2010. Available at: http://www.mini-sentinel.org/work_products/HealthOutcomes/MS_HOI_SuicideReport.pdf. Accessed April 2012
- 23.US Dept of Justice Drug Enforcement Administration Controlled Substance Schedule (CSA), DEA Office of Diversion Control. CSA Schedule Drug List. 2010. Available at: http://www.deadiversion.usdoj.gov/schedules/orangebook/e_cs_sched.pdf. Accessed September 2012
- 24.Centers for Disease Control and Prevention Injury Prevention & Control: Data & Statistics—Web-based Injury Statistics Query and Reporting System (WISQARS). 2002–2006. Available at: http://www.cdc.gov/injury/wisqars/fatal.html. Accessed September 2012
- 25.Rosenbaum P, Rubin D The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70(1):41–55
- 26.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin D. Estimating causal effects from large data sets using propensity scores. Ann Int Med 1997;127(8, pt 2):757–763 [DOI] [PubMed]
- 28.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158(3):280–287 [DOI] [PubMed] [Google Scholar]
- 29.Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161(9):891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Team RCR. A language and environment for statistical computing. 2012. Available at: http://www.r-project.org. Accessed September 2012
- 31.SAS Institute SAS Business Analytics and Business Intelligence Software, Version 9.2. 2012. Available at: http://www.sas.com. Accessed September 2012
- 32.Du DT, Zhou EH, Goldsmith J, Nardinelli C, Hammad TA. Atomoxetine use during a period of FDA actions. Med Care. 2012;50(11):987–992 [DOI] [PubMed] [Google Scholar]
- 33.Wolraich M, Brown L, Brown RT, et al. ; Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management . ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702–709 [DOI] [PubMed] [Google Scholar]
- 35.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2014;35(7):448–457 [DOI] [PubMed] [Google Scholar]
- 36.US Census Bureau Census 2000 data. 2000. Available at: http://www.census.gov/main/www/cen2000.html. Accessed September 2012
- 37.Bright RA, Avorn J, Everitt DE. Medicaid data as a resource for epidemiologic studies: strengths and limitations. J Clin Epidemiol. 1989;42(10):937–945 [DOI] [PubMed] [Google Scholar]
- 38.Crystal S, Akincigil A, Bilder S, Walkup JT. Studying prescription drug use and outcomes with medicaid claims data: strengths, limitations, and strategies. Med Care. 2007;45(10 suppl 2):S58–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie DA, Semradek J, McFarland BH, Mullooly JP, McCamant LE. The validity of medicaid pharmacy claims for estimating drug use among elderly nursing home residents: the Oregon experience. J Clin Epidemiol. 2000;53(12):1248–1257 [DOI] [PubMed] [Google Scholar]
- 40.Goldsmith S, Pellmar T, Kleinman A, Bunney W. Reducing Suicide: A National Imperative Institute of Medicine of the National Academies; 2002. Available at: http://www.nap.edu/openbook.php?record_id=10398&page=R1. Accessed September 2012 [PubMed]
- 41.James A, Lai FH, Dahl C. Attention deficit hyperactivity disorder and suicide: a review of possible associations. Acta Psychiatr Scand. 2004;110(6):408–415 [DOI] [PubMed] [Google Scholar]
- 42.Monk M. Epidemiology of suicide. Epidemiol Rev. 1987;9:51–69 [DOI] [PubMed] [Google Scholar]
- 43.Brent DA, Perper JA, Allman CJ. Alcohol, firearms, and suicide among youth. Temporal trends in Allegheny County, Pennsylvania, 1960 to 1983. JAMA. 1987;257(24):3369–3372 [PubMed] [Google Scholar]
- 44.Mościcki EK. Epidemiology of suicidal behavior. Suicide Life Threat Behav. 1995;25(1):22–35 [PubMed] [Google Scholar]