Abstract
BACKGROUND AND OBJECTIVE:
Care for neonatal abstinence syndrome (NAS), a postnatal drug withdrawal syndrome, remains variable. We designed and implemented a multicenter quality improvement collaborative for infants with NAS. Our objective was to determine if the collaborative was effective in standardizing hospital policies and improving patient outcomes.
METHODS:
From 2012 to 2014, data were collected through serial cross-sectional audits of participating centers. Hospitals assessed institutional policies and patient-level data for infants with NAS requiring pharmacotherapy, including length of pharmacologic treatment and length of hospital stay (LOS). Models were fit, clustered according to hospital, to evaluate changes in patient outcomes over time.
RESULTS:
Among 199 participating centers, the mean number of NAS-focused guidelines increased from 3.7 to 5.1 of a possible 6 (P < .001), with improvements noted in all measured domains. Among infants cared for at participating centers, decreases occurred in median (interquartile range) length of pharmacologic treatment, from 16 days (10 to 27 days) to 15 days (10 to 24 days; P = .02), and LOS from 21 days (14 to 33 days) to 19 days (15 to 28 days; P = .002). In addition, there was a statistically significant decrease in the proportion of infants discharged on medication for NAS, from 39.7% to 26.5% (P = .02). After adjusting for potential confounders, standardized NAS scoring process was associated with shorter LOS (–3.3 days,95% confidence interval, –4.9 to –1.4).
CONCLUSIONS:
Involvement in a multicenter, multistate quality improvement collaborative focused on infants requiring pharmacologic treatment for NAS was associated with increases in standardizing hospital patient care policies and decreases in health care utilization.
What’s Known on This Subject:
The incidence of neonatal abstinence syndrome, a drug withdrawal syndrome experienced by substance-exposed infants shortly after birth, grew substantially over the last decade. Hospital care for infants with the syndrome varies, resulting in uneven outcomes.
What This Study Adds:
This large, multicenter quality improvement collaborative was effective in standardizing care among participating centers. Centers were effective in reducing length of pharmacotherapy treatment and length of stay while reducing the number of infants discharged from the hospital on medication tapers.
Neonatal abstinence syndrome (NAS) is a drug withdrawal syndrome experienced by opioid-exposed infants shortly after birth. Infants with NAS exhibit a constellation of clinical signs, including irritability, sleep disturbances, poor feeding, hypertonia, and tremors.1 Over the last decade, the number of infants with NAS grew nearly 5-fold, in parallel with an increase in opioid use in pregnancy.2–6 Although the number of infants with NAS grew, several studies reported variable approaches to diagnosis and treatment of the syndrome.7,8 In response to the growth and variable treatment of these infants with NAS, the American Academy of Pediatrics (AAP) released a policy statement in 2012 calling for standardization of care delivered to infants with the syndrome.1
In 2012, the Vermont Oxford Network (VON) embarked on a multicenter quality improvement collaborative that aimed to standardize NAS hospital care. The objective of the quality improvement collaborative was to determine if implementation of evidence-based potentially better practices (PBPs), supported by interactive webinars, real-time feedback of outcomes, and sharing of improvement practices through electronic forums, was effective in standardizing hospital care and improving infant outcomes. The primary objective of the present article was to evaluate if centers participating in the quality improvement collaborative were effective in standardizing hospital care for infants with NAS and if this outcome was associated with improvements in length of pharmacotherapy treatment (LOT) and hospital length of stay (LOS) among infants who required pharmacotherapy for the syndrome.
Methods
Study Design and Setting
This prospective cohort study was conducted by using serial cross-sectional audits of centers enrolled in the VON NAS Internet-Based Quality Improvement Collaborative (iNICQ). VON is a not-for-profit organization that aims to improve the quality, safety, and value of medical care delivered to infants and their families through research, education, and quality improvement.9 Each year VON chooses a topic or care process that the community believes merits intensive focus.10 In 2012, the network launched a quality improvement collaborative focused on standardizing care and improving outcomes of infants and families affected by NAS. The iNICQ recruited centers from the VON who elected to participate, paid a fee, and began enrolling in November 2012. In addition, several states partnered with VON to create statewide NAS improvement collaboratives. This structure allowed participation by all levels of hospitals, including Level 1 (ie, birth hospitals with no NICU), Level 2, and Level 3 centers. The Massachusetts collaborative (the Neonatal Quality Improvement Collaborative of Massachusetts) enrolled 42 of 50 birthing hospitals in the state. Michigan enrolled 19 of 21 NICUs and included an additional 5 Level 1 nurseries. The Northern New England Perinatal Quality Collaborative enrolled 8 NICUs and birthing centers from New Hampshire and Vermont. In total, 223 centers participated in the collaborative, of which 199 participated in at least 1 audit (Supplemental Tables 5 and 6).
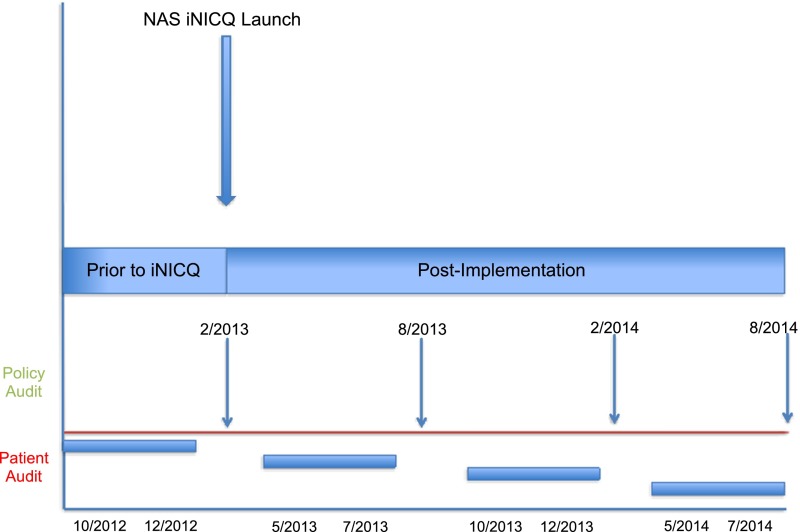
To assess the primary outcomes, serial preplanned quality audits were performed in February 2013, August 2013, February 2014, and August 2014 (Fig 1). The audit included both hospital- and patient-level measures; questions were derived primarily from published AAP guidelines. Auditors were instructed to review records for all discharged infants who received pharmacotherapy for NAS during a 3-month period before the audit and to submit data on the most recent 30 patients discharged in the time period. Infants were included in the cohort if they were diagnosed with NAS, identified by using the International Classification of Diseases, Ninth Revision, Clinical Modification, code 779.5, and required pharmacotherapy (Supplemental Appendix A). Auditors received manuals of operation with standardized definitions for each measure. This project was classified as not human research and considered exempt by the institutional review board of the University of Vermont. Each participating center was provided with institutional review board sample materials and instructed to obtain the necessary determinations that may be required at their local site.
FIGURE 1.
Time line for NAS quality improvement collaborative and audits.
Interventions for the quality improvement collaborative focused on the following: (1) an extensive quality improvement toolkit; (2) a series of Internet-based interactive webinars (Supplemental Appendix B); (3) exposure to a center of innovation via a virtual video visit and facilitator’s guide; (4) standardized data collection; and (5) iterative coaching and feedback on process and outcome measures.
The VON NAS toolkit served as a blueprint for centers to evaluate their institutional processes and apply evidence-based PBPs. The literature does not support a single best practice for care provided to infants with NAS; therefore, practices were evaluated in the literature to craft PBPs. The term PBP is used because a practice is not “better” until it is shown to work in the local context and culture of a unit.11 Teams were encouraged to set a clinical aim, value aim, and family-centered care aim, and to use the model for improvement, implementing small practice changes by using a Plan-Do-Study-Act methodology, to measure and evaluate the impact of the PBPs in their local context. The NAS toolkit focused on 3 specific PBPs: (1) develop and implement a standardized process for the identification, evaluation, treatment, and discharge management of an infant with NAS; (2) develop and implement a standardized process for measuring and reporting rates of NAS and drug exposure; and (3) create a culture of compassion, understanding, and healing for the mother–infant dyad affected by the issue of NAS.
The curriculum included case studies and data-driven improvement stories from institutions to highlight successful PBP implementation. For example, for PBP 3, a virtual video visit was conducted at 2 centers in Vancouver, British Columbia. “Sheway” is a comprehensive community-based outpatient center for pregnant women with substance use that is carefully linked to the hospital-based unit. “Families in Recovery” is a comprehensive inpatient program that uses a rooming-in model of care, as well as intense coaching and support, to provide care for substance-exposed infants. The video offered teaching and guidance around creating nonpunitive, trauma-informed, family-centered services for women who are pregnant or newly parenting.
Audit data were collected by each center and reported to VON through the use of an online data portal. Local data were immediately reported back to the center at the completion of data entry. The aggregate data were reported back to centers, comparing their processes and patient-level outcomes versus those of their peers.
Exposure Measurement
Several hospital- and patient-level outcomes were assessed throughout the collaborative to quantify the degree of care standardization. Hospital standardization was measured through serial assessment of hospital policies addressing the following collaborative-focused guidelines: maternal substance use screening; NAS evaluation and treatment; scoring practices; pharmacologic and nonpharmacologic treatment; and the use of human milk for substance-exposed infants.
Outcomes
Patient-level outcomes for NAS included LOT, LOS, discharge on human milk, discharge with a parent, and discharge on a medication. The audit process is described in Supplemental Appendix A and Supplemental Figure 2.
Data Analysis
Descriptive statistics were generated. Separate time series analyses were then conducted for hospital- and patient-level outcomes. All patient-level models used generalized estimating equations, controlling for potential patient-level confounders (eg, gestational age, transfer status, discharge home on medication) and clustering of multiple observations at the hospital level. To account for differences in data structure, categorical measures used the binomial distribution, LOS measures used a negative binomial distribution, and the number of policies used Poisson distribution. Models were fit, clustered according to hospital, to evaluate changes in patient outcomes over time. To assess if the results were influenced by initiation or termination of a center, a sensitivity analysis was performed that was restricted to centers that reported data in audits 1 and 4. Lastly, we sought to explore the relationship between hospital policies and our key outcomes (LOT and LOS). We fit negative binomial models by using generalized estimating equations to evaluate the relationship of institutional policies with our outcomes after accounting for patient-level confounders and clustering according to hospital and audit number. Interactions were tested between hospital policies and audit number. P values < .05 were considered statistically significant. Analyses were completed by using SAS version 9.3 (SAS Institute, Inc, Cary, NC).
Results
A total of 199 centers participated in a VON iNICQ quality audit. Overall, 82.9% of centers housed a NICU and 42.2% were classified as “level B” (no restrictions on assisted ventilation, performed most major surgeries, except cardiac surgery requiring bypass). Most participating centers (98.5%) were in the United States, with the northeast (34.7%) being the most common geographical region represented. Centers from the United Kingdom and Canada also participated in the collaborative (Supplemental Table 7).
During our study period, the 199 centers audited 3458 infants with NAS. Most audited infants (77.6%) were born at term and treated with morphine (83.3%). Overall, 34% were discharged from the hospital on a medication taper; however, the proportion of infants on a medication taper decreased throughout the course of the collaborative from 39.7% to 26.5% (P = .02). Although most infants (70.6%) were discharged from the hospital with their parent, nearly one- quarter was discharged to foster care (Table 1).
TABLE 1.
Patient Characteristics, Process Measures, and Treatment and Disposition of Audited Infants
| Infant Characteristic | No. (N = 3458) | % |
|---|---|---|
| Gestational Age | ||
| >37 wk | 2684 | 77.6 |
| 34–37 wk | 647 | 18.7 |
| <34 wk | 127 | 3.7 |
| Inborn | 2753 | 79.6 |
| Process measures and treatment | ||
| Pharmacologic agents administered for the treatment of NAS | ||
| Morphine | 2880 | 83.3 |
| Methadone | 522 | 15.1 |
| Buprenorphine | 0 | 0 |
| Clonidine | 297 | 8.6 |
| Phenobarbital | 840 | 24.3 |
| Paregoric | 1 | 0 |
| Diluted tincture of opium | 102 | 2.9 |
| At the time of discharge from your hospital, infant was receiving medications for NAS | 1194 | 34.5 |
| Disposition | ||
| Home with parent | 2440 | 70.6 |
| Home with a guardian or foster parent | 856 | 24.8 |
| Transferred to another hospital | 96 | 2.8 |
| Other | 66 | 1.9 |
Participating centers were successful in standardizing care, increasing their mean number of policies from 3.7 to 5.1 of 6 total measured policies (P < .001). Centers improved in polices focused on maternal substance screening from 75.4% to 89.8% (P = .002), evaluation and treatment of substance-exposed infants from 76.2% to 95.0% (P < .001), standardized NAS scoring from 44.8% to 76.5% (P < .001), inclusion of nonpharmacologic treatment strategies for NAS from 59.1% to 84.0% (P < .001), standardizing the pharmacologic treatment of NAS from 68.0% to 91.6% (P < .001), and policies regarding the provision of human milk for substance-exposed infants from 48.6% to 72.3% (P < .001) (Table 2).
TABLE 2.
Hospital Policies and Guidelines Pertaining to Maternal Substance Use, Evaluation, and Treatment of Substance-Exposed Infants, 2013–2014
| Variable | February 2013 | August 2013 | February 2014 | August 2014 | P |
|---|---|---|---|---|---|
| %a | %a | %a | %a | ||
| Maternal substance use screen | 75.4 | 77.8 | 80.5 | 89.8 | .002 |
| Evaluation and treatment of substance-exposed infant | 76.2 | 82.9 | 88.0 | 95.0 | <.001 |
| Standardization of NAS scoring | 44.8 | 59.4 | 67.2 | 76.5 | <.001 |
| Nonpharmacologic treatment of NAS | 59.1 | 65.9 | 68.8 | 84.0 | <.001 |
| Pharmacologic treatment of NAS | 68.0 | 81.2 | 84.0 | 91.6 | <.001 |
| Breastfeeding or provision of expressed human milk for substance-exposed infants | 48.6 | 55.3 | 56.8 | 72.3 | <.001 |
Total hospital sample: February 2013, n = 181; August 2013, n = 170; February 2014, n = 125; and August 2014, n = 119.
Percentage of participating hospitals with policy.
After accounting for differences in patient characteristics, there were decreases in median (interquartile range) LOT from 16 days (10 to 27 days) to 15 days (10 to 24 days; P = .02) and LOS from 21 days (14 to 33 days) to 19 days (15 to 28 days; P = .002) (Table 3). Results of supplemental analysis restricted to institutions that reported data only in audits 1 and 4 were no different from the main analysis and are presented in Supplemental Tables 8 and 9.
TABLE 3.
LOT and LOS for Infants With NAS, 2013–2014
| Variable | February 2013, Audit 1 | August 2013, Audit 2 | February 2014, Audit 3 | August 2014, Audit 4 | Pa | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| LOT, d | 16 | 10–27 | 15 | 10–23 | 15 | 10–24 | 15 | 10–24 | .02 |
| LOS, d | 21 | 14–33 | 20 | 14–28 | 20 | 14–29 | 19 | 15–28 | .002 |
For LOT and LOS: Audit 1, n = 1050; Audit 2, n = 991; Audit 3, n = 797; and Audit 4, n = 620. For length of NICU stay: Audit 1, n = 922; Audit 2, n = 890; Audit 3, n = 669; and Audit 4, n = 534 . IQR, interquartile range.
After accounting for gestation age, transfer status, discharge home on medications, and clustered according to hospital.
An exploratory analysis was performed to determine if specific hospital policies were associated with improvements in LOT or LOS. After accounting for gestational age, inborn status, being discharged from the hospital on pharmacotherapy, and other NAS policies audited, we found that policies to standardize NAS scoring were associated with changes in LOT of –2.1 days (95% confidence interval [CI], –3.6 to –0.6) and LOS of –3.1 days (95% CI, –4.9 to –1.4). In this exploratory analysis, there were no other statistically significant changes in adjusted LOT or LOS, and the interaction of policies according to audit number was not statistically significant (Table 4).
TABLE 4.
Difference in Predicted Mean LOT and LOS According to Policy Implemented
| Hospital Policy | LOT, d (95% CI) | LOS, d (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Maternal substance use screen | −1.4 (–3.5 to 0.6) | −1.8 (–3.8 to0.3) | −1.4 (–3.5 to 0.6) | −1.5 (–3.6 to 0.6) |
| Evaluation and treatment of substance-exposed infant | −1.0 (–3.5 to 1.5) | 1.6 (–1.5 to 4.6) | −2.3 (–4.9 to 0.3) | 1.2 (–1.6 to 4.0) |
| Standardization of NAS scoring | −2.4 (–4.1 to –0.8) | −2.1 (–3.6 to –0.6) | −3.3 (–5.1 to –1.6) | −3.1 (–4.9 to –1.4) |
| Nonpharmacologic treatment of NAS | −2.4 (–4.9 to 0.0) | −2.0 (–4.4 to 0.5) | −3.0 (–5.5 to –0.6) | −2.5 (–5.1 to 1.2) |
| Pharmacologic treatment of NAS | −2.2 (–5.0 to 0.5) | −1.4 (–4.3 to 1.5) | −2.9 (–0.5 to –0.7) | −1.3 (–3.7 to 1.2) |
| Breastfeeding or provision of expressed human milk for substance-exposed infants | −0.9 (–2.4 to 0.6) | −0.7 (–2.1 to 0.7) | −1.1 (–2.6 to 0.4) | −0.5 (–2.1 to 1.1) |
Adjusted for gestational age, inborn status, discharged on medication, all other policies, clustering of infants in hospitals, and repeated measurements by hospitals and audit number.
Discussion
Among 199 centers across the United States, Canada, and the United Kingdom, participation in a quality improvement collaborative focused on evidence-based and process-oriented standardization was associated with increasing hospital-level processes with reductions in LOT and LOS. This large-scale collaborative was focused on building interdisciplinary teams to foster engagement in structured quality improvement. Teams developed and implemented a process for identification, evaluation, treatment, and discharge management of infants with NAS. Each center’s efforts were augmented and supported by a toolkit of PBPs informed by the literature, bimonthly webinars, standardized data collection, and near real-time feedback on outcomes relative to peer institutions. Our study reinforces findings from smaller studies that standardizing care to infants with NAS improves clinical outcomes.12
Over the last decade, the rate of NAS has grown substantially across the United States; however, LOS has remained stagnant.2,6 Data suggest that hospitals vary in identification8 and management7,8 of infants with NAS. There have been several innovations over the last decade in the management of opioid-dependent mothers13 and infants with NAS,14,15 but it is unclear how broadly these innovations have been adopted. In 2012, the AAP issued a policy statement focused on management of substance-exposed infants. The AAP called for all centers that might care for infants at risk for NAS to establish guidelines for the following: (1) screening for maternal substance use; (2) nonpharmacologic treatment of infants with NAS; (3) scoring signs of NAS; (4) breastfeeding; (5) pharmacologic management; and (6) duration of observation of exposed infants.1 Rather than relying on NICUs to respond individually to the AAP’s policy, VON sought to facilitate guideline uptake. Participating NICUs shared common interests, worked collaboratively, shared information and ideas, and developed standardized practices. The collaborative was effective in standardizing overall practice in each domain.
In serial cross-sectional audits of patient outcomes, we found improvements in the median LOT (16 to 15 days) and LOT (21 to 19 days) even as the proportion of infants discharged from the hospital on medication decreased substantially from 39.7% to 26.5% (P < .001). Two recent studies from the Ohio NAS collaborative found that standardization, defined as stringent adherence to a treatment protocol, was more effective in reducing LOS than choice of a specific opioid (ie, methadone versus morphine).12,16 In addition to protocol adherence, the evidence for treatment improvements for NAS is building. For example, data suggest that breastfeeding an infant who has NAS, when medically appropriate,17 can decrease the need for NAS treatment18; however, breastfeeding rates among infants with NAS are reportedly low.19 Even after participation in the collaborative, >25% of participating institutions lacked a protocol to address breastfeeding for substance-exposed infants.
Each participating hospital served as their control group by using participation in the collaborative as a natural experiment. Hospitals audited patients and policies before beginning their improvement process (Fig 1), followed their progress forward, and were able to compare findings with those of their peers. In our exploration of the association of hospital policies with LOT and LOS, we find that standardizing the NAS-scoring process was associated with decreases in LOT of –2.1 days (95% CI, –3.6 to –0.6) and LOS of –3.1 days (95% CI, –4.9 to –1.4), after accounting for infant characteristics and other hospital policies. Several scoring systems for NAS have been shown to suffer from problems of interrater reliability,20 making it an optimal target for improvement. Importantly, the interaction of standardizing scoring according to audit number was tested and was not statistically significant, suggesting that either our hospital sample was not large enough to detect a change or that this finding was associated mainly with hospitals that had a policy in place for the duration of the collaborative.
In February 2015, the Government Accountability Office released a report that highlighted gaps which exist in research and the care delivery of mothers with opioid dependency and infants with NAS.21 Despite these well-documented knowledge gaps, we found that care can be improved through rapid-cycle quality improvement efforts utilizing PBPs. In the future, large networks such as VON could be used to test and implement NAS treatment innovations, including protocol and quality metric development. Our collaborative was unique in the existing literature in its rapid-cycle, real-time center data feedback, inclusion of birth hospitals with no associated NICU, and in its ability to address the maternal–infant dyad in a holistic fashion. The collaborative was not limited to improving processes solely targeting infant medical care and included understanding and engaging families by use of principles of trauma-informed care. Such an approach may offer useful, large-scale improvement efforts that can target vulnerable populations.
We found that nearly 30% of infants were not discharged from the hospital with their mothers, highlighting the syndrome’s societal toll. NAS increased nationwide2 as the number of women using opioid pain relievers during pregnancy also grew.3,4 Furthermore, several recent studies directly associate use of opioid pain relievers in pregnancy and NAS.22–24 Primary prevention strategies aimed at reducing unnecessary prescribing to women of childbearing age are needed.
Several states joined the VON collaborative as a statewide initiative. These state collaboratives provided additional support and coaching to participating hospitals, and they conducted statewide meetings to foster greater collaboration among centers. State collaboratives developed strong and lasting partnerships with their state health departments and other state organizations, including payers, child protection agencies, and early intervention services. States play an important role in improving care delivered to infants who have NAS. Medicaid, the primary payer for an estimated 80% of infants with NAS,2,6 is jointly financed by the federal and state governments25 and is administered at the state level. In response to rising rates of NAS (largely associated with use of opioid pain relievers),22,24,26 states developed several strategies to combat the problem, including public reporting of NAS,22 statewide task forces,27 and state-funded perinatal collaboratives.28 Such partnerships are vital in improving care for complex problems such as NAS. Investment in this collaborative was likely cost-saving for states that supported center engagement. Using 2012 estimates, we estimated that reducing the hospital LOS by 2 days nationwide would result in a savings of an estimated $170 million in hospital charges.6
As a prospective serial cross-sectional analysis, the present study has important limitations to consider. First, we have limited data on differences in maternal antenatal drug use, which can affect NAS LOT and LOS.13,24 Next, as a serial cross-sectional analysis, it is possible that an unmeasured confounder (eg, patient-level genotype)29 may have influenced our results. Because some state collaboratives enrolled as a group, these geographic areas (eg, the northeast region) are overrepresented in our sample, perhaps limiting the generalizability of our findings. In addition, given the challenges with accurately identifying all substance-exposed infants, only audited infants who were pharmacologically treated were included in the audit, potentially biasing our results to the null. Errors of omission and commission (ie, misclassification bias) are possible in data collection. This collaborative was not designed to measure compliance to hospital policies centrally implemented; however, teams were encouraged to do so locally. Lastly, there was no control group of hospitals that did not participate in the collaborative to compare results over time, raising the possibility that the changes in LOS and LOT we observed were not due to the collaborative; however, recent studies suggest that LOS for NAS has not changed2,6 or increased5 in recent years.
Conclusions
Involvement in a multicenter, multistate quality improvement collaborative that was focused on infants with NAS was associated with increases in standardizing hospital patient care policies and decreases in health care utilization. The data provide support for the AAP’s call for standardizing NAS care, which we have shown is associated with improved outcomes. Furthermore, it provides evidence supporting the use of a novel model to promote rapid-cycle adoption of practice guidelines. State governments and health agencies play an important role in improving neonatal care and have the potential to garner significant savings by partnering with structured systematic quality improvement collaboratives.
Acknowledgments
We are grateful to the patients and families who made this research possible. We acknowledge the Scientific Steering Committee for the VON iNICQ NAS Quality Improvement Collaborative, including: Ronald Abrahams, MD, Karol Kaltenbach, PhD, Lenora Marcellus, RN, BSN, MN, PhD, Lauren M. Jansson, MD, and Walter K. Kraft, MD. We also acknowledge the FIR Square and Sheway programs for sharing their practice, and the women who bravely shared their stories for our Virtual Video Visit.
We are also grateful to the statewide perinatal quality improvement collaboratives and their leaders, who were critical to the statewide demonstration projects: Alan Picarillo, MD, and Munish Gupta, MD, Neonatal Quality Improvement Collaborative of Massachusetts; Padu Karna, MD, Michigan Collaborative Quality Initiative; Victoria A. Flanagan, RN, MS, Bonny Whalen, MD, William Edwards, MD, Northern New England Quality Improvement Network, Vermont/New Hampshire. We also thank Ann Stark, MD for her contributions to this manuscript, and all the participating centers for their commitment to improve the care for newborns in their NICUs (Supplemental Tables 5 and 6).
Glossary
- AAP
American Academy of Pediatrics
- CI
confidence interval
- iNICQ
Internet-Based Quality Improvement Collaborative
- LOS
length of stay
- LOT
length of treatment
- NAS
neonatal abstinence syndrome
- PBP
potentially better practice
- VON
Vermont Oxford Network
Footnotes
Dr Patrick participated in the collaborative’s webinars, drafted the toolkit used by participating centers, crafted the analytic plan for this study, drafted the initial manuscript, and participated in interpretation of the results and revision of the manuscript; Dr Schumacher participated in the collaborative’s webinars, drafted the toolkit used by participating centers, and participated in interpretation of the results and revision of the manuscript; Dr Horbar participated in the collaborative’s webinars, led the collaborative, and participated in interpretation of the results and revision of the manuscript; Dr Buus-Frank participated in the collaborative’s webinars, led the collaborative, contributed to the audit content and design, and participated in interpretation of the results and revision of the manuscript; Dr Edwards crafted the analytic plan for the study, conducted the analysis, and participated in interpretation of the results and revision of the manuscript; Ms Morrow conducted serial institutional and patient audits and analyzed their results, conducted the analysis, and participated in interpretation of the results and revision of the manuscript; Ms Ferrelli conducted serial institutional and patient audits and analyzed their results, and participated in interpretation of the results and revision of the manuscript; Drs Picarillo and Gupta participated in the collaborative’s webinars and participated in interpretation of the results and revision of the manuscript; Dr Soll participated in the collaborative’s webinars, conducted serial institutional and patient audits and analyzed their results, led the collaborative, and participated in interpretation of the results and revision of the manuscript; and all authors approved the manuscript as written.
FINANCIAL DISCLOSURE: Drs Patrick and Schumacher were consultants for the Vermont Oxford Network (VON). Dr Horbar, Dr Soll, Dr Buus-Frank, Ms Morrow, and Ms Ferrelli are employees of VON. Dr Edwards receives salary support from VON. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Patrick was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000446 and the National Institute on Drug Abuse of the National Institutes of Health under award number K23DA038720. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics . Neonatal drug withdrawal. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e54022291123 [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934–1940 [DOI] [PubMed] [Google Scholar]
- 3.Epstein RA, Bobo WV, Martin PR, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol. 2013;23(8):498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118–2126 [DOI] [PubMed] [Google Scholar]
- 6.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012 [published correction appears in J Perinatol. 2015;35(8):667]. J Perinatol. 2015;35(8):650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004-2011. J Perinatol. 2014;34(11):867–872 [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17 [DOI] [PubMed] [Google Scholar]
- 9.Horbar JD, Soll RF, Edwards WH. The Vermont Oxford Network: a community of practice. Clin Perinatol. 2010;37(1):29–47 [DOI] [PubMed] [Google Scholar]
- 10.Horbar JD. The Vermont Oxford Network: evidence-based quality improvement for neonatology. Pediatrics. 1999;103(1 suppl E):350–359 [PubMed] [Google Scholar]
- 11.Plsek PE. Quality improvement methods in clinical medicine. Pediatrics. 1999;103(1 suppl E):203–214 [PubMed] [Google Scholar]
- 12.Hall ES, Wexelblatt SL, Crowley M, et al. ; OCHNAS Consortium . A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agthe AG, Kim GR, Mathias KB, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5). Available at: www.pediatrics.org/cgi/content/full/123/5/e849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MS, Hayes MJ, Thornton LM. Methadone versus morphine for treatment of neonatal abstinence syndrome: a prospective randomized clinical trial. J Perinatol. 2015;35(4):278–283 [DOI] [PubMed] [Google Scholar]
- 16.Hall ES, Wexelblatt SL, Crowley M, et al. ; OCHNAS Consortium . Implementation of a neonatal abstinence syndrome weaning protocol: a multicenter cohort study. Pediatrics. 2015;136(4). Available at: www.pediatrics.org/cgi/content/full/136/4/e803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson LM; Academy of Breastfeeding Medicine Protocol Committee . ABM clinical protocol #21: Guidelines for breastfeeding and the drug-dependent woman. Breastfeed Med. 2009;4(4):225–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welle-Strand GK, Skurtveit S, Jansson LM, Bakstad B, Bjarkø L, Ravndal E. Breastfeeding reduces the need for withdrawal treatment in opioid-exposed infants. Acta Paediatr. 2013;102(11):1060–1066 [DOI] [PubMed] [Google Scholar]
- 19.Wachman EM, Byun J, Philipp BL. Breastfeeding rates among mothers of infants with neonatal abstinence syndrome. Breastfeed Med. 2010;5(4):159–164 [DOI] [PubMed] [Google Scholar]
- 20.Bagley SM, Wachman EM, Holland E, Brogly SB. Review of the assessment and management of neonatal abstinence syndrome. Addict Sci Clin Pract. 2014;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prenatal Drug Use and Newborn Health Federal Efforts Need Better Coordination and Planning. Washington, DC: United States Government Accountability Office; 2015 [Google Scholar]
- 22.Warren MD, Miller AM, Traylor J, Bauer A, Patrick SW; Centers for Disease Control and Prevention (CDC) . Implementation of a statewide surveillance system for neonatal abstinence syndrome—Tennessee, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(5):125–128 [PMC free article] [PubMed] [Google Scholar]
- 23.Lind JN, Petersen EE, Lederer PA, et al. ; Centers for Disease Control and Prevention (CDC) . Infant and maternal characteristics in neonatal abstinence syndrome—selected hospitals in Florida, 2010-2011. MMWR Morb Mortal Wkly Rep. 2015;64(8):213–216 [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrick SW, Davis MM. Reformulating the federal match as a key to the sustainability of Medicaid. JAMA Pediatr. 2013;167(3):218–220 [DOI] [PubMed] [Google Scholar]
- 26.Kellogg A, Rose CH, Harms RH, Watson WJ Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstet Gynecol 2011;204(3):259.e1–4 [DOI] [PubMed]
- 27.Statewide Task Force on Prescription Drug Abuse & Newborns Available at: http://myfloridalegal.com/pages.nsf/Main/CFC9846F8D7790FC85257A10004AE67E. Accessed March 2, 2015 [PubMed]
- 28.Massachusetts Neonatal Abstinence Syndrome Improvement Project Available at: www.neoqic.org/nas-project. Accessed March 2, 2015
- 29.Wachman EM, Hayes MJ, Brown MS, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309(17):1821–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]