Abstract
In this report we investigated the role and regulation of FOXM1 in breast cancer and epirubicin resistance. We generated epirubicin resistant MCF-7 breast carcinoma (MCF-7-EPIR) cells and found FOXM1 protein levels to be higher in MCF-7-EPIR compared to MCF-7 cells, and that FOXM1 expression is down-regulated by epirubicin in MCF-7 but not in MCF-7-EPIR cells. We also established that there is a loss of p53 function in MCF-7-EPIR cells and that epirubicin represses FOXM1 expression at transcription and gene promoter levels through activation of p53 and repression of E2F activity in MCF-7 cells. Using p53-/- MEFs, we showed that p53 is important for epirubicin sensitivity. Moreover, transient promoter transfection assays demonstrated that epirubicin and its cellular effectors p53 and E2F1 modulate FOXM1 transcription through an E2F-binding site located within the proximal promoter region. Chromatin immunoprecipitation analysis also revealed that epirubicin treatment increases pRB and decreases E2F1 recruitment to the FOXM1 promoter region containing the E2F-site. We also found Ataxia-telangiectasia mutated (ATM) protein and mRNA to be overexpressed in the resistant MCF-7-EPIR cells compared to MCF-7 cells and that epirubicin can activate ATM to promote E2F activity and FOXM1 expression. Furthermore, inhibition of ATM in U2OS cells with caffeine or depletion of ATM in MCF-7-EPIR with siRNAs can re-sensitise these resistant cells to epirubicin, resulting in down-regulation of E2F1 and FOXM1 expression and cell death. In summary, our data show that ATM and p53 coordinately regulate FOXM1 via E2F to modulate epirubicin response and resistance in breast cancer.
Keywords: ATM, p53, FOXM1, E2F1, epirubicin, resistance
Introduction
Breast cancer is the most common cancer in women and one of the most prevalent causes of women cancer death worldwide (1, 2). Endocrine agents, including anti-estrogens and aromatase inhibitor (AI), have become the primary adjuvant treatment for breast cancer (3). However, in addition to endocrine agents, cytotoxic chemotherapeutic drugs taxanes and anthracyclines have also been used more frequently in the neoadjuvant and adjuvant settings to reduce tumour size prior to surgery and to reduce the chance of cancer relapse or metastasis, respectively (4, 5). Moreover, cytotoxic chemotherapy is also used to treat breast cancer patients that are resistant to or not suitable for hormonal therapy and it is particularly important in the treatment of advanced or metastatic solid cancers, as it is sometimes the sole treatment option (6, 7).
Anthracyclines, including doxorubicin (also called Adriamycin) and epirubicin (Fig. S1), are a group of Streptomyces peucetius bacteria-derived antibiotics commonly used in cancer chemotherapy. These compounds have been shown to be effective for the treatment of a broad spectrum of cancers such as breast, lung, and ovary carcinomas as well as leukaemia (8, 9). Despite being some of the most effective and widely used anticancer drugs in the clinic, anthracycline treatment will eventually fail and patients relapse because of the development of acquired drug resistance (10–12). The exact mechanism of action of anthracyclines is still not completely understood, but likely to involve inducing DNA intercalation and damage (13, 14). For DNA targeting anticancer drugs, such as anthracyclines, enhanced DNA repair can confer resistance and hamper the efficacy of the chemotherapeutic drugs. Consistently, DNA repair gene network signature has been found to be associated with anthracycline response in triple negative metastatic breast cancer (15). A better understanding of the molecular mechanisms of anthracycline action and resistance will be required for the development of novel strategies for the treatment of advanced or metastatic breast cancer and for overcoming the resistance to anthracyclines.
Forkhead box M1 (FOXM1), also previously called HNF-3, HFH-11, WIN, MPP2 or Trident, is a transcription factor of the Forkhead box (FOX) protein superfamily characterised by a conserved winged helix DNA-binding domain (16). FOXM1 is required for normal G1-S, G2 and M cell cycle phase transitions. Besides its involvement in cell cycle transitions, FOXM1 is also a key regulator of mitotic spindle integrity (17), angiogenesis (18), metastasis (18, 19), apoptosis (16, 19), DNA damage repair (20, 21) and tissue regeneration (22). FOXM1 is frequently overexpressed in a diversity of human cancers, including colorectal (23), lung (24), prostate (25), liver (26) and breast (27) carcinomas. In agreement, a microarray study also found FOXM1 expression to be elevated in carcinomas of the prostate, lung, ovary, colon, pancreas, stomach, bladder, liver, kidney and breast, compared with their normal counterparts. Besides its potential involvement in tumorigenesis, FOXM1 dysregulation has also been implicated in drug resistance in breast cancer. For example, FOXM1 dyregulation has been shown to be involved in the development of cisplatin resistance in breast cancer (20). Accordingly, FOXM1 overexpression has been shown to confer resistance to the humanized anti-epidermal growth factor receptor 2 (HER2) monoclonal antibody Herceptin (also called trastuzumab) and microtubule-stabilizing drug taxene paclitaxel (taxol) (28). In addition, FOXM1 has also been found to be a transcriptional target of ERα and play key role in breast cancer endocrine therapy resistance (29). In this report, we investigated the expression of FOXM1 and its regulation in response to epirubicin treatment in drug sensitive and resistant MCF-7 breast carcinoma cell lines.
Materials and Methods
Cell culture and transfections
The human breast carcinoma cell lines MCF-7 and U2OS cell lines originated from the American Type Culture Collection and were acquired from Cancer Research UK, in which they were tested and authenticated. Knock-out MEFs for p21Cip1 and p53 have previously been described (30, 31). Fig. S0.
Western blot analysis
Cells were lysed and SDS-PAGE gel electrophoresis was performed as described (32).
Antibodies
Real-Time quantitative PCR (RT-qPCR)
Transfection and luciferase assay
The human FOXM1 promoter constructs have previously been described (29) Cells were transfected with the human FOXM1 promoter and Renilla (pRL-TK; Promega, Southampton, UK) as internal transfection control using Fugene-6 (Qiagen, Crawley, UK) as described (20)alone or in combination with pCMV-E2F1 or pcDNA3-Flag-p53. The FOXM1 promoter reporter constructs have previously been described (29). Putative forkhead site mutagenesis was performed using a Stratagene QuikChange site-directed mutagenesis kit and oligonucleotides mE2F1F (5’-GGAATGCCGAGACAAGGCCGGATCCGATTGCGCACGTTCC-3’), mE2F1R (5’-GGAACGTCGCCAATCGGATCCGGCCTTGTCTCGGCATTCC-3’); mE2F2F (5’-CGTGACCTTAACGCTCCGCCGGATCCAATTTCAAACAGCGGAAC-3’), mE2F2R (5’-GTTCCGCTGTTTGAAATTGGATCCGGCGGAGCGTTAAGGTCACG -3’).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as described previously (33) using MCF-7 cells grown to 70% confluence. DNA fragments were purified using the QIAquick Spin Kit (Qiagen, Crawley, UK). Antibody for E2F1 (KH95) was purchased from Abcam and pRB antibody (C-15) was purchased from Santa Cruz Biotechnology (Autogen Bioclear). For PCR, one-twenty-fifth of the extracted DNA was used and amplified in 33 PCR cycles using FOXM1 primers: FOXM1-F 5’-CCACTTCTTCCCCCACAAG-3’, FOXM1-R 5’-CCGGAGCTTTCAGTTTGTTC-3’ ,
Sulforhodamine B assay and cell cycle analysis
Sulforhodamine B assays and cell cycle analysis were performed and read as described (31).
Phospho-γH2AX immunofluorescent staining and quantification
Phospho-γH2AX immunofluorescent staining was performed as described (19) and the images acquired using confocal or ImageXpress (Molecular Devices).
RESULTS
Dysregulated FOXM1 expression is associated with epirubicin resistance in breast cancer
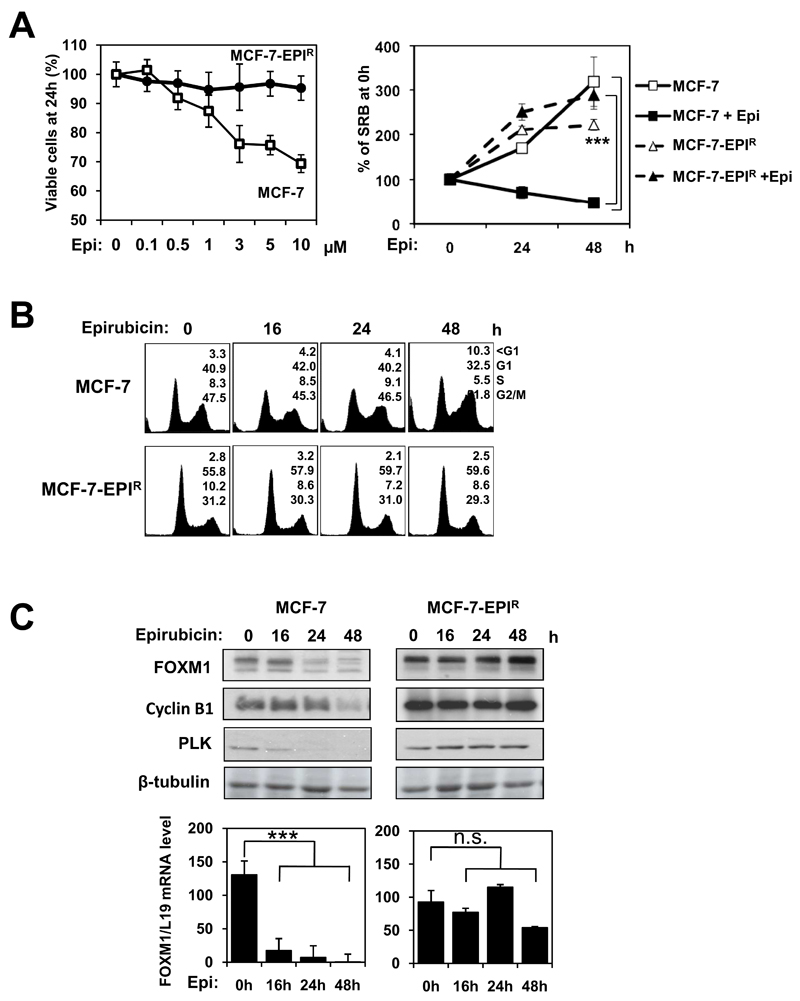
The involvement of FOXM1 in DNA damage response and chemotherapeutic drug resistance led us to hypothesise that FOXM1 has a role in anthracycline sensitivity as well as resistance in breast cancer. In order to test this conjecture, we established an epirubicin resistant breast cell line MCF-7-EPIR by chronic exposure of the parental drug sensitive MCF-7 to stepwise increases in epirubicin concentration, until a concentration of resistance up to 10µmol/L was achieved. SRB proliferation assays showed the MCF-7-EPIR displayed strong resistance to epirubicin compared the parental MCF-7 cells (Fig. 1A). We next examined the effect of epirubicin on the proliferation of the MCF-7 and the MCF-7-EPIR cells at 1µmol/L, a concentration generally used in cancer therapy, and the SRB assay revealed that the proliferation of the MCF-7 cells was significantly inhibited following epirubicin treatment, while the growth of the MCF-7-EPIR cells was relatively unaffected in the presence of epirubicin (Fig. 1A). There was also a notable significant difference in the rates of proliferation between the epirubicin-treated MCF-7 and MCF-7-EPIR cells at both 24 h and 48 h. Cell cycle analysis showed that epirubicin exposure (1µmol/L) induced an accumulation of MCF-7 cells at G2/M and sub-G1 phases, indicative of G2/M delay and cell death, whereas no significant changes in cell cycle profile are observed for the MCF-7-EPIR cells (Fig. 1B). Subsequent western blot analysis revealed no significant changes in the levels of FOXM1 and FOXM1 protein targets cyclin B1 and PLK, following 48 h treatment with epirubicin at 1µmol/L in MCF-7-EPIR cells. In contrast, FOXM1 protein expression decreased within 24 h and was completely abrogated at 48 h in MCF-7 cells (Fig. 1C). Consistently, RT-qPCR analysis revealed no significant decrease in FOXM1 transcript level in the MCF-7-EPIR cells, while epirubicin induced a drastic reduction of FOXM1 mRNA level in MCF-7 cells (Fig. 1C). Collectively, these results show that FOXM1 is down-regulated at mRNA and protein levels in response to epirubicin in the sensitive MCF-7 cells, while FOXM1 expression is deregulated in epirubicin resistant cells (Fig. S2), suggesting that FOXM1 has a role in epirubicin sensitivity and resistance.
Figure 1. Epirubicin resistant MCF-7-EPIR cell line shows elevated FOXM1 protein and mRNA levels.
A. MCF-7 and MCF-7-EPIR cells were treated with increasing concentrations of epirubicin and their proliferation rates were measured by SRB assay. SRB assay was also performed on MCF-7 and MCF-7-EPIR cells treated with 1µmol/L of epirubicin for 0, 24 and 48 h. Representative data from three independent experiments are shown. Statistical analyses were done using Student’s t test. ***, P≤0.001 significant. Significant differences between the epirubicin-treated MCF7 cells and MCF7-EPIR cells were detected at both 24 h and 48 h. B. MCF-7 and MCF-7-EPIR cells were treated with 1µmol/L of epirubicin for 0, 16, 24 and 48 h and FACS analysis carried out after propidium iodide staining. Percentage of cells in each phase (sub-G1, G1, S, G2/M) is indicated. C. MCF-7 and MCF-7-EPIR cells were treated with 1µmol/L of epirubicin for 0, 16, 24 and 48 h. At indicated time, cells were collected and analysed by western blotting to determine the protein expression levels of FOXM1, Cyclin B1, PLK and β-tubulin, and by RT-qPCR to determine FOXM1 mRNA transcript levels. Columns, means derived from three independent experiments; bars, SD.
The loss of FOXM1 repression by p53 contributes to epirubicin resistance
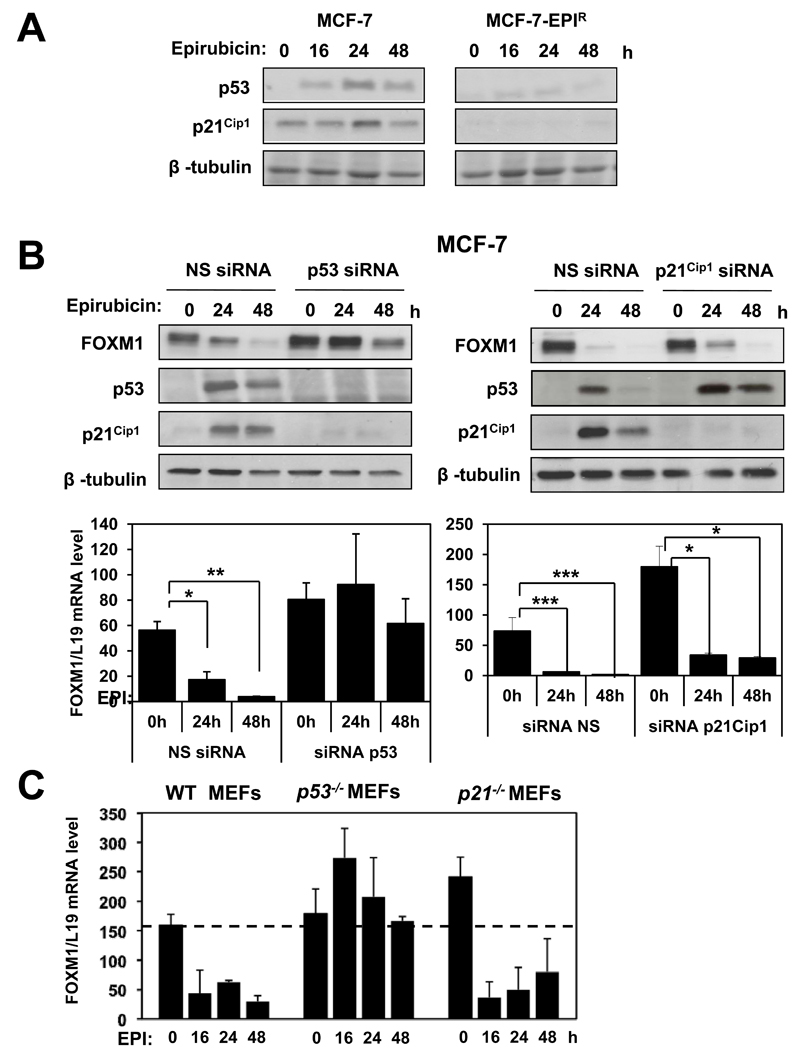
The recent observation that p53 represses FOXM1 expression following daunorubicin treatment (34) led us to predict that epirubicin also activates p53 to repress FOXM1 expression in breast cancer cells. To assess the role of p53 in mediating the epirubicin response in breast cancer cells, we first examined the expression of p53 and its target p21Cip1 in the sensitive and resistant MCF-7 cell lines in response to epirubicin treatment. Western blot analysis revealed that epirubicin treatment strongly induced the expression of the p53 protein and its target, the cyclin-dependent kinase inhibitor p21Cip1 in the MCF-7 cells. In contrast, p53 and p21Cip1 expression was undetectable in the MCF-7-EPIR cells before and after epirubicin treatment (Fig. 2A). RT-qPCR analysis also showed an induction in p21Cip1 transcript level in the MCF-7, but not in the MCF-7-EPIR cells, in response to epirubicin treatment (data not shown). To test if p53 is responsible for the down-regulation of FOXM1 expression in MCF-7 cells following epirubicin treatment, MCF-7 cells were transiently transfected with non-targeting or p53-targeting siRNA, treated with epirubicin and FOXM1 expression examined. Western blot and RTq-PCR analysis showed that silencing of p53 attenuated FOXM1 down-regulation at both protein and mRNA levels in response to epirubicin (Fig. 2B). The inability of p53 depletion to completely abolish the down-regulation of FOXM1 also suggests that p53 might not be the sole regulator of FOXM1 expression in response to epirubicin. A previous study showed that p53 represses FOXM1 expression via pRB following daunorubicin treatment (34). Thus, one mechanism by which p53 can repress FOXM1 expression is through its ability to induce p21Cip1, which can in turn repress cyclin-CDK-mediated pRB hyperphosphorylation, resulting in the repression of E2F transcriptional activity. Surprisingly, although p53 knockdown abrogated the induction of p21Cip1 and the down-regulation of FOXM1 by epirubicin, silencing of p21Cip1 had little effects on the epirubicin-induced FOXM1 down-regulation, suggesting that epirubicin can also repress FOXM1 expression via p21Cip1-independent mechanisms (Fig. 2B). To investigate further the role of p53 and p21Cip1 in regulating FOXM1 expression in response to epirubicin, wild-type (WT), p53-deficient (p53-/-), and p21-deficient (p21Cip1-/-) mouse embryo fibroblasts (MEFs) were subjected to epirubicin treatment and the expression of FOXM1 investigated. Treatment of the WT, p21Cip1-/- MEFs with epirubicin resulted in a reduction of FOXM1 expression within 16 h, further confirming that p21Cip1 is not essential for the repression of FOXM1 expression by epirubucin. In contrast, epirubicin did not cause a down-regulation of FOXM1 expression in the p53-deficient MEFs. Together these data support the idea that epirubicin represses FOXM1 expression at the transcriptional level through p53.
Figure 2. Activation of p53 in MCF-7 cells represses FOXM1 protein and mRNA levels.
A. MCF-7 and MCF-7-EPIR cells were treated with 1µmol/L of epirubicin for 0, 16, 24 and 48 h. At indicated times, cells were collected for western blot analysis to determine the protein expression levels of p53, p21Cip1 and β-tubulin. B. MCF-7 cells were either transfected with non-specific (NS) siRNA, siRNA smart pool against p53, or siRNA smart pool against p21Cip1 (100nmol/L). Twenty-four hours after transfection, MCF-7 cells were treated with 1µmol/L of epirubicin and harvested for western blot and RT-qPCR analysis at 0, 24 and 48h. The protein expression levels were determined for FOXM1, p53, p21Cip1 and β-tubulin and the mRNA level for FOXM1. Columns, means derived from three independent experiments; bars, SD. C. Wild-type, p53-/- and p21Cip1-/- MEF cells were treated with 1µmol/L of epirubicin for 0, 16, 24 and 48 h, and RT-qPCR was performed to determine FOXM1 mRNA transcript levels.
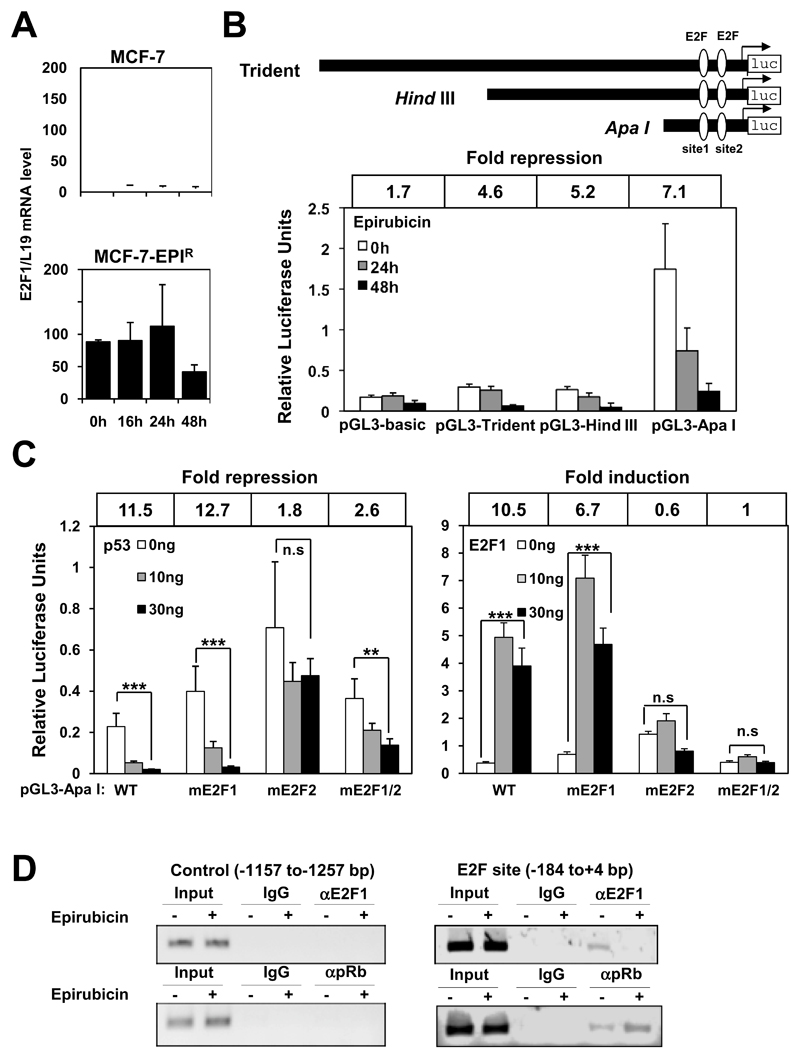
p53 can repress FOXM1 expression through an E2F site in its promoter
The pRb/E2F transcription factors are principal regulators of the cell cycle and function downstream of the p53 canonical pathway. To assess whether the E2F transcription factors are involved in the p53-dependent FOXM1 repression, we analysed the expression pattern of E2F1, a well-characterized E2F-responsive gene product as well as a subunit of the E2F transcription factor dimers. Treatment of the MCF-7 cells with epirubicin markedly reduced E2F1 mRNA levels within 16 h (Fig. 3A), whereas the E2F1 transcript level remained relatively constant in the MCF-7-EPIR cells in response to epirubicin (Fig. 3A). Furthermore, the close correlation between the expression pattern of E2F1 and FOXM1, suggests that p53 is likely to down-regulate FOXM1 expression through repressing E2F activity. We next analysed the involvement of the putative E2F-binding sites in the FOXM1 promoter in FOXM1 repression upon epirubicin treatment. To this end, the MCF-7 cells were transiently transfected with a luciferase reporter driven by either a 2.4kbp (Trident), a 1.4kbp (HindIII), or a 300bp (ApaI) FOXM1 promoter, and the promoter activity assayed at 0, 24 and 48 h after epirubicin treatment. The activity of all three FOXM1 promoter constructs was markedly reduced following exposure to 1µmol/L epirubicin, consistent with the fact that the putative E2F-binding sites (site 1:-58bp and site 2:-24bp) locate inside all three FOXM1 promoter constructs (Fig. 3B). We next examined whether p53 exerts its repression on the FOXM1 promoter activity through these putative E2F-binding sites. To this end, we co-transfected into MCF-7 cells increasing amounts of p53 together with either the wild-type ApaI FOXM1 promoter-reporter (WT-luc) or the ApaI FOXM1 promoter lacking one (mE2F1-luc or mE2F2-luc) or both (mE2F1/2-luc) putative E2F-sites. The results showed p53 caused a drastic (12.7 fold) reduction in mE2F1-luc activity, comparable to that (11.5 fold) observed for WT-luc (Fig 3C). By contrast, the repression by p53 was considerably reduced in both the mE2F2-luc and the mE2F1/2-luc, suggesting the second putative E2F-binding site (site 2) mediates the repression of the FOXM1 promoter by p53. Next, the activity of the wild-type (WT-luc) as well as the mutated ApaI-luc constructs (mE2F1-, mE2F2- and mE2F1/2-luc) was examined by co-transfection assays in MCF-7 cells with different amounts of E2F1 expression vector. The results showed that the mE2F1-luc construct showed similar responsiveness to E2F1 as the WT-luc. In contrast, both the mE2F2-luc and the mE2F1/2-luc mutants lost the majority of their responsiveness to E2F1. Together these co-transfection results provide strong evidence that the E2F-binding element located at −24 bp confers the responsiveness to p53 and E2F, further confirming that p53 represses FOXM1 expression through E2F activity.
Figure 3. p53 represses FOXM1 expression through an E2F-binding site located in the proximal FOXM1 promoter region.
A. MCF-7 and MCF-7-EPIR cells were treated with 1µmol/L of epirubicin for 0, 16, 24 and 48 h and RT-qPCR was performed to determine E2F1 transcript levels. Columns, means derived from three independent experiments; bars, SD. B. Schematic representation of the full-length Trident, HindIII and ApaI FOXM1-luciferase reporter constructs and the E2F-binding sites 1 and 2 (upper panel). MCF-7 cells were transiently transfected with 20 ng of either the empty pGL3-basic, pGL3-Trident, pGL3-HindIII or the pGL3-ApaI, and cells were treated with 1µmol/L of epirubicin. Cells were then harvested at 0, 24 and 48h after treatment and assayed for luciferase activity. All relative luciferase activity values are corrected for co-transfected Renilla activity. The fold of repression were calculated between 0h and 48h of epirubicin treatment. Columns, means derived from three independent experiments; bars, SD. C. MCF-7 cells were transiently transfected with 20 ng of either the pGL3-ApaI(WT), pGL3-ApaI-mE2F1, pGL3-ApaI-mE2F2, or pGL3-ApaI-mE2F1/2 together with increasing amounts (0, 10 and 30ng) of p53 expression vector in the left panel and E2F1 in the right panel. Cells were harvested after 24h transfection and assayed for luciferase activity. All relative luciferase activity values are corrected for co-transfected Renilla activity. The fold of repression and activation were calculated indicated between 0h and 48h of epirubicin treatment. Columns, means derived from three independent experiments; bars, SD. Statistical analyses were done using Student’s t test. **, P≤0.01 and ***, P≤0.001, significant. D. MCF-7 cells untreated or treated 1µmol/L with epirubicin for 24 h were used for ChIP assays using IgG negative control, anti-E2F1 and anti-pRb antibodies as indicated. After crosslink reversal, the co-immunoprecipitated DNA was amplified by PCR using primers amplifying the FOXM1 E2F-binding sites containing region (-184/+4) and a control region (-1157/-1257), and resolved in 2% agarose gel. Inverted images were shown.
To provide further evidence that epirubicin represses FOXM1 expression through inhibition of E2F activity, MCF-7 cells were treated with epirubicin for 0 and 24 h, followed by chromatin immunoprecipitation (ChIP) analyses of E2F1 and its negative regulator pRB on the FOXM1 promoter (Fig. 3D; Fig. S3). The ChIP assay showed that the in vivo occupancy of the proximal FOXM1 promoter by E2F1 decreased and pRB increased after epirubicin treatment, indicating epirubicin causes the depletion of the transactivator E2F1 and the accumulation of the transcriptionally repressive pRB/E2F complex on the FOXM1 promoter (Fig. 3D; Fig. S3). Taken together, these results indicate that epirubicin can induce p53 to repress FOXM1 through modulating E2F activity on the FOXM1 promoter.
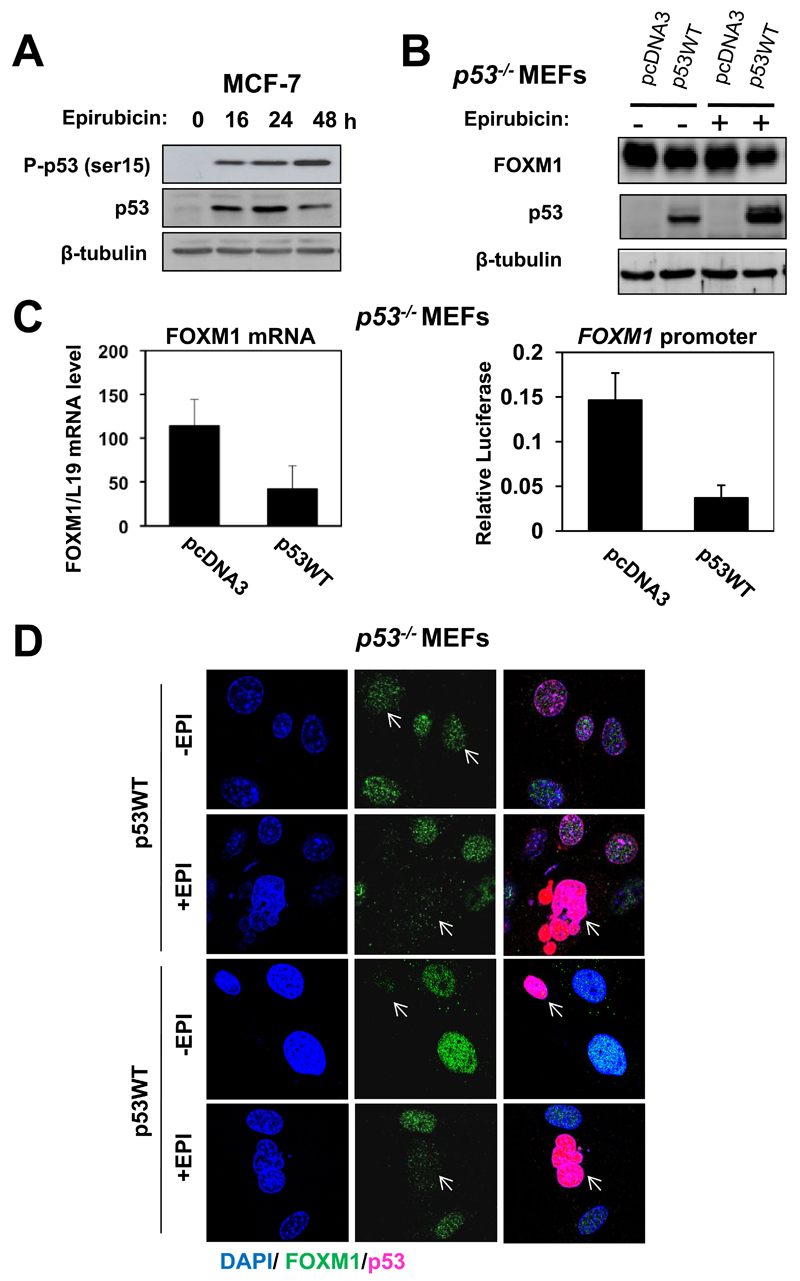
p53 is involved in repression of FOXM1 expression and induction of cell death in response to epirubicin
Much evidence has indicated that p53 is activated through phosphorylation on serine 15 by ATM upon DNA damage (35). Consistent with this, western blot analysis also demonstrated that concomitant with an increase in its expression level, p53 was phosphorylated at serine 15 in the MCF-7 cells after epirubicin treatment (Fig. 4A). To investigate the mechanism by which FOXM1 expression is repressed by epirubicin, p53-/- MEFs were transfected with an empty vector or the wild-type p53 and FOXM1 expression assessed in absence or presence epirubicin treatment. Western blot analysis showed that in the presence of epirubicin treatment transfection of p53 repressed FOXM1 expression at the protein level (Fig. 4B). Consistently, p53 could also repress FOXM1 mRNA expression and promoter activity, suggesting epirubicin induces p53 to repress FOXM1 expression at transcription and gene promoter levels (Fig. 4C). Notably, the moderate repression of FOXM1 expression observed in these transfection studies probably reflects the low transfection efficiencies. To circumvent this problem, immunofluorescence staining was performed on the p53-/- MEFs transfected with p53 in the presence or absence of epirubicin treatment (Fig. 4D). The staining results demonstrated an inverse correlation between p53 and FOXM1 expression, with p53-/- MEFs with high levels of p53 showing low FOXM1 expression and vice versa. The results also showed that p53 was capable of repressing FOXM1 expression and inducing apoptosis in response to epirubicin treatment, as only the cells expressing ectopic p53 displayed apoptotic morphologies in response to epirubicin. Collectively, these results suggest that p53 is required for the repression of FOXM1 expression and the induction of cell death upon epirubicin treatment.
Figure 4. p53 represses FOXM1 expression and induces apoptosis in the presence of epirubicin.
A. MCF-7 cells treated with 1µmol/L with epirubicin were collected for western blot analysis to determine the expression levels of FOXM1, p53, P-p53 (ser15) and β-tubulin. B. p53-/- MEF cells were transiently transfected with the empty vector pcDNA3 or the pcDNA3 wild-type p53 and treated with or without 1µmol/L of epirubicin for 24 h. Cells were collected to determine the protein level of FOXM1, p53 and and β-tubulin. C. p53-/- MEF cells transiently transfected with the empty vector pcDNA3 or the pcDNA3 wild-type p53 with or without 1µmol/L of epirubicin for 24 h were analysed for their FOXM1 mRNA levels using RT-qPCR (left panel) . Columns, means derived from three independent experiments; bars, SD. These p53-/- MEF cells were also co-transfected with pGL3-ApaI FOXM1 and assayed for luciferase activity. Relative luciferase activity values are corrected for co-transfected Renilla activity. Columns, means derived from three independent experiments; bars, SD. D. p53-/- MEF cells transiently transfected with the empty vector pcDNA3 or the pcDNA3 wild-type p53 and treated with or without epirubicin were stained for FOXM1, p53 and DAPI using specific antibodies, followed by the addition of ALEX488 (green) and ALEX567 (pink) labelled anti-rabbit and goat antisera (Molecular Probes, Eugene, OR), respectively. Nuclei were also stained with DAPI (blue). Images were visualized by confocal microscopy and the average integrated fluorescence intensity quantified by Zeiss Axiovert 100 confocal laser scanning microscope using Zeiss LSM 500 software. Images: original magnification X 100. Cells expressing p53 displayed apoptotic morphologies in response to epirubicin treatment.
Increased DNA repair in epirubicin resistant cells
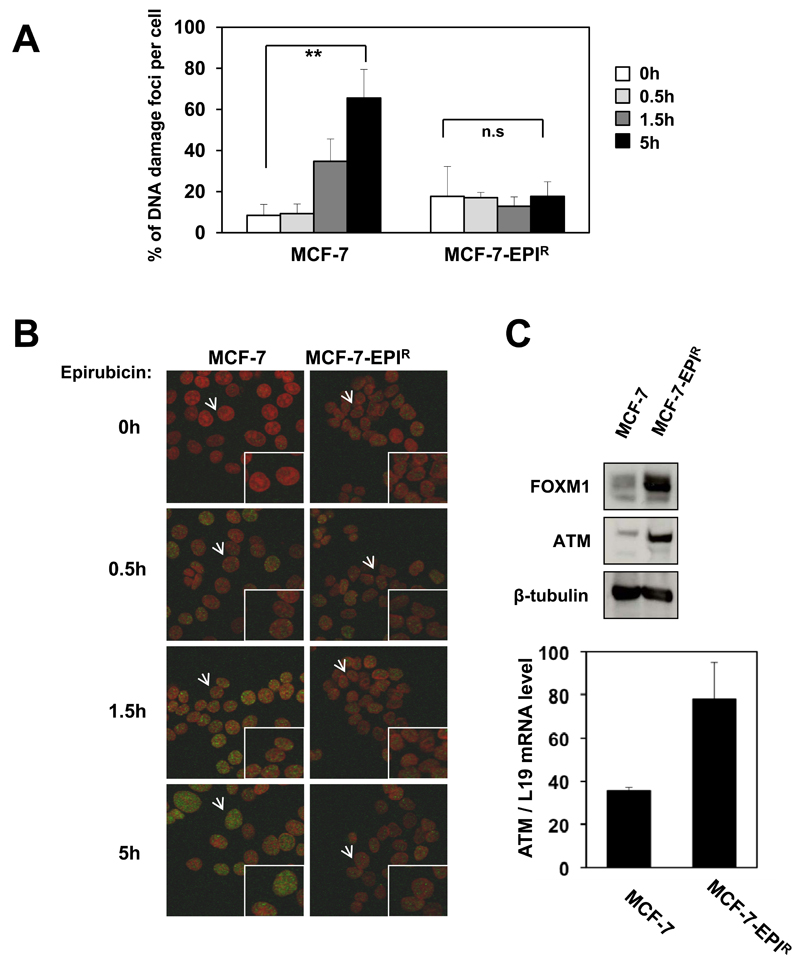
Next, we sought to determine the molecular mechanism that confers epirubicin resistance in the MCF-7-EPIR cells. It has been shown previously that FOXM1 expression is associated with the cisplatin-induced DNA damage response and drug resistance (20). We therefore examined the DNA damage foci formation by P-γH2AX staining in MCF-7 and MCF-7-EPIR cells in response to epirubicin treatment. The results showed an increase in the mean number of γH2AX foci/cell over time after epirubicin treatment in the MCF-7 cells, while the level of P-γH2AX foci/cell remained relatively constant in the MCF-7-EPIR cells, suggesting higher DNA repair activities in these cells (Fig. 5A and B). To investigate this further, we evaluated the expression level of the DNA repair protein ATM in the MCF-7 and MCF-7-EPIR cells. The western blot and RT-qPCR analysis demonstrated that the ATM protein and mRNA levels were strongly up-regulated in the MCF-7-EPIR cells compared to MCF-7 cells (Fig. 5C), thus suggesting a role of ATM in mediating the increase in DNA repair activity in the resistant cells. Notably, no measurable changes in ATR expression levels were detected in MCF-7-EPIR cells (data not shown).
Figure 5. The epirubicin resistant MCF-7-EPIR cells show a reduction of DNA damage in response to epirubicin treatment and expresses higher levels of ATM.
A. MCF-7 and MCF-7-EPIR cells treated with 1µmol/L of epirubicin for 0, 0.5, 1.5 and 5 h were stained with P-γH2AX antibody and DAPI. Images were visualized and scored by ImageXpress (Molecular Devices). The results are the average of three independent experiments. Mean ± SD. Statistical analyses were performed using Students’s test. **, P ≤ 0.01 significant; n.s non significant. B. MCF-7 and MCF-7-EPIR cells treated with 1µmol/L of epirubicin were stained with P-γH2AX antibody (green) and DAPI (red). Images visualized by confocal microscopy. Images: magnification: x 20; insets x 80. C. MCF-7 and MCF-7-EPIR cells were analysed for ATM, FOXM1 and and β-tubulin by western blotting and ATM mRNA levels using RT-qPCR.
ATM is involved in FOXM1 regulation and drug resistance in the epirubicin resistant cells
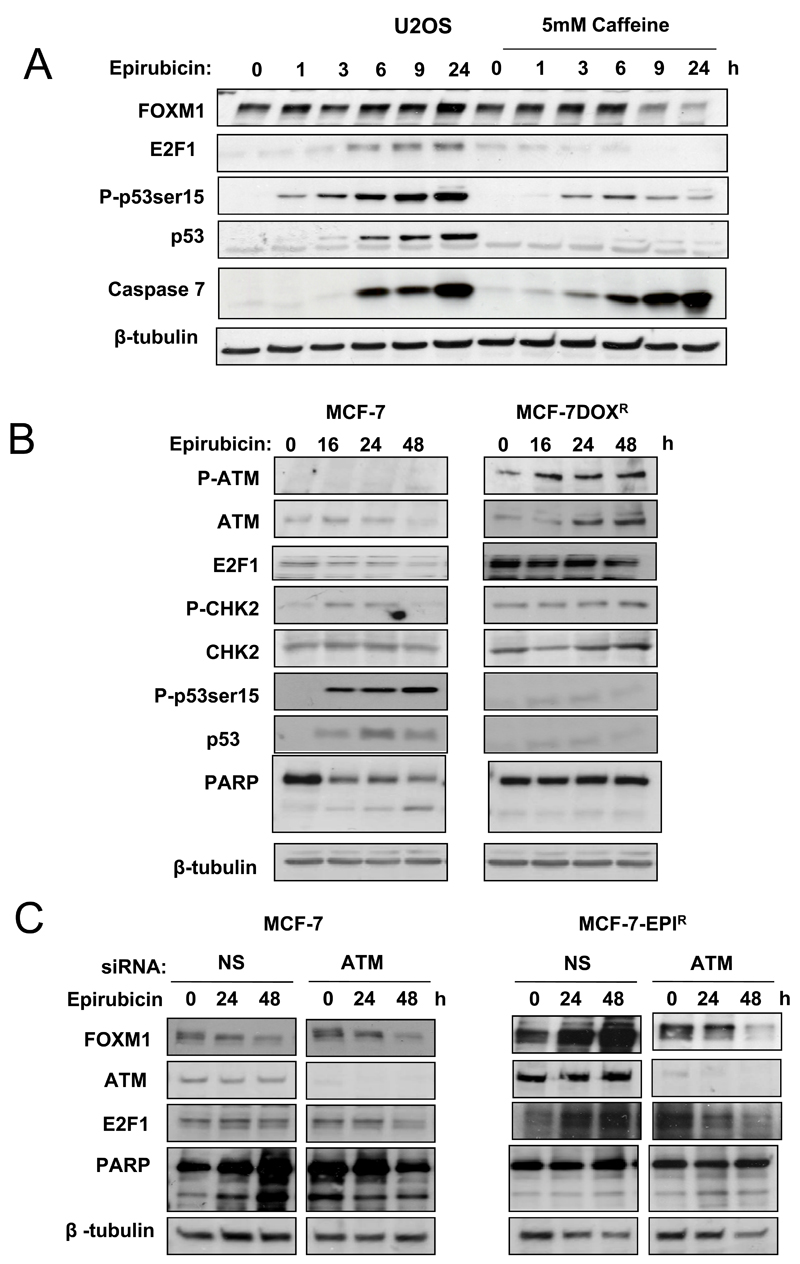
To determine whether the ATM pathway is involved in FOXM1 regulation in response to epirubicin, we treated the U2OS p53-positive osteosarcoma cells with epirubicin in the absence or presence of caffeine, a known ATM inhibitor. Western blot analysis demonstrated that epirubicin induced p53 activation, but did not affect FOXM1 protein level, suggesting that FOXM1 expression is not sensitive to p53 induction in these cells (Fig. 6A). However, when the U2OS cells were pretreated with caffeine, which inhibits ATM as well as p53 activity, epirubicin strongly represses FOXM1 expression. Paradoxically, it was noted that in the caffeine treated cells, p53 expression was significantly down-regulated, suggesting that FOXM1 repression by epirubicin is independent of p53 in these caffeine-treated cells. Our results also suggested that the ATM DNA damage response pathway could be involved in FOXM1 regulation in a p53 independent-manner. Consequently, ATM expression and activity was investigated in the MCF-7 and MCF-7-EPIR cells by western blot analysis (Fig. 6B). Treatment with epirubicin induced the phosphorylation (serine1981) and expression of ATM in the MCF-7-EPIR within 24 h, while this induction was not detectable in the MCF-7 cells (Fig. 6B). Phosphorylation of the ATM downstream target CHK2 was strongly enhanced in the MCF-7-EPIR and to a much lesser extent in the MCF-7 cells. Accumulation of E2F1, another ATM target (36, 37), was detected in the MCF-7-EPIR cells, while E2F1 level decreased in the MCF-7 cells in response to epirubicin treatment. It was also noticeable that epirubicin induced apoptosis as revealed by PARP cleavage in MCF-7, but not in the MCF-7-EPIR cells. These data indicate a pathway linking ATM with E2F1 and FOXM1 expression independent of p53 in MCF-7-EPIR cells. To determine whether ATM is involved in E2F1 and FOXM1 regulation in MCF-7-EPIR cells, we silenced ATM expression using siRNA in both MCF-7 and MCF-7-EPIR cells and studied E2F1 and FOXM1 expression in response to epirubicin (Fig. 6C). Western blot analysis showed that the knockdown of ATM had little effects on E2F1 and FOXM1 expression in the MCF-7 cells. In contrast, whereas the expression level of E2F1 and FOXM1 increased in the control MCF-7-EPIR cells upon epiribicin treatment, epirubicin caused a decrease in E2F1 and FOXM1 protein expression in the MCF-7-EPIR cells with ATM silenced, suggesting that the induction of ATM in the MCF-7-EPIR is responsible for the induction of E2F1 and FOXM1 in the MCF-7-EPIR cells. Moreover, silencing of ATM using siRNA abrogated the induction of FOXM1 mRNA by epirubicin, suggesting that ATM regulates FOXM1 at the transcriptional level (Fig. S4). Consistent with this, inhibition of ATM by Ku-55933 repressed E2F1 and FOXM1 induction and re-sensitised the resistant MCF-7-EPIR cells to epirubicin (Fig. S5). Moreover, the role of FOXM1 in epirubicin sensitivity and resistance is further supported by the observations that overexpression of FOXM1 in MCF-7 cells can decrease the sensitivity of MCF-7 cells to epirubicin (Fig. S6) and that FOXM1 knockdown in MCF-7-EPIR cells mimics the anti-proliferative effects of epirubicin on MCF-7 cells (Fig. S7).
Figure 6. The epirubicin resistant MCF-7-EPIR cells express higher levels of ATM in response to epirubicin to promote E2F1 and FOXM1 expression, and cell survival.
A. U2OS cells were treated for 0 to 24 h with 1µmol/L of epirubicin in the presence or absence of 5mmol/L of caffeine. At indicated time, cells were collected for western blot analysis to determine the protein expression levels of FOXM1, E2F1, P-p53 (ser15), p53, Cleaved caspase 7 and β-tubulin. B. MCF-7 and MCF-7-EPIR were treated with 1µmol/L of epirubicin and the protein expression levels of P-ATM, ATM, E2F1, P-CHK2, CHK2, P-p53 (ser15), p53, PARP and β-tubulin were analysed by western blot analysis. C. MCF-7 and MCF-7-EPIR cells were either transfected with non-specific (NS) siRNA (100nmol/L) or siRNA smart pool against ATM (100nmol/L). Twenty-four hours after transfection, cells were treated with 1µmol/L of epirubicin and harvested for western blot at 0, 24 and 48h. The protein expression levels were determined for FOXM1, ATM, E2F1, PARP and β-tubulin.
DISCUSSION
The FOXM1 transcription factor plays a crucial part in the regulation of a diversity of cellular functions, including cell proliferation, cell survival, and immortalisation, which are essential for tumorigenesis. Consistent to this notion, FOXM1 has been found to be frequently upregulated in a host of human cancers, including colorectal (23), lung (24), prostate (25), liver (26), stomach (38), breast (27) and basal cell carcinomas (39) as well as glioblastoma (40). Recently emerging evidence reveals that FOXM1 also has a role in cancer drug resistance. In concordance, latest studies demonstrate that FOXM1 expression level is an important determinant of sensitivity to breast cancer chemotherapeutic drugs, such as herceptin (28), gefitinib (41), lapatinib (27), paclitaxel (28) and cisplatin (20). Consistent with these findings, we established in this study that FOXM1 is a crucial cellular target of the anthracycline epirubicin in breast cancer cells. Moreover, FOXM1 protein levels are higher in the epirubicin resistant MCF-7-EPIR cells relative to the sensitive MCF-7 cells, and FOXM1 expression is down-regulated by epirubicin in the sensitive MCF-7 cells but not in the resistant MCF-7-EPIR cells, suggesting further that FOXM1 also has a role in epirubicin resistance. In agreement, a recent study revealed that the anthracyclin daunorubicin can repress FOXM1 expression through the sequential activation of p53, p21Cip1 and the retinoblastoma (pRB) family of proteins (34). We confirmed and extended these findings in breast cancer cell lines, and showed that the MCF-7-EPIR cells failed to induce p53 expression and activity in response to epirubicin treatment. Using p53-/- mouse embryo fibroblasts (MEFs), we established that FOXM1 expression is negatively regulated by p53. In contrast, epirubicin can effectively repress FOXM1 expression in the p21Cip1-/- MEFs. This finding indicates that p53 can repress E2F activity and FOXM1 expression through mechanisms independent of the cyclin-dependent kinase inhibitor p21Cip1, despite previous studies showing that the activation of pRB by the anthracyclin daunorubicin is mediated at least partially through p21Cip1 (34). Consistently, anthracyclines have been shown to activate the forkhead transcription factor FOXO3a to induce another cyclin-dependent kinase inhibitor (CKI) p27Kip1, which can in turn inhibit CDKs and activate pRB proteins to repress E2F activity. (16, 42). It is notable that E2F1 is an E2F-regulated gene and therefore, its expression level reflects the cellular E2F activity. Transient promoter reporter transfection assays indicate that the effects of epirubicin and its cellular targets p53 and E2F1 are mediated through a proximal E2F-binding site on the FOXM1 promoter. In agreement, a recent study revealed that a great majority of genes repressed by p53 and p73 contain E2F-binding sites, suggesting that p53 proteins repress gene expression through inhibiting E2F activity (43). The direct binding of pRB and E2F1 to the FOXM1 promoter was confirmed in vivo by chromatin immunoprecipitation (ChIP) analysis. ChIP assays also revealed that upon epirubicin treatment there were increased levels of pRB and decreased levels of E2F1 recruited to the FOXM1 promoter region containing the E2F-binding site. Collectively, these findings indicate that epirubicin can repress FOXM1 expression through induction of p53, which in turn represses E2F activity through activating pRB and down-regulating E2F1 expression. Transient transfection experiments in which p53 was reintroduced into deficient cells also demonstrated that p53 activity is required for the cytotoxic function of epirubicin, confirming that the loss of p53 contributes towards the development of epirubicin resistance.
However, despite our finding that the loss of p53 in the drug resistant MCF-7-EPIR cells have a role in epirubicin resistance, it is improbable that loss of functional p53 is the primary or sole cause for the development of epirubicin resistance, considering loss of p53 function is highly prevalent in cancer. In accordance with this idea, we obtained evidence that DNA damage-sensing kinase Ataxia-telangiectasia mutated (ATM) also has a role in regulating FOXM1 expression and epirubicin sensitivity, independent of p53. For instance, in the osteosarcoma cell line U2OS with wild-type and functional p53 and pRB, epirubicin triggered the accumulation of p53 as well as ATM but failed to induce cell death. Furthermore, caffeine treatment attenuated p53 and ATM induction and yet sensitised the U2OS cells to epirubicin-induced cell death. It is also notable that E2F1 and FOXM1 levels were maintained, if not increased, after epirubicin treatment in the U2OS cells, but decreased in the presence of the ATM inhibitor caffeine, suggesting ATM also has a role in regulating E2F1 and FOXM1 expression as well as epirubicin sensitivity. Like FOXM1, ATM is overexpressed in the drug resistant MCF-7-EPIR cells compared with the MCF-7 cells and its expression is upregulated in response to epirubicin treatment. This FOXM1 induction is antagonised by the effects of p53 activation in the epirubicin-sensitive cells with functional p53, but is unopposed in the p53-deficient cells. In consequence, low levels of epirubicin will cause an induction of FOXM1 expression in the resistant cells. Together these findings suggest that ATM is activated in response to epirubicin to enhance E2F activity and consequently FOXM1 expression to promote cell survival in drug-resistant cancer cells (Fig. S8). Consistently, ample evidence has demonstrated that ATM regulates E2F1 expression in response to DNA damage, although the mechanism involved is not completely understood (36, 44). For example, genotoxic stress has been reported to upregulate E2F1 expression at the transcriptional level through the activation of ATM (36). On the contrary, a previous study has also showed that E2F1 expression is upregulated in response to DNA damage because of an increase in protein stability and not at the transcriptional level (44). Current evidence indicates that E2F1 expression can be involved in proliferation and tumorigenesis as well as apoptosis and tumour suppression (45, 46). However, in the context of cancer chemotherapy, the current observations evidently suggest that E2F1 is linked to cell survival through promoting FOXM1 expression. In a previous microarray study, E2F1-3 have been shown to promote the expression of genes involved in DNA replication, DNA repair and mitosis (47), and interestingly, some of these E2F-regulated genes identified, including cdc2, cyclin B1, and MCM members are also transcriptional targets of FOXM1 (16, 48). Consistently, a number of recent studies have demonstrated that E2F1 expression is induced by a variety of DNA damaging agents and genotoxic chemotherapeutic drugs and mirrors that of p53, further supporting a possible involvement of E2F and FOXM1 in the DNA damage response and drug resistance (44, 49, 50).
Based upon our current findings that ATM induces E2F activity and FOXM1 expression in response to DNA damage and that E2F can promote FOXM1 transcription, we propose that ATM enhances E2F1 expression and activates E2F-dependent FOXM1 expression at transcriptional level in response to DNA damaging agents, such as epirubicin. In addition, it has previously been shown that FOXM1 protein is phosphorylated by checkpoint kinase 2 (CHK2) on serine 361 in response to DNA damage and this phosphorylation has been proposed to increase the stability of the FOXM1 protein to promote expression of DNA repair genes (21). Given that CHK2 functions directly downstream of ATM in DNA damage response, it is predicted that the induction of FOXM1 expression by ATM may therefore also occur through post-translational mechanisms in response to DNA damage (21). Irrespective of the mechanism by which ATM regulates FOXM1 expression, these observations also indicate that in the MCF-7-EPIR cells the increased ATM expression may promote DNA repair to counteract the DNA damage-induced cell death triggered by genotoxic chemotherapeutic drugs. Consistent with this, the levels of DNA damage sustained by the MCF-7-EPIR cells after epirubicin treatment is significantly reduced when compared with the drug sensitive MCF-7 cells, as revealed by the γH2AX staining. Moreover, this idea is further supported by our finding that depletion of ATM activity by siRNA or the specific inhibitor Ku-55933 sensitised the resistant MCF-7-EPIR cells to epirubicin-induced cell death and abolished the accumulation of FOXM1, which has a role in DNA damage repair.
In summary, our data suggest that genotoxic chemotherapeutic agents, such as epirubicin, trigger the accumulation and activation of p53 and ATM, and it is the antagonistic signals of activated ATM and p53 that converge on E2F to control FOXM1 expression, DNA damage repair and cell survival (Fig. S8). Specifically, p53 represses while ATM enhances E2F activity, FOXM1 expression, DNA repair and cell survival in response to genotoxic drugs. In consequence, the development of epirubicin resistance can be due to the loss of p53 function and/or an increase in ATM expression and activity. The finding that ATM as well as p53 modulates FOXM1 expression may have important implications for the diagnosis and treatment of drug resistant cancers, particularly those lacking functional p53. For example, ATM and FOXM1 inhibitors can be important cancer therapeutics as they can cause cell death independent of p53 status. These ATM and FOXM1 inhibitors can also be used in combination with conventional genotoxic therapeutics to enhance the drug efficacy and for overcoming resistance. Furthermore, p53, ATM and FOXM1 could be useful biomarkers for the prediction of epirubicin sensitivity in cancer patients.
Supplementary Material
Acknowledgements
Grant support: Breast Cancer Campaign (J. Millour, E.W-F. Lam), Cancer Research UK (N. de Olano, E.W-F. Lam) and Spanish Ministry of Science (N. de Olano).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H. Current research topics in endocrine therapy for breast cancer. Int J Clin Oncol. 2008;13:380–3. doi: 10.1007/s10147-008-0818-7. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, et al. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003;14:833–42. doi: 10.1093/annonc/mdg260. [DOI] [PubMed] [Google Scholar]
- 5.(EBCTCG) EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez RH. Present and future evolution of advanced breast cancer therapy. Breast Cancer Res. 2010;12(Suppl 2):S1. doi: 10.1186/bcr2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmieri C, Krell J, James CR, Harper-Wynne C, Misra V, Cleator S, et al. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol. 2010;7:561–74. doi: 10.1038/nrclinonc.2010.122. [DOI] [PubMed] [Google Scholar]
- 8.Lown JW. Anthracycline and anthraquinone anticancer agents: current status and recent developments. Pharmacol Ther. 1993;60:185–214. doi: 10.1016/0163-7258(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen D, Maare C, Skovsgaard T. Cellular resistance to anthracyclines. Gen Pharmacol. 1996;27:251–5. doi: 10.1016/0306-3623(95)02013-6. [DOI] [PubMed] [Google Scholar]
- 10.Broxterman HJ, Gotink KJ, Verheul HM. Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resist Updat. 2009;12:114–26. doi: 10.1016/j.drup.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 12.Zelnak A. Overcoming taxane and anthracycline resistance. Breast J. 2010;16:309–12. doi: 10.1111/j.1524-4741.2010.00911.x. [DOI] [PubMed] [Google Scholar]
- 13.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 14.Rivera E. Implications of anthracycline-resistant and taxane-resistant metastatic breast cancer and new therapeutic options. Breast J. 2010;16:252–63. doi: 10.1111/j.1524-4741.2009.00896.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez AA, Makris A, Wu MF, Rimawi M, Froehlich A, Dave B, et al. DNA repair signature is associated with anthracycline response in triple negative breast cancer patients. Breast Cancer Res Treat. 2010;123:189–96. doi: 10.1007/s10549-010-0983-z. [DOI] [PubMed] [Google Scholar]
- 16.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 17.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69:3501–9. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok JM, Myatt SS, Marson CM, Coombes RC, Constantinidou D, Lam EW. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol Cancer Ther. 2008;7:2022–32. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 20.Kwok JM, Peck B, Monteiro LJ, Schwenen HD, Millour J, Coombes RC, et al. FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol Cancer Res. 2010;8:24–34. doi: 10.1158/1541-7786.MCR-09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol. 2007;27:1007–16. doi: 10.1128/MCB.01068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, et al. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol. 2006;20:1853–66. doi: 10.1210/me.2006-0056. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida Y, Wang IC, Yoder HM, Davidson NO, Costa RH. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132:1420–31. doi: 10.1053/j.gastro.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–61. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 25.Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, et al. Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res. 2006;66:1712–20. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830–50. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis RE, Myatt SS, Krol J, Hartman J, Peck B, McGovern UB, et al. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer. Int J Oncol. 2009;35:57–68. doi: 10.3892/ijo_00000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr JR, Park HJ, Wang Z, Kiefer MM, Raychaudhuri P. FoxM1 mediates resistance to herceptin and paclitaxel. Cancer Res. 2010;70:5054–63. doi: 10.1158/0008-5472.CAN-10-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millour J, Constantinidou D, Stavropoulou AV, Wilson MS, Myatt SS, Kwok JM, et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–95. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnouin K, Dubuisson ML, Child ES, Fernandez de Mattos S, Glassford J, Medema RH, et al. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J Biol Chem. 2002;277:13761–70. doi: 10.1074/jbc.M111123200. [DOI] [PubMed] [Google Scholar]
- 31.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–55. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 32.Krol J, Francis RE, Albergaria A, Sunters A, Polychronis A, Coombes RC, et al. The transcription factor FOXO3a is a crucial cellular target of gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther. 2007;6:3169–79. doi: 10.1158/1535-7163.MCT-07-0507. [DOI] [PubMed] [Google Scholar]
- 33.Essafi A, Gomes AR, Pomeranz KM, Zwolinska AK, Varshochi R, McGovern UB, et al. Studying the subcellular localization and DNA-binding activity of FoxO transcription factors, downstream effectors of PI3K/Akt. Methods Mol Biol. 2009;462:201–11. doi: 10.1007/978-1-60327-115-8_13. [DOI] [PubMed] [Google Scholar]
- 34.Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–305. doi: 10.1038/onc.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa K, Taya Y, Tamai K, Yamaizumi M. Requirement of ATM in phosphorylation of the human p53 protein at serine 15 following DNA double-strand breaks. Mol Cell Biol. 1999;19:2828–34. doi: 10.1128/mcb.19.4.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carcagno AL, Ogara MF, Sonzogni SV, Marazita MC, Sirkin PF, Ceruti JM, et al. E2F1 transcription is induced by genotoxic stress through ATM/ATR activation. IUBMB Life. 2009;61:537–43. doi: 10.1002/iub.197. [DOI] [PubMed] [Google Scholar]
- 37.Guo R, Chen J, Zhu F, Biswas AK, Berton TR, Mitchell DL, et al. E2F1 localizes to sites of UV-induced DNA damage to enhance nucleotide excision repair. J Biol Chem. 2010;285:19308–15. doi: 10.1074/jbc.M110.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng J, Wang L, Li Q, Li W, Bjorkholm M, Jia J, et al. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1. J Pathol. 2009;218:419–27. doi: 10.1002/path.2530. [DOI] [PubMed] [Google Scholar]
- 39.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–80. [PubMed] [Google Scholar]
- 40.Leyton J, Alao JP, Da Costa M, Stavropoulou AV, Latigo JR, Perumal M, et al. In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography. Cancer Res. 2006;66:7621–9. doi: 10.1158/0008-5472.CAN-05-3962. [DOI] [PubMed] [Google Scholar]
- 41.McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, et al. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8:582–91. doi: 10.1158/1535-7163.MCT-08-0805. [DOI] [PubMed] [Google Scholar]
- 42.Hui RC, Francis RE, Guest SK, Costa JR, Gomes AR, Myatt SS, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670–8. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 43.Scian MJ, Carchman EH, Mohanraj L, Stagliano KE, Anderson MA, Deb D, et al. Wild-type p53 and p73 negatively regulate expression of proliferation related genes. Oncogene. 2008;27:2583–93. doi: 10.1038/sj.onc.1210898. [DOI] [PubMed] [Google Scholar]
- 44.Blattner C, Sparks A, Lane D. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol Cell Biol. 1999;19:3704–13. doi: 10.1128/mcb.19.5.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emmrich S, Putzer BM. Checks and balances: E2F-microRNA crosstalk in cancer control. Cell Cycle. 2010;9 doi: 10.4161/cc.9.13.12061. [DOI] [PubMed] [Google Scholar]
- 46.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–48. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- 47.Polager S, Kalma Y, Berkovich E, Ginsberg D. E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene. 2002;21:437–46. doi: 10.1038/sj.onc.1205102. [DOI] [PubMed] [Google Scholar]
- 48.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–11. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 49.Hofferer M, Wirbelauer C, Humar B, Krek W. Increased levels of E2F-1-dependent DNA binding activity after UV- or gamma-irradiation. Nucleic Acids Res. 1999;27:491–5. doi: 10.1093/nar/27.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connor DJ, Lu X. Stress signals induce transcriptionally inactive E2F-1 independently of p53 and Rb. Oncogene. 2000;19:2369–76. doi: 10.1038/sj.onc.1203540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.