Abstract
The developmental pathways of regulatory T cells (Treg) generation in the thymus are not fully understood. Here, we reconstituted thymic development of Zap70 deficient thymocytes with a tetracycline inducible Zap70 transgene to allow temporal dissection of Treg development. We find that Treg develop with distinctive kinetics, first appearing by day 4 amongst CD4 single positive (SP) thymocytes. Accepted models of CD25+FoxP3+ Treg selection suggest development via CD25+FoxP3− CD4 SP precursors. In contrast, our kinetic analysis revealed the presence of abundant CD25− FoxP3+ cells that are highly efficient at maturing to CD25+FoxP3+ cells in response to IL-2. CD25− FoxP3+ cells more closely resembled mature Treg both with respect to kinetics of development and avidity for self peptide MHC. These population also exhibited distinct requirements for cytokines during their development. CD25−FoxP3+ cells were IL-15 dependent while generation of CD25+FoxP3+ specifically required IL-2. Finally, we found that IL-2 and IL-15 arose from distinct sources in vivo. IL-15 was of stromal origin, while IL-2 was of exclusively from haemopoetic cells that depended on intact CD4 lineage development but not either antigen experienced or NKT cells.
Keywords: regulatory T cells, cytokines, thymus
Introduction
Regulatory T cells (Treg) are a subset of mature CD4 T cells, characterised by expression of signature transcription factor Forkhead Box protein 3 (FoxP3), and are essential for normal immune homeostasis. Treg can develop from mature naive T cells in the presence of TGFβ and are so term inducible Treg. However, Treg are also generated in the thymus (1, 2) and cells developing from this route are termed thymic or natural Treg. While most Class II restricted thymocytes develop into CD4 single positive thymocytes (SP) a small proportion are induced to express FoxP3 and mature into Treg. This developmental fate is driven by strong TCR and costimulatory CD28 signals during selection and thymic Treg in general recognise self with a high avidity than conventional CD4 SP (3-7). The precise developmental steps involved in generating mature CD25+FoxP3+ Treg remain controversial. A two step model (8) suggests that TCR signals are required to first induce expression of CD25 on selecting CD4 SP thymocytes and that IL-2 then stimulates these cells in a second step that results in the induction of FoxP3. However, more recent studies show that CD25−FoxP3+ cells present in normal thymus can be stimulated with IL-2 in vitro, or transferred i.v. in vivo to give rise to CD25+FoxP3+ Treg (9). In addition, the precise role of cytokines in development of thymic Treg is not fully understood. IL-2 is required for maintenance of mature peripheral Treg (10, 11, 12) and generation of FoxP3+ cells in the thymus (8, 9, 13). However, while some suggest that IL-2 is required for induction of FoxP3 gene expression (8), others suggest that cytokine signaling plays a permissive role in development by promoting survival of FoxP3+ cells, since FoxP3 also induces a pro-apoptotic gene expression pattern. Furthermore, there is a degree of redundancy between members of the common gamma chain family of cytokines, since IL-2 deficient mice have only a 50% reduction in Treg numbers (12) and cytokines such as IL-15 and IL-7 have also been implicated in Treg development (14-16).
A further complication to the study of thymic Treg development is that not all FoxP3+ cells present in the thymus are necessarily de novo generated. In Rag2p-GFP mice, a substantial proportion of thymic Treg are GFP negative suggestive of an aged or extrathymic origin (17). A more recent study suggests that the majority of thymic Treg are ‘resident’ and dependent on IL-2 production induced by CD40-CD40L interactions within the thymus (18). Therefore, it is not straightforward when studying Treg in steady state conditions to determine whether changes in thymic Treg are due to alterations in de novo development or are instead effecting maintenance of thymic resident or recirculated Treg. Here, we specifically analysed de novo development of thymic Treg using a mouse model in which thymic development, arrested in Zap70 deficient mice, can be restored in adult mice by induction of an inducible Zap70 transgene. Since these mice lack mature peripheral T cells, there are no thymic resident or recirculated Treg present, so genuine de novo Treg development can be observed. Therefore, this system permits specific and time dependent analysis of thymic Treg development. Using this approach, we confirm the importance of early TCR and co-stimulatory signals via CD28 for development of thymic Treg. We identify distinct roles for, and sources of, the cytokines IL-15 and IL-2 for the development of Treg. Of significance, we observe that de novo CD25+ FoxP3+ Treg development occurs via two routes, involving either CD25+FoxP3− or CD25−FoxP3+ intermediates. We propose a model in which independent regulation of CD25 and FoxP3 expression results in the apparent bifurcated routes of Treg development.
Materials and Methods
Mice
C57BL/6J (WT) and congenic C57BL/6J.SJL-Ptprca (WT CD45.1 hereon) were used as control strains. Zap70−/− tetracycline-inducible Zap70 mice (TetZap70 hereon) have been described previously (19). The Nur77-GFP reporter allele (7) was additionally bred onto the TetZap70 background to generate a Nur77-GFP TetZap70 mice. B6.Cg-Foxp3tm1Mal/J, in which GFP is expressed from an IRES sequence downstream of FoxP3 open reading frame (20), was also bred onto the TetZap70 background to generate a FoxP3-GFP TetZap70 mice. These strains, together with Il15ra−/− Rag1−/−, OT-I Rag1−/−, OT-II Rag1−/−, Ikk2fx/fx R26REYFP CD4Cre mice were bred and housed in specific pathogen free (SPF) conditions at the MRC National Institute for Medical Research (London, UK), and experiments were performed in accordance with UK home office regulations. All mice were analyzed at 5-12 weeks of age. To induce Zap70 expression in different temporal windows, FoxP3-GFP TetZap70 chimeras were either fed 3% (w/w) doxycycline-containing diet continuously (dox) or were given a single intraperitoneal (i.p.) injection of 2mg methacycline hydrochloride (Vetranal, Sigma Fluka) dissolved in dH2O and neutralized to ~pH 7 (met).
Bone marrow chimeras
All experiments with FoxP3GFP TetZap70 and Nur77GFP TetZap70 strains were performed with thymocytes obtained from bone marrow (BM) chimeric mice. Chimeras were generated by transferring 5×106 T depleted BM cells into sublethally irradiated (500 Rads) Rag1−/− or other specified Rag1−/− hosts and allowing ≥6 weeks for reconstitution. Mixed bone marrow chimeras were generated using 1:1 mix of 107 BM from FoxP3GFP TetZap70 and the indicated donors. TetZap70 donor populations were distinguished from different partners using: CD45.2 vs CD45.1 when mixed with C57Bl6/J CD45.1 partner; huCD2+ vs huCD2-Va2+ in the presence of OTI and OTII partners, EYFP-vs EYFP+ in the presence of Ikk2fx/fx R26REYFP CD4Cre donor BM.
Antibodies, flow cytometry and cell sorting
The following antibodies were used in this study, and purchased from eBioscience or Biolegend unless otherwise indicated; Biotinylated antibody against CD45.1, CD45.2 and CD24 (HSA), Fluorescein isothiocyanate (FITC)-conjugated antibodies against CD5, HSA, CD45.1 and CD45.2. Phycoerythrin-conjugated antibodies against GITR, PE Texas Red® conjugated antibody against CD4 and CD8, PeCy7-conjugated antibodies against CD25 (clone 7D4), CD5 and CD8, APC-conjugated antibodies against TCR-β chain, Efluor® 450-conjugated antibody against CD4, CD8 and FoxP3, Pacific Orange™-conjugated antibody against CD8. Biotinylated antibodies were detected with Streptavidin conjugated to Pacific Orange™ (invitrogen).
Detection of surface antigens was performed with 2-5×106 cells, stained in 100μL PBS containing 0.1% (v/v) bovine serum albumin (BSA) on ice in the dark for one hour as described previously (21). Detection of FoxP3 (eBioscience) was performed using the FoxP3 Detection kit according to the manufacturer’s instructions.
Flow cytometry was performed using a BD FACSCantoII (Becton Dickinson) or Cyan ADP (Beckman Coulter) analyzer. Cell sorting was performed on a BD FACSAriaII (Beckton Dickinson) or MoFlo XDP (Beckman Coulter) instrument. Data was analysed using FlowJo software (v9.4.11, TreeStar).
The following mAb were used for blockade experiments in vivo at days −1, 1, 3 and 5 after dox feeding;. anti-CD154 (MR-1) (0.5mg/injection), anti-CD80 (16-10A1) (1mg/injection), anti-CD86 (GL-1) (1mg/injection) and anti-IL-2 (JES6-1A12) (1mg/injection). All mAb from Bio X Cell.
Intrathymic injections
Intrathymic injection by blind injection into the thoracic cavity has been described (22). Briefly, host mice were anaesthetised via inhalation of isofluorane (Isoflow, Abbott). A ~1cm incision was made along the midline overlying the lower cervical and upper thoracic region. Cells were suspended at 2-3×108 in air buffered Iscove’s Modified Dulbecco’s Medium, and 10μL of the suspension was injected into the anterior superior portion of each thymic lobe using a Tridak stepper pipette (Tridak). Mice were treated with the local anesthetic Bupivacaine Hydrochloride (0.25%, Marcain Polyamp®, AstraZeneca), and the incision was closed with 9mm wound clips (BD).
Results
De novo Treg development gives rise to both CD25+ and CD25− FoxP3+ cells
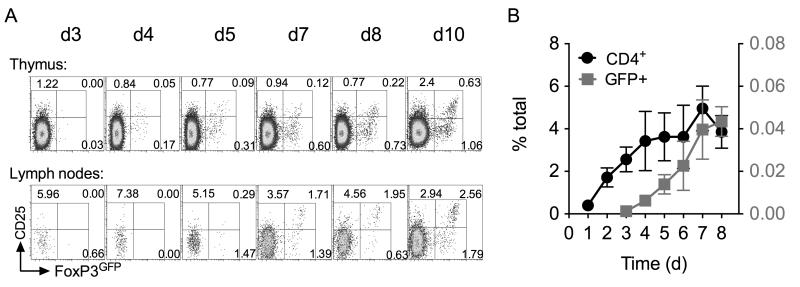
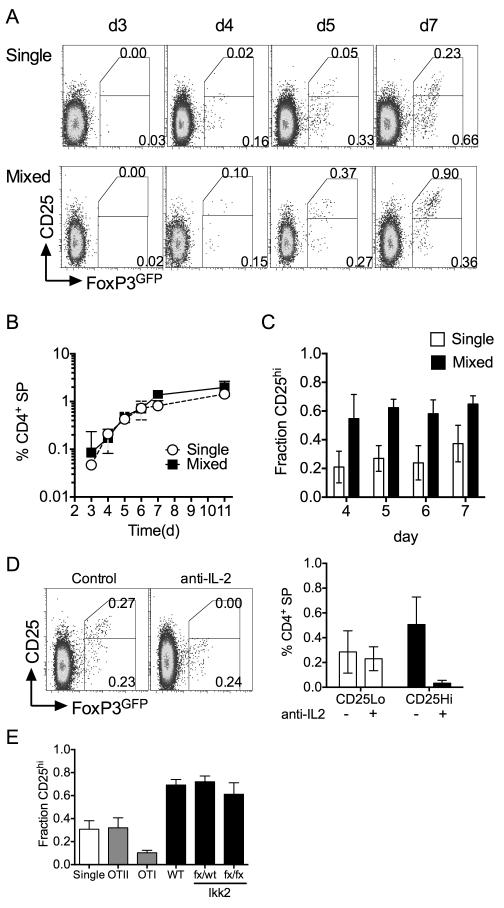
In order to investigate de novo generation of Treg in the thymus, we took advantage of a previously described mouse model in which tyrosine kinase Zap70 is under control of a tetracycline regulated promotor. A reverse tetracycline transactivator construct (rtTA) is constitutively expressed in T cells using huCD2 expression elements (rtTAhuCD2) (23). Expression of a Tetracycline regulated Zap70 transgene (Zap70Tre) (19) is induced by administration of tetracycline derivative doxycycline (dox). Mice were additionally bred onto a Zap70 deficient background to generate Zap70Tre rtTAhuCD2 Zap70−/− mice such that Zap70 expression in T cells is exclusively regulated by dox administration. For some experiments, detection of Treg development was facilitated by use of the Foxp3tm1Mal/J strain (20) in which GFP is expressed from an IRES sequence downstream of the endogenous FoxP3 locus. In the absence of Zap70 expression, thymocytes are unable to transduce TCR signals and therefore thymic development is arrested at the DP stage (24). Feeding mice dox results in rapid restoration of Zap70 expression and thymic development as previously described (19, 25). For consistency throughout, we studied thymic development in irradiation bone marrow chimeras of Rag1−/− hosts reconstituted with bone marrow from Zap70Tre rtTAhuCD2 Zap70−/− Foxp3tm1Mal mice (FoxP3GFP TetZap70 chimeras hereon). Zap70 was induced in chimeras following reconstitution at six weeks post irradiation. Analysing FoxP3GFP expression amongst CD4 SP thymocytes at different days following dox feeding revealed the first appearance of FoxP3 expressing CD4 T cells. While CD4 SP are readily detectable by day 2 after Zap70 induction (22), FoxP3GFP expressing cells were not evident until later, from day 4 onward (Figs. 1A and 1B). CD4 SP population reached equilibrium by day 5. FoxP3GFP+ cell frequencies did not peak until approximately day 7-8 (Fig. 1B). Analysing T cell numbers in peripheral lymph nodes revealed that FoxP3+ cells were readily detectable by day 7 (Fig. 1A). These data are consistent with the dynamics of Treg development observed in neonatal mice (26) suggesting that Treg development in TetZap70 mice was representative of that in WT mice.
Figure 1. Reconstitution of Treg development in FoxP3GFP TetZap70 chimeras following induction of Zap70 expression.
FoxP3GFP TetZap70 chimeras were generated by reconstituting Rag1−/− hosts with bone marrow of FoxP3GFP TetZap70 mice (see Materials and Methods). Following reconstitution, after 6 weeks, mice were placed on dox food to induce Zap70 expression and thymi and lymph nodes from groups of mice (for days 2-8, n=7, 8, 15, 13, 17,12 and 9 respectively) analysed at different times. (A) Density plots are of CD25 vs FoxP3GFP amongst CD4 SP thymocytes (top row) or CD4+ TCRhi lymph node cells (bottom row) at the days indicated after dox food administration started. Numbers indicate percentage of cells that were CD25+FoxP3GFP−, CD25+FoxP3GFP+ or CD25−FoxP3GFP+. (B) Line graphs show frequency of CD4 SP (black lines, left Y axis scale) and FoxP3GFP+ CD4 SP (red lines, right Y axis scale) amongst total thymocytes at different days after inducing Zap70 expression. Data are pooled from six independent experiments.
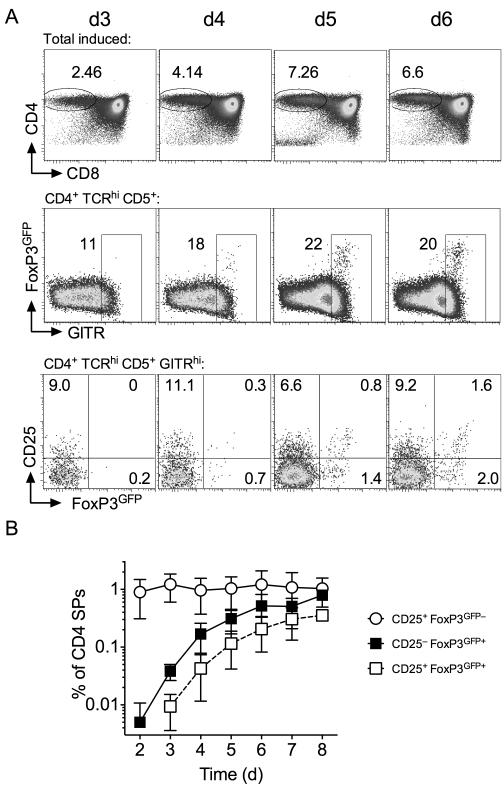
Analysing the phenotype of FoxP3GFP+ CD4 SP thymocytes revealed clear populations of both CD25+ and CD25− FoxP3GFP+ cells throughout the time course (Fig. 1A). CD25− cells appeared in greater relative abundance than CD25+ FoxP3GFP+ counterparts. While CD25+FoxP3GFP+ cells expressed uniformly high levels of GFP, CD25− FoxP3GFP+ cells expressed a broader range of GFP that was not greater than observed in CD25+ FoxP3GFP+ population. Analysis of peripheral FoxP3GFP+ CD4 T cells revealed similar pattern of expression of GFP and CD25. Previous studies show that Treg can develop from CD25+FoxP3− CD4 SP precursor population (8) and that both precursor and product populations express high levels of GITR. We therefore also focused our kinetic analysis specifically on GITRhi CD4 SP thymocytes. FoxP3GFP+ thymocytes were GITRhi throughout the timecourse (Fig. 2A). Specific analysis of GITRhi CD4 SP thymocytes helped visualise the dynamics with which CD25+ FoxP3GFP−, CD25+ FoxP3GFP+ and CD25− FoxP3GFP+ populations developed. Interestingly, CD25+ FoxP3GFP− cells were readily detectable amongst the first wave of developing CD4 SPs, but their abundance did not increase further, having reached a plateau already by day 2 (Fig. 2B). In contrast, both CD25− FoxP3GFP+ and CD25+ FoxP3GFP+ populations appeared more gradually, with numbers not reaching equilibrium until around day 7-8. Although CD25− FoxP3GFP+ and CD25+ FoxP3GFP+ cells were both detectable by day 4, CD25− FoxP3GFP+ appeared in greater abundance than CD25+ FoxP3GFP+ cells and the time course of population growth suggested that development of CD25− FoxP3GFP+ cells may precede that of CD25+ FoxP3GFP+ cells.
Figure 2. Treg and their precursors develop with distinct kinetics.
Following their reconstitution, FoxP3GFP TetZap70 chimeras were placed on dox food to induce Zap70 expression and thymi analysed at between days 3 and 6. (A) Density plots are of CD4 vs CD8 by total live cells (top row), FoxP3GFP vs GITR by CD4 SP TCRhi thymocytes, CD25 vs FoxP3GFP by CD4SP TCRhi GITRhi thymocytes. (B) Line graph shows frequency of CD25+ FoxP3GFP− (open circles), CD25− FoxP3GFP+ (black squares) and CD25+ FoxP3GFP+ cells (open squares) amongst TCRhi CD5+ CD4 SP thymocytes. Data are pooled from six independent experiments.
CD25+ and CD25− subsets of FoxP3+ cells have overlapping developmental requirements for co-stimulatory and TCR signals
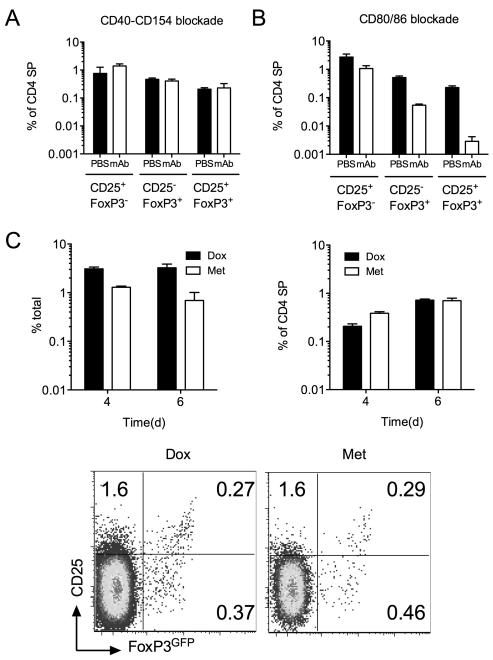
We next examined the role of co-stimulatory signaling in the de novo development of different Treg and precursor populations. CD40 signaling has been implicated in development of Treg, though more recent studies suggest such requirement may be confined to ‘resident’ thymic and peripheral Treg populations (18). To address the role of CD40-CD154 signaling in de novo generated Treg, we fed FoxP3GFP TetZap70 chimeras dox and treated a cohort with anti-CD40L blocking mAb. Treatment of WT mice for just 7 days resulted in a two-fold reduction of both peripheral and thymic Treg (data not shown) as previously described (18, 27). Analysing Treg abundance at day 6 after Zap70 induction revealed that CD40-CD154 blockade had no significant effect on the development of either FoxP3GFP+ or CD25+FoxP3GFP− populations (Fig. 3A). Co-stimulatory signals through CD28 are also thought to be critical for Treg development (3, 4, 6). Treating dox fed TetZap70 mice with blocking mAb specific for CD80 and CD86 resulted in reductions in all three populations. Abundance of the CD25+FoxP3GFP− population was modestly affected, while CD25+FoxP3GFP+ subset was most severely reduced by blocking mAb treatment (Fig. 3B).
Figure 3. Regulation of Treg development by co-stimulatory and TCR signaling.
FoxP3GFP TetZap70 chimeras were placed on dox food to induce Zap70 expression. Groups of mice were treated with either anti-CD154 (CD40L) blocking mAb or anti-CD80 and anti-CD86 blocking mAb in combination on d-1, d1, d3 and d5 after dox feeding. Controls received PBS injections. Thymi were analysed on day 6. (A) Bar chart shows the frequency of CD25+ FoxP3GFP−, CD25− FoxP3GFP+ and CD25+ FoxP3GFP+ cells amongst CD4 SP thymocytes of chimeras treated with anti-CD154 (open bars), six days after Zap70 induction as compared with controls treated with PBS (filled bars). (B) Bar chart shows the frequency of CD25+FoxP3GFP−, CD25− FoxP3GFP+ and CD25+ FoxP3GFP+ amongst CD4 SP thymocytes of chimeras treated with anti-CD80 and anti-CD86 (open bars), six days after Zap70 induction compared with controls given PBS injection (filled bars). (C) Zap70 induction was transiently induced in FoxP3GFP TetZap70 chimeras following a single i.p. injection of Met. Control chimeras were fed dox food. At days four and six after Zap70 induction, thymi were analysed for the presence of FoxP3GFP+ Treg. Left bar chart shows frequency of CD4 SP thymocytes in dox or met treated mice at days four and six after treatment. Right bar chart shows frequency of FoxP3GFP+ cells amongst CD4 SP TCRhi thymocytes at days four and six after Zap70 induction. Density plots are of CD25 vs FoxP3GFP amongst CD4 SP TCRhi thymocytes from dox and Met treated chimeras, six days after induction of Zap70. Data are pooled from three (A-B) or two (C) independent experiments.
Finally, we asked whether persistent TCR signaling was required for induction of FoxP3 by cells at day 4. To do this, we took advantage of the ability to rapidly reverse transgene expression in the Tet-inducible Zap70 system following removal of inducer. We have previously shown that a single injection of dox derivative methacycline (Met) results in a short window of Zap70 protein expression lasting ~36h (21). More recently, we have confirmed that downstream signaling pathways are reduced to background levels by 48h after peak Zap70 expression by measuring expression of CD5, Nur77 and Egr1 (28). Therefore, induction of Zap70 expression by Met limits TCR signaling to a window ~48h window between 12h and 60h post injection. However, following loss of Zap70 expression, CD4 SP numbers decline as cells die in the absence of Zap70 expression (28) (Fig. 3C). Nevertheless, analysing emergence of FoxP3GFP+ cells at d4 and d6 after Met injection revealed that FoxP3GFP+cells were readily detectable in met treated mice, in spite of declining CD4 SP numbers (Fig. 3C). Furthermore, even though no new CD4 SP development was possible after loss of Zap70 expression in Met treated mice, the relative abundance of FoxP3GFP+ cells increased between day 4 and 6. This could either be a consequence of continued development of FoxP3GFP+ cells from CD4 SP precursors following loss of Zap70 expression or that FoxP3GFP+ cells survived relatively better than CD4 SP in the absence of Zap70. Which ever is the case, the data clearly showed that a short 48h window of TCR signaling was sufficient for subsequent development of FoxP3+ thymocytes 4 days after onset of thymic development and sustained TCR signaling was not necessary for induction or maintenance of FoxP3 expression.
Both overlapping and distinct requirements for cytokine signaling during generation of CD25+ and CD25− FoxP3+ cells
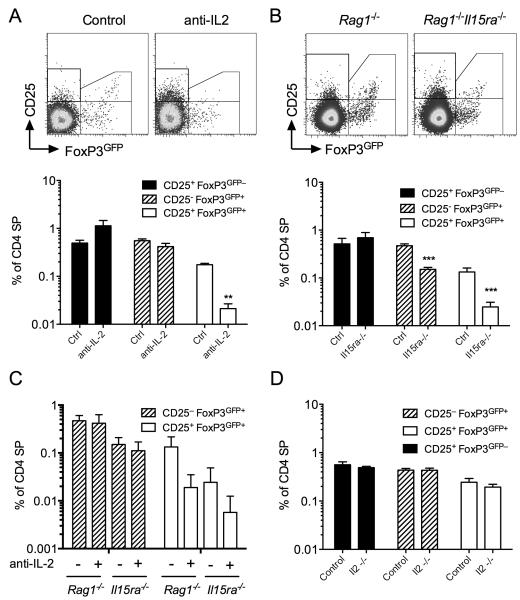
There is abundance evidence that gamma-chain (γc) family of cytokines are required for both development and maintenance of peripheral FoxP3+ Treg populations. We therefore examined the specific role of IL-2 and IL-15 in the de novo development and generation of precursor and FoxP3+ subsets in the thymus. To do this, we fed TetZap70 chimeras dox to induce Zap70 expression then treated cohorts of mice with mAb to block the activity of IL-2 in vivo and analysed Treg development by day 6. Consistent with previously studies, IL-2 blockade had no significant effect on the appearance of CD25+FoxP3GFP− precursor population (Fig. 4A). Interestingly, IL-2 blockade had distinct effects on development of CD25+FoxP3GFP+ and CD25−FoxP3GFP− populations. Appearance of CD25+ FoxP3GFP+ cells was almost completely inhibited by IL-2 blockade, while the generation of CD25− FoxP3GFP+ cells was unaffected (Fig. 4A). Given that CD25− FoxP3GFP+ cells lack high expression of the high affinity receptor for IL-2, we asked whether other γc cytokines, such as IL-15, could instead be required for their survival. Other studies have suggested redundancy between γc family cytokines for the maintenance of Treg (14). We therefore generated irradiation chimeras in which Rag1−/− Il15ra−/− hosts were reconstituted with bone marrow from FoxP3GFP TetZap70 mice. In the absence of IL-15Rɑ receptor, cells cannot transpresent IL-15 (29) and Rag1−/− Il15ra−/− hosts are functionally deficient for IL-15. In mixed irradiation chimeras, this deficiency is restricted to stromal and radio-resistant but not FoxP3GFP TetZap70 bone marrow derived cells. Analysing development of TetZap70 Treg in thymus at day 6 after feeding chimeras dox revealed that both CD25− FoxP3GFP+ and CD25+FoxP3GFP+ cells were reduced in abundance. In contrast, CD25+ FoxP3GFP− cells were unaffected. Although we found that CD25− FoxP3GFP+ cells were unaffected by anti-IL-2 blockade, it was possible that in Il15ra−/− chimeras, the remaining CD25− FoxP3GFP+ cells were IL-2 dependent in the absence of IL-15. To test this, we additionally treated groups of Il15ra−/− chimeras with anti-IL-2. As before, generation of CD25+FoxP3GFP+ cells was prevented in all anti-IL-2 treated groups, while development CD25− FoxP3GFP+ cells was not affected by anti-IL-2 either in the presence of absence of IL-15 activity (Fig. 4C). Therefore, although IL-15 of stromal origin was required for efficient development of both CD25− and CD25+FoxP3GFP+ populations, there was not an absolute requirement for either IL-2 or IL-15 signaling for induction of FoxP3 expression. CD25− FoxP3GFP+ cells still developed in the absence of both cytokines, albeit in reduced abundance.
Figure 4. Differential roles for IL-2 and IL-15 for the development of Treg and precursor populations.
FoxP3GFP TetZap70 chimeras were generated using either Rag1−/−, Il15ra−/−Rag1−/− or Il2−/−Rag1−/− hosts. (A-C) FoxP3GFP TetZap70 chimeras of Rag1−/− and Il15ra−/−Rag1−/− hosts were placed on dox food. Groups of mice were additionally injected with anti-IL-2 blocking mAb or PBS as control at days −1, 1, 3 and 5 after dox feeding. Thymi were analysed at day 6. (A) Density plots are of CD25 vs FoxP3GFP by CD4 SP TCRhi thymocytes from FoxP3GFP TetZap70 chimeras of Rag1−/− hosts. Bar chart shows frequencies of CD25+ FoxP3GFP− (black fill bars), CD25− FoxP3GFP+ (hatched bars) and CD25+ FoxP3GFP+ (empty bars) amongst CD4 SP thymocytes of chimeras treated with anti-IL-2, six days after Zap70 induction. (B) Density plots are of CD25 vs FoxP3GFP by CD4 SP TCRhi thymocytes from chimeras of Rag1−/− or Il15ra−/−Rag1−/− hosts six days after dox feeding. Bar chart shows frequencies of CD25+FoxP3GFP− (black fill bars), CD25− FoxP3GFP+ (hatched bars) and CD25+ FoxP3GFP+ (empty bars) amongst CD4 SP thymocytes from Rag1−/− or Il15ra−/−Rag1−/− FoxP3GFP TetZap70 chimeras. (C) Groups of Rag1−/− or Il15ra−/−Rag1−/− FoxP3GFP TetZap70 chimeras were additionally treated with anti-IL-2. Bar chart shows frequencies of CD25− FoxP3GFP+ (hatched bars) and CD25+ FoxP3GFP+ (empty bars) amongst CD4 SP thymocytes from Rag1−/− or Il15ra−/−Rag1−/− FoxP3GFP TetZap70 chimeras treated or not with anti-IL-2 blocking mAb. (D) Il2−/−Rag1−/− and Rag1−/− FoxP3GFP TetZap70 chimeras were fed dox and Treg development analysed at day 6. Bar chart shows frequencies of CD25+FoxP3GFP− (black fill bars), CD25− FoxP3GFP+ (hatched bars) and CD25+ FoxP3GFP+ (empty bars) cells amongst CD4 SP thymocytes from the indicated chimeras. Data are representative of two (D) or three (A-C) independent experiments.
Since we identified a stromal source of IL-15 required for development of CD25− FoxP3GFP+ cells, we also asked whether IL-2 that is required for generation of CD25+FoxP3GFP+ Treg development arose from a similar source. To test this, irradiation chimeras were made in which Il2−/− Rag1−/− hosts were reconstituted with bone marrow from FoxP3 TetZap70 mice. Following reconstitution, mice were fed dox for six days and Treg development examined. Significantly, abundance of Treg and precursor populations was unaffected by IL-2 gene ablation in stromal cells (Fig. 4D). Together, these data suggest that IL-15 from stromal cells supports development of both CD25− FoxP3GFP+ through which it supports onward development of CD25+ FoxP3GFP+ Treg, while IL-2 of haematopoetic origin is required specifically for development of CD25+ FoxP3GFP+ but not CD25− FoxP3GFP+ Treg.
CD25+ FoxP3+ Treg develop from both CD25−GFP+ and CD25+GFP− subsets
Previous studies suggest that CD25+FoxP3+ Treg develop from CD25+FoxP3− precursors. However, following the time course of do novo generation of Treg revealed development of CD25−FoxP3+ cells whose kinetics and abundance matched development of CD25+FoxP3+ Treg more closely that did those of CD25+FoxP3− cells. Therefore, we wished to examine the developmental relationships between these subsets more closely, to determine what developmental relationships exist between these subsets.
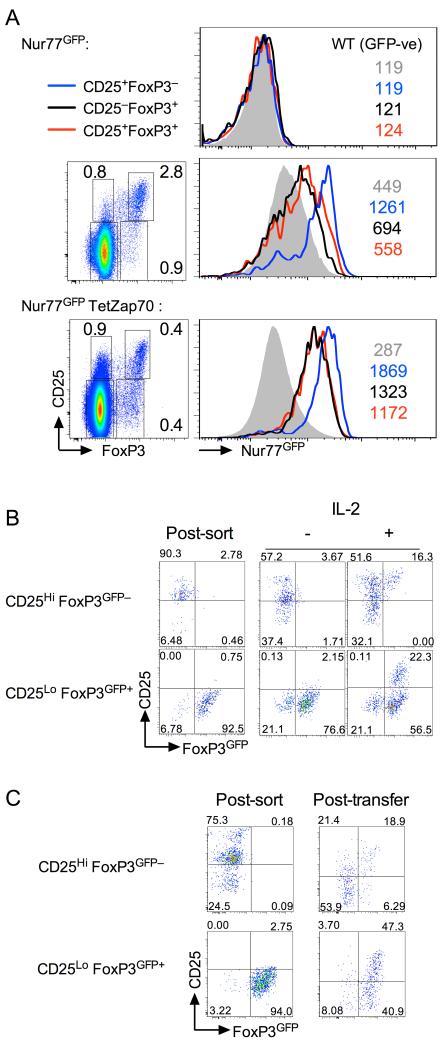
First, we examined the TCR avidity for self of each population by measuring Nur77 expression using recently described Nur77GFP reporter strain (7). Thymic Treg are known to have TCR with high functional avidity for self, as revealed by Nur77GFP expression. We therefore compared Nur77GFP expression by CD25−FoxP3+ and CD25+FoxP3− thymic populations as compared with fully mature CD25+FoxP3+ cells in both WT and TetZap70 cells. Compared to CD25−FoxP3− cells, the other CD25 and FoxP3 expressing populations of CD4 SPs all had higher levels of Nur77 in both Zap70WT Nur77GFP and Nur77GFP TetZap70 mice. Interestingly, Nur77GFP expression by CD25+FoxP3− populations in both WT and TetZap70 strains, was greater than either CD25−FoxP3+ or CD25+FoxP3+ populations, which instead expressed similar Nur77GFP levels to one another. These data suggest that CD25+ FoxP3− and FoxP3+ populations have distinct self avidities and that CD25+ and CD25−FoxP3+ cells share a similar self reactivity to one another that might imply a common origin.
Second, to directly assess precursor product relations of CD25+FoxP3−, CD25−FoxP3+ cells and CD25+FoxP3+ cells, we analysed developmental behaviour of purified populations in vitro and in vivo. CD25+FoxP3− and CD25− FoxP3+ populations were sorted from WT FoxP3GFP mice and cultured for 24h in the presence and absence of IL-2. Culture of CD25+FoxP3− cells with IL-2 caused a small proportion of cells to induce expression of FoxP3 and become CD25+FoxP3+. This was in a manner and to an extent essentially as reported previously (8, 9). However, culturing CD25−FoxP3+ cells with IL-2 also resulted in development of a more substantial CD25+FoxP3+ Treg population (Fig. 5B). Finally, to assess the developmental fates of these subsets in vivo, purified populations were transferred by direct intrathymic injection into congenic WT host thymi. After 48h, donor cell phenotype was analysed. Following transfer, the majority of CD25+FoxP3− cells lost CD25 expression and became CD25−FoxP3−, as previously described (8). Nevertheless, almost a fifth of recovered cells were CD25+FoxP3+ confirming these previous reports that this population does indeed include precursors of thymic Treg. Strikingly, following transfer, CD25−FoxP3+ cells maintained their FoxP3 expression and significantly, more than half of recovered cells also upregulated CD25 to give rise to mature CD25+FoxP3+ Treg.
Figure 5. Mature Treg arise from both CD25+ FoxP3GFP− and CD25− FoxP3GFP+ precursors.
(A) Nur77GFP reporter expression was analysed amongst subsets of CD4 SP thymocytes from either Nur77GFP mice, Nur77GFP TetZap70 chimeras fed dox food for 6 days, or WT control mice as control for GFP detection. Thymocytes were fixed and stained for intracellular FoxP3 protein. Density plots are of FoxP3 vs CD25 amongst CD4SP of the indicated strain. Histograms are of Nur77GFP expression by CD25−FoxP3− (grey fill), CD25+FoxP3− (blue line), CD25−FoxP3+ (black line) and CD25+FoxP3+ cells (red line) from Nur77GFP− WT control mice (top), Nur77GFP mice (middle row) and Nur77GFP TetZap70 chimeras (bottom row). Colour coded numbers indicate MFI of GFP for the corresponding histogram on each plot. Data are representative of four independent experiments. (B) CD25+ FoxP3GFP− and CD25− FoxP3GFP+ populations were purified by cell sorting from CD4 SP of Zap70WT FoxP3GFP mice and cultured with or without exogenous IL-2 for 24h and phenotype assessed. Density plots are of CD25 vs FoxP3GFP immediately following sorting (post sort), and after 24 h culture with (+) and without (−) IL-2. Data are representative of three independent experiments. (C) Purified CD25+ FoxP3GFP− and CD25− FoxP3GFP+ CD4 SP thymocytes were sorted from Zap70WT FoxP3GFP mice and injected directly into the thymus of CD45.1 congenic control hosts. 48h later, thymi were recovered and phenotype of donor populations determined. Density plots are of CD25 vs FoxP3GFP immediately following cell sorting (Post-sort), and 48h after transfer (Post-transfer). Data are representative of two independent experiments.
Pre-existing thymic development enhances de novo Treg development
Zap70 induction restores thymic development of all major populations of T cells in TetZap70 mice. However, it takes more than a week for development to reach equilibrium (22). Full maturation of mTEC, that are important in Treg maturation (30), requires the presence of mature SP thymocytes (31). In addition, we identified a haempoetic source of IL-2 as critical for de novo development of CD25+FoxP3+ Treg in TetZap70 mice. Therefore, it was possible that do novo Treg development could be modulated in the context of fully reconstituted thymic development. To investigate this, we compared TetZap70 Treg development in single chimeras to that in mixed bone marrow chimeras reconstituted with a 1:1 mix of FoxP3 TetZap70 and CD45.1 WT bone marrow. In such mixed chimeras, TetZap70 Treg development could be observed in the presence of pre-existing fully reconstituted WT thymocyte development. Following dox administration to chimeras, TetZap70 FoxP3GFP+ cells appeared with similar kinetics in mixed and single chimeras (Fig. 6A). The size of the total FoxP3GFP+ population was similar until day 6, after which cell frequencies reached a slightly higher maximum in mixed chimeras compared with those in single chimeras (Fig. 6B). While the overall abundance of FoxP3+ cells was similar in mixed and single chimeras, analysis of CD25 expression revealed that the representation of CD25+ and CD25− FoxP3+ cells was substantially altered in mixed chimeras. The fraction of FoxP3GFP+ cells that were CD25+ was increased in mixed chimeric mice (Fig. 6A and 6C). The increase was apparent as soon as FoxP3+ cells were detectable by day 5 after dox feeding, even when absolute levels of FoxP3+ cells were similar between mixed and single chimeras. To see if development of CD25+FoxP3GFP+ cells was strictly IL-2 dependent in mixed chimeras as we had observed in single chimeras, we treated mixed chimeras with anti-IL-2. Analysis of TetZap70 Treg development at day six confirmed that the enhanced development of CD25+FoxP3+ cells observed in mixed chimeras was also IL-2 dependent (Fig. 6D).
Figure 6. CD25+FoxP3+ Treg development is most efficient in the presence established CD4 lineage development.
Chimeras were generated by reconstituting irradiated Rag1−/− hosts with either bone marrow from FoxP3GFP TetZap70 donors alone (single) or a 1:1 mixture of bone marrow from CD45.1 WT and FoxP3GFP TetZap70 donors (mixed). Following reconstitution, chimeras were fed dox food and groups of mice analysed at different times after dox feeding. (A) Density plots are of CD25 vs FoxP3GFP amongst CD45.2 CD4 SP TCRhi TetZap70 donor populations in single or mixed chimeras. (B) The line graph is frequency of FoxP3GFP cells amongst CD45.2 TetZap70 donor CD4 SP thymocytes in either single (open circles) or mixed (filled square) chimeras. (C) Bar chart shows the fraction of TetZap70 CD4 SP FoxP3GFP+ cells that are CD25hi in either single or mixed bone marrow chimeras at the days indicated. Data are pool of four independent experiments. (D) Mixed chimeras were additionally treated with anti-IL-2 blocking mAb at days −1, 1, 3 and 5 after dox feeding, and thymus analysed at day 6. Density plot is of CD25 vs FoxP3GFP amongst CD45.2 TetZap70 donor CD4 SP thymocytes from control or anti-IL-2 treated mice. Bar chart shows mean frequency of CD25−FoxP3GFP+ and CD25+FoxP3GFP+ cells amongst CD45.2 TetZap70 donor CD4 SP thymocytes from control or anti-IL-2 treated mice. Data are representative of three independent experiments. (E) Mixed bone marrow chimeras were generated using bone marrow from FoxP3GFP TetZap70 donors together with bone marrow from either OTII Rag1−/−, OTI Rag1−/−, CD45.1 WT, Ikk2fx/fx R26REYFP CD4Cre or control Ikk2fx/WT R26REYFP CD4Cre donors. Following reconstitution, mice were fed dox food and thymus analysed at day 6. Bar chart shows the fraction of TetZap70 donor FoxP3GFP+ CD4 SP thymocytes that were CD25+. Data are pooled from six independent experiments.
Finally, we wanted to investigate which aspects of WT development contributed to enhanced development of CD25+FoxP3GFP+ cell development. WT development gives rise to multiple cell types that could in principle be responsible. We tested this by generating mixed chimeras in which TetZap70 bone marrow was partnered with bone marrow of mice with specific developmental properties. After reconstitution, chimeras were fed Dox and development of CD25+FoxP3GFP+ cells assessed by measuring the fraction of FoxP3GFP+ cells that were CD25+ by day six. We first tested whether positive selection of Class I or Class II restricted monoclonal populations was sufficient to support enhanced CD25+FoxP3GFP+ cell development. Mixed chimeras employing bone marrow from OTII mice had similar CD25+FoxP3GFP+ TetZap70 cell development as single chimeras controls. In contrast, the presence of Class I restricted OTI development resulted in a modest impairment of CD25+FoxP3+ development (FIg. 6E). Lastly, we tested whether the presence of WT Treg, NKT or CD4 memory cell populations was required for enhanced development CD25+FoxP3+ TetZap70 Treg. NKT and memory populations are potential sources of IL-2. We tested this using bone marrow from CD4Cre Ikk2fx/fx mice to make mixed bone marrow chimeras. Ikk2 is a critical component of inhibitor of kappa B kinase (IKK) complex required for canonical NF-κB signaling. In the absence of Ikk2, naive CD4 and CD8 SP development is normal, but development of Treg and NK T cells is almost completely absent in these mice, and generation of memory cells in the periphery is also profoundly inhibited (32, 33). Significantly, analysing TetZap70 Treg development in mixed bone marrow chimeras of TetZap70 and CD4Cre Ikk2fx/fx cells, revealed that enhanced development of CD25+FoxP3GFP+ cells was observed even in the specific absence of established NKT, CD4 memory or WT Treg (Fig. 6E). Therefore, these data suggest that pre-existing polycloncal selection of conventional naive T cells is sufficient for optimal development of CD25+FoxP3GFP+cells.
Discussion
In the present study, we investigated the developmental pathways, signaling and cytokine requirements during de novo generation of CD25+ FoxP3+ Treg in the thymus. We confirm that costimulatory signals from CD28 are essential for development of both precursor and mature FoxP3+ subsets but that CD40 signaling is redundant. Consistent with previous models, TCR signaling was only transiently required to induce Treg development. However, we find evidence for bifurcated routes of development to mature CD25+FoxP3+ Treg. While CD25+ FoxP3− cells are enriched for precursors, we observed that CD25+FoxP3+ Treg developed efficiently via CD25−FoxP3+ cells. Interestingly, these different precursor pools had distinct requirements for IL-2 and IL-15 during their development and these cytokines arise from distinct sources.
Previous studies propose a two step model of Treg development in which TCR signaling initially induces development of CD25+FoxP3− precursors that develop to CD25+FoxP3+ Treg following IL-2 dependent induction of FoxP3 expression (8). Consistent with this view, we found that blockade of CD28 costimulation profoundly inhibited CD25+FoxP3+ Treg development, as reported elsewhere (3, 4, 6), and that TCR signaling was only transiently required for the development of CD25+ FoxP3+ Treg. Also consistent with the two step model, approximately 20% of CD25+FoxP3− cells could develop into CD25+ FoxP3+ Treg in vitro, with exogenous IL-2, or in vivo, following intrathymic transfer. However, we also found evidence that this was not the sole pathway of Treg development. Following restoration of thymopoesis in FoxP3GFP TetZap70 mice, we not only observed development of the previously described CD25+FoxP3− precursor and mature CD25+FoxP3+ Treg populations, but also an abundant CD25−FoxP3+ population. Such cells are generally apparent in 2D plots of CD25 vs FoxP3, but have been specifically noted in Il2rb−/− and WT mice (13, 14) in which they are observed to be FoxP3locompared with CD25+FoxP3+ Treg. More recent studies have suggested these cells are also capable of acquiring a mature CD25+ phenotype (9) following intravenous adoptive transfer. De novo generated CD25−FoxP3+ cells in TetZap70 mice also appeared to have lower levels of FoxP3 expression than CD25+FoxP3+ Treg. During timecourses of development, expression of both FoxP3 and CD25 amongst CD25−FoxP3+ and CD25+FoxP3+ subsets appeared as a continuum rather than discrete populations, suggestive of a developmental progression first involving up regulation of FoxP3 but followed after by induction of CD25 to give rise to mature Treg. Direct proof of this developmental progression came from the observation that IL-2 was capable of efficiently inducing CD25 expression on WT CD25−FoxP3+ cells in vitro and, most importantly, following their intrathymic injection into WT thymus, where they developed into mature Treg by induction of CD25 and further upregulation of FoxP3. Therefore, CD25−FoxP3+ cells are a natural developmental precursor of CD25+FoxP3+ Treg in the thymus in vivo.
The observation that mature Treg can arise from both CD25−FoxP3+ and CD25+FoxP3− precursors raises the questions of whether these subsets represent genuinely distinct pathways of Treg development and, if so, which is the predominating route? There were several pieces of evidence pointing to CD25−FoxP3+ cells as the more significant precursor of mature Treg. Firstly, Nur77 expression by CD25−FoxP3+ and CD25+FoxP3− subsets show that these cells experience distinct TCR signaling during selection, since CD25+FoxP3− expressed the highest level of Nur77. Significantly, Nur77 expression by mature Treg was most similar to that of CD25−FoxP3+ cells suggestive of a common origin amongst the selecting repertoire. Secondly, developmental appearance and growth of CD25−FoxP3+ and CD25+FoxP3+ Treg populations occurred with similar kinetics in TetZap70 mice, and were consistent with a precursor product relationship between CD25−FoxP3+ and CD25+FoxP3+ cells. In contrast, the kinetics of CD25+FoxP3− cell development was far more rapid than that of either FoxP3+ subsets. The rapid kinetics were suggestive of a high death rate within the subset, since the CD25+FoxP3− population grew and reached equilibrium quickly but without evidence of onward development to a mature CD25+FoxP3+ phenotype with a correspondingly fast kinetic. The high level of Nur77 expression by CD25+FoxP3− cells may indicate that this population represents thymocytes expressing TCRs with the highest extent of self-recognition and therefore at or near the boundaries of negative selection. Such a view would be consistent with an anticipated high death rate amongst these cells. Furthermore, both our results and previous studies show that many CD25+FoxP3− cells loose CD25 expression upon adoptive transfer (8, 9), so it is also possible that the rapid plateau of population growth could be due to a combination of death by negative selection and/or reversion to a CD25− state. Significantly, both ours and other studies consistently report only a fraction of CD25+FoxP3− cells up regulate FoxP3 either in vitro or in vivo (8, 9). Nevertheless, while Nur77 expression levels were higher on CD25+FoxP3− cells, there was still substantial overlap with that of CD25+FoxP3+ Treg cells, and it is also possible that this overlap represents the subset of CD25+FoxP3− cells capable of onward development to CD25+FoxP3+ Treg.
Analysis of TetZap70 mice also revealed differential requirements for cytokine signaling during Treg development. CD122 is required for both IL-2R and IL-15R function and significantly, Il2rb knockout mice have fewer Treg than IL-2 knockout mice (12, 34), suggesting that IL-2 and IL-15 cooperate in promoting Treg development. The precise role of these cytokines in Treg development has been less clear. While it was thought that IL-2 signaling via STAT5 was required for specific induction of FoxP3 gene expression (8, 15), more recent studies suggest that γc cytokines play a permissive role in development by counteracting the pro-apoptotic effects of FoxP3 expression (9). We found that development of CD25+FoxP3+ cells was strictly IL-2 dependent but that IL-2 was not required for either survival or induction of FoxP3 expression in CD25−FoxP3+ cells or development of CD25+FoxP3− precursors. We did find evidence that CD25−FoxP3+ cells had a requirement for IL-15 produced by thymic stroma. However, even in the absence of IL-15, FoxP3 expression was still induced in this population, albeit in a reduced population of cells, revealing that neither IL-15 nor IL-2 signaling were absolute requirements for FoxP3 gene induction. Rather, IL-15 may be providing survival signals for CD25−FoxP3+ cells prior to their IL-2 dependent induction of CD25. The reduced abundance of CD25+FoxP3+ cells in IL-15 deficient hosts also supports the view that CD25−FoxP3+ cells represent a major input to the mature Treg pool, since a reduction in precursors would be expected to result in reduced abundance of progeny, as observed here.
Our data show that IL-2 is critical for development of CD25+FoxP3+ Treg. We do not unequivocally pinpoint the source of this cytokine in the thymus. Our data do, however, provide strong indications. Although mTEC play an important roles in Treg development (30), production of IL-2 does not appear to be one of them. Rather, we found that IL-2 most efficiently promoted Treg development in conditions of established thymopoeisis. Memory/effector cells are implicated as a source if IL-2 for maintenance of Treg in peripheral lymphoid organs (35) and can recirculate to the thymus. However, we found that neither memory nor indeed NK T cells, another potent source of cytokines (36), were required for this activity. Interestingly, positive selection of monoclonal populations was insufficient for optimal CD25+FoxP3+ TetZap70 Treg development. In contrast, established polyclonal selection of naive thymocytes in Ikk2 deficient thymocytes was sufficient for optimal TetZap70 Treg development. Taken together, these data suggest that a subset of selecting thymocytes, not present in a monoclonal settings, are required. This may represent a subset of IL-2 producing thymocytes undergoing negative selection. Certainly, Treg development is observed side by side with negative selection in TCR transgenic mice in the presence of cognate antigen (37). However, given that selected T cells are also required for full maturation of mTEC (31), we cannot rule out a more complex scenario in which a subset of selected T cells licenses IL-2 production by another cell of haemopoetic origin. Interestingly, the presence or absence of a substantial pre-existing WT Treg compartment in the thymus had no impact on the size of the FoxP3+ TetZap70 population development, which was even marginally larger in the presence of WT development. This suggests that the number of Treg that develop in the thymus is not limited by a specific cytokine niche but rather, is a function of the selecting repertoire and what fraction of those cells have a TCR compatible with induction of FoxP3 and development to the Treg lineage.
Why are there apparently two distinct routes of Treg development? It is possible that the Treg developing from either CD25−FoxP3+ or CD25+FoxP3− precursors represent subtlety different subsets with distinct functional properties. Testing this idea will require a means to distinguish the developmental origin of mature Treg, which currently does not exist. Alternatively, these apparent distinct developmental pathways could be rationalised if CD25 and FoxP3 expression were fact independent and stochastic in developing Treg. This being the case, asynchronous expression of CD25 and FoxP3 in developing Treg could account for the presence of both CD25−FoxP3+ and CD25+FoxP3− intermediates. In such a scheme, the pro-apoptotic properties of FoxP3 would dictate the apparent dependence of subsets on different cytokines, which would play a permissive role by counteracting FoxP3 induced apoptosis. The distributions of times to expression of CD25 and FoxP3 by developing Treg need not be the same and differences would account for the apparent preference of one developmental route over another. Also, if TCR signal strength also influences these distributions, this would account for the distinct Nur77 levels in different precursor populations. Regardless of how these precursors are regulated, future studies trying to unravel the complex regulation of thymic Treg development will need to consider and account for both CD25−FoxP3+ cells and CD25+FoxP3− intermediates.
Acknowledgements
We thank Biological Services staff for assistance with mouse breeding, and the NIMR flow cytometry facility.
This work was supported by the Medical Research Council UK under programme code U117573801.
References
- 1.Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J Exp Med. 1996;184:2393–2398. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annual review of immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 3.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nature immunology. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 4.Lio CW, Dodson LF, Deppong CM, Hsieh CS, Green JM. CD28 facilitates the generation of Foxp3 (-) cytokine responsive regulatory T cell precursors. J Immunol. 2010;184:6007–6013. doi: 10.4049/jimmunol.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vang KB, Yang J, Pagan AJ, Li LX, Wang J, Green JM, Beg AA, Farrar MA. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J Immunol. 2010;184:4074–4077. doi: 10.4049/jimmunol.0903933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinterberger M, Wirnsberger G, Klein L. B7/CD28 in central tolerance: costimulation promotes maturation of regulatory T cell precursors and prevents their clonal deletion. Frontiers in immunology. 2011;2:30. doi: 10.3389/fimmu.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer DS, Singer A. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38:1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4 (+)CD25 (+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nature immunology. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G, Yu A, Dee MJ, Malek TR. IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol. 2013;190:1567–1575. doi: 10.4049/jimmunol.1201218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, −7, and −15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuss SM, Green EA. Abrogation of CD40-CD154 signaling impedes the homeostasis of thymic resident regulatory T cells by altering the levels of IL-2, but does not affect regulatory T cell development. J Immunol. 2012;189:1717–1725. doi: 10.4049/jimmunol.1200588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal. 2010;3:ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Kissenpfennig A, Mingueneau M, Richelme S, Perrin P, Chevrier S, Genton C, Lucas B, DiSanto JP, Acha-Orbea H, Malissen B, Malissen M. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J Immunol. 2008;180:1565–1575. doi: 10.4049/jimmunol.180.3.1565. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair C, Saini M, Schim van der Loeff I, Sakaguchi S, Seddon B. The long-term survival potential of mature T lymphocytes is programmed during development in the thymus. Sci Signal. 2011;4:ra77. doi: 10.1126/scisignal.2002246. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proc Natl Acad Sci U S A. 2013;110:E2905–2914. doi: 10.1073/pnas.1304859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, Williams K, Norton T, Kioussis D, Zamoyska R. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity. 2000;12:537–546. doi: 10.1016/s1074-7613(00)80205-8. [DOI] [PubMed] [Google Scholar]
- 24.Gelfand EW, Weinberg K, Mazer BD, Kadlecek TA, Weiss A. Absence of ZAP-70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J Exp Med. 1995;182:1057–1065. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bains I, van Santen HM, Seddon B, Yates AJ. Models of self-peptide sampling by developing T cells identify candidate mechanisms of thymic selection. PLoS Comput Biol. 2013;9:e1003102. doi: 10.1371/journal.pcbi.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105:973–978. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair C, Seddon B. Overlapping and Asymmetric Functions of TCR Signaling during Thymic Selection of CD4 and CD8 Lineages. J Immunol. 2014;192:5151–5159. doi: 10.4049/jimmunol.1303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 30.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210:675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, Lane PJ, Jenkinson EJ, Anderson G. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. Journal of immunology. 2010;185:4769–4776. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 35.O’Gorman WE, Dooms H, Thorne SH, Kuswanto WF, Simonds EF, Krutzik PO, Nolan GP, Abbas AK. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–339. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 37.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]