Abstract
Recently RAD51C mutations were identified in families with breast and ovarian cancer1. This observation prompted us to investigate the role of RAD51D in cancer susceptibility. We identified eight inactivating RAD51D mutations in unrelated individuals from 911 breast-ovarian cancer families compared with one in 1060 controls (P=0.01). The association was principally with ovarian cancer with three mutations identified in the 59 pedigrees with three or more ovarian cancer cases (P=0.0005). The relative risk of ovarian cancer for RAD51D mutation carriers was estimated to be 6.30 (95%CI: 2.86-13.85; P=4.8×10−6). By contrast, the relative risk of breast cancer was estimated to be 1.32 (95%CI: 0.59-2.96; P=0.50). These data indicate that RAD51D mutation testing may have clinical utility in individuals with ovarian cancer and their families. Moreover, we show that cells deficient in RAD51D are sensitive to treatment with a PARP inhibitor, suggesting a possible therapeutic approach for cancers arising in RAD51D mutation carriers.
Homologous recombination (HR) is a mechanism for repairing stalled replication forks, DNA interstrand crosslinks and double-strand breaks2. Constitutional inactivating mutations in several genes that encode proteins crucial for DNA repair by HR have been shown to predispose to cancer3. In particular, there is a strong association with female cancers and mutations in genes such as BRCA1, BRCA2, ATM, BRIP1, CHEK2, PALB2, RAD50 and RAD51C have been shown to confer susceptibility to breast and/or ovarian cancer1,4. Indeed, the analysis of families with breast and ovarian cancer was crucial to the mapping of the BRCA1 gene5. For many years, it was widely believed that the genetic contribution to families with breast and ovarian cancer was largely attributable to mutations in BRCA1 and BRCA26-8. However, last year Meindl et al. identified mutations in RAD51C in breast-ovarian cancer families1. This suggested that analysis of such families may still have utility in cancer predisposition gene discovery.
In eukaryotic cells, DNA repair by HR involves several proteins of which a central player is the DNA recombinase RAD51, the ortholog of bacterial RecA9. RAD51 forms helical filaments on DNA and catalyzes DNA strand invasion and exchange. Multiple other proteins are involved in these processes including five RAD51 paralogs: RAD51B, RAD51C, RAD51D, XRCC2 and XRCC310. Here, through a case-control mutation study, we demonstrate that mutations in RAD51D (also known as RAD51L3) predispose to cancer in humans.
We sequenced the full coding sequence and intron-exon boundaries of RAD51D in DNA from unrelated probands from 911 breast-ovarian cancer families and 1060 population controls (Supplementary Table 1). The breast-ovarian cancer families included at least one case of breast cancer and at least one case of ovarian cancer and all were negative for mutations in BRCA1 and BRCA2 (Supplementary Table 2).
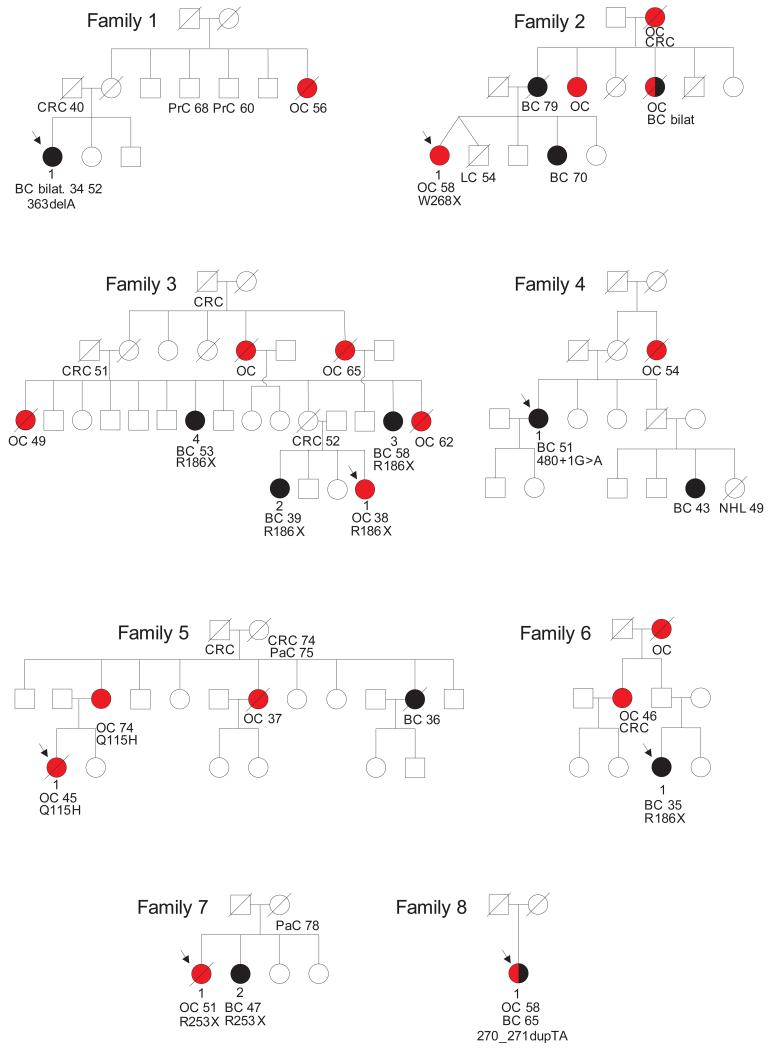
We identified inactivating mutations in RAD51D in eight of 911 cases and one of 1060 controls (P=0.01) (Table 1). The mutations were not equally distributed within the series, with a higher prevalence in families with more than one ovarian cancer; four mutations were detected in 235 families with two or more cases of ovarian cancer (P=0.005) and three mutations were detected in the 59 families with three or more cases of ovarian cancer (P=0.0005) (Fig. 1).
Table 1. Cancer history and pathology in RAD51D mutation carriers.
| Family ID | Mutation* | Person ID | Cancer history (age at which cancer occurred, in years) | Pathology | Tumor analysis |
|---|---|---|---|---|---|
| FAM1 | c.363delA | 1 | Breast cancer, left (34) | Invasive ductal carcinoma of no special type, grade 3 | NA |
| Breast cancer, right (52) | Invasive ductal carcinoma of no special type, grade 3 | Loss of wildtype allele | |||
| FAM2 | c.803G>A;W268X | 1 | Ovarian cancer (58) | Bilateral serous adenocarcinoma | Loss of wildtype allele |
| FAM3 | c.556C>T;R186X | 1 | Ovarian cancer (38) | NA | NA |
| 2 | Breast cancer (39) | High grade ductal comedo carcinoma in situ | NA | ||
| 3 | Breast cancer (58) | Invasive carcinoma with medullary features | NA | ||
| 4 | Breast cancer (53) | Invasive ductal carcinoma of no special type | NA | ||
| FAM4 | c.480+1G>A | 1 | Breast cancer (51) | Invasive ductal carcinoma of no special type, grade 3 | NA |
| FAM5 | c.345G>C;Q115H** | 1 | Ovarian cancer (45) | Bilateral serous adenocarcinoma | NA |
| 2 | Ovarian cancer (74) | NA | NA | ||
| FAM6 | c.556C>T;R186X | 1 | Breast cancer (35) | Invasive ductal carcinoma of no special type, grade 3 | NA |
| FAM7 | c.757C>T;R253X | 1 | Ovarian cancer (51) | Differentiated endometrioid adenocarcinoma | NA |
| 2 | Breast cancer (47) | NA | NA | ||
| FAM8 | c.270_271dupTA | 1 | Ovarian cancer (58) | Differentiated adenocarcinoma | Loss of mutant allele |
| Breast cancer (65) | Invasive ductal carcinoma of no special type, grade 3 | Reduction of wildtype allele | |||
| Control | c.748delC | NA | NA | NA |
Mutation nomenclature corresponds to Ensembl Transcript ID ENST00000345365
This mutation is at the final base of exon 4, disrupts the splice-site and results in skipping of exons 3 and 4. Person IDs correspond to Fig. 1. NA: not available.
Figure 1.
Abridged pedigrees of eight families with RAD51D mutations. Individuals with ovarian cancer are shown as red circles, individuals with breast cancer are shown as black circles, other cancers are shown as unfilled circles or squares. Where known, the age of cancer diagnosis is under the individual, with two ages given for metachronas bilateral breast cancers. The relevant RAD51D mutation is given under the affected individuals analysed but not the unaffected individuals, to preserve confidentiality. BC, breast cancer; BC bilat., bilateral breast cancer; OC, ovarian cancer; CRC, colorectal cancer; LC, lung cancer; NHL, non-Hodgkin lymphoma; PaC, pancreatic cancer; Pr, prostate cancer.
All the mutations are predicted to result in protein truncation through frameshifting insertions or deletions (n=3), the generation of nonsense codons (n=4) or splice defects (n=2) (Table 1).We also identified 5 intronic, 3 synonymous and 15 non-synonymous variants. Three coding variants, rs9901455 (S78S), rs4796033 (R165Q) and rs28363284 (E233G) have minor allele frequency >1% and no association was observed for any of these variants (Supplementary Table 3). Of the remaining rare variants, three were present in both cases and controls, nine were detected in a single case and eight were detected in a single control (Supplementary Table 4). There was thus no overall difference in the frequency of non-truncating RAD51D variants between cases and controls. Moreover, there was no difference in the position or predicted functional effects of these variants and it is noteworthy that an equal number (n=5) of non-synonymous variants detected in cases and controls are predicted to affect function (Supplementary Fig. 1 and Supplementary Table 4). These data indicate that mutations that result in inactivation of RAD51D function predispose to cancer, but that variants with less significant functional effects are likely to be non-pathogenic.
We tested for the family mutation in samples from 13 relatives. This revealed that five of five individuals affected with ovarian or breast cancer carried the family mutation, whereas six of eight unaffected relatives did not carry the family mutation. Several other cancers were present in relatives, such as pancreatic, prostate and colorectal cancer (Fig. 1). However, the mutation status of these individuals is not known and additional studies will be required to evaluate whether RAD51D mutations predispose to other cancers. Pathology information was available for four ovarian cancers from RAD51D mutation carriers; three were serous adenocarcinoma and one was an endometrioid cancer. Pathology information was available for eight breast cancers of which seven were ductal in origin and one was a carcinoma with medullary features. Receptor status was available from five breast cancers of which three were estrogen receptor positive and two were negative. Tumor material was available from two ovarian cancers and two breast cancers. We detected loss of the wild-type allele in one ovarian and one breast cancer and reduction of the proportion of the wild-type allele in a further breast cancer. In the final ovarian cancer the mutant allele was lost and the wildtype allele was retained (Table 1 and Supplementary Fig. 2).
These characteristics are typical of the intermediate-penetrance cancer predisposition genes that we, and others, have described in breast cancer1,4,11-14. To estimate directly the risks associated with RAD51D mutations we undertook modified segregation analysis, by modelling the risks of ovarian and breast cancer simultaneously and incorporating the information from the controls and full pedigrees of both mutation-positive and mutation-negative breast-ovarian cancer families. The ovarian cancer relative risk for RAD51D mutation carriers was estimated to be 6.30 (95%CI: 2.86-13.85; P=4.8×10−6) (Fig.2). By contrast, the association with breast cancer risk was not statistically significant (RR= 1.32 (95%CI: 0.59-2.96; P=0.50).
Figure 2.
Average age-related cumulative risk of ovarian cancer in RAD51D mutation carriers, BRCA1 and BRCA2 mutation carriers22 and the population23 .
To further explore the role of RAD51D mutations in breast cancer predisposition, we sequenced the gene in an additional series of 737 unrelated individuals from pedigrees in which there was familial breast cancer but no ovarian cancer. We did not identify any inactivating mutations (0/737 cases vs 1/1060 controls P=1.0). Although at first glance these data may seem surprising, they are consistent with the results of the segregation analysis. This is because if RAD51D mutations confer a sizeable relative risk of ovarian cancer but only a small, or no, increase in breast cancer risk, the frequency of RAD51D mutations in a series of breast cancer families selected on the basis of not containing ovarian cancer would be anticipated to be very low. The data are also consistent with the detection of RAD51D mutations in seven individuals with breast cancer in the breast-ovarian cancer families, as we specifically ascertained the ovarian cancer cases because of their close family history of breast cancer. This will inevitably result in an enrichment of breast cancer in relatives of RAD51D mutation-positive ovarian cancer cases, irrespective of whether such mutations confer a risk of breast cancer. To formally refine the risk of breast cancer associated with RAD51D mutations will likely be very challenging because the population frequency of RAD51D mutations is so low. Assuming a population mutation frequency of 0.1% and a relative risk of breast cancer of 1.3, full gene mutational analysis of RAD51D in 275,000 cases and 275,000 controls would be required to have 90% power to demonstrate the association.
Our data clearly demonstrate that RAD51D is an ovarian cancer predisposition gene but further studies in familial and sporadic ovarian cancer series would be of value to further clarify the risks of ovarian cancer. RAD51D mutation analysis in individuals with Fanconi anemia and Fanconi-like disorders would also be of interest, given that biallelic mutations in BRCA2, PALB2, BRIP1 and RAD51C have been demonstrated to cause these phenotypes15-18.
Our discovery has potential clinical utility both for individuals with cancer and their relatives. Cancer patients with RAD51D mutations may benefit from specific therapies such as Poly (ADP-Ribose) Polymerase (PARP) inhibitors, which have shown efficacy in patients with impairment of HR due to mutations in BRCA1 or BRCA219. To investigate this we used RNA interference (RNAi) and assessed the relationship between RAD51D loss of function and the sensitivity of tumor cells to a clinical PARP inhibitor, olaparib (AstraZeneca). Short interfering (si) RNAi reagents targeting RAD51D caused olaparib sensitivity of a magnitude similar to that achieved using silencing of BRCA2 (Fig. 3a,b), an observation in keeping with the HR defect observed in RAD51D null rodent cell lines20. To extend this analysis, we also observed the RAD51D selective effect of olaparib in RAD51D deficient CHO cells in which both alleles of RAD51D have been rendered dysfunctional by gene targeting (Fig. 3c)20. These data suggest that PARP inhibitors may have clinical utility in individuals with RAD51D mutations. We estimate that only ~0.6% of unselected individuals with ovarian cancer will harbour RAD51D mutations, but as we enter an era in which genetic testing will become routine, such individuals will be readily identifiable. Their identification will also be of potential value to female relatives, as those with mutations will be on average at ~6 fold increased risk of ovarian cancer, which equates to an ~10% cumulative risk by age 80. An appreciable proportion of women at this level of risk may consider strategies such as laprascopic oophorectomy, which is well-tolerated and undertaken in many women with BRCA mutations21.
Figure 3.
Effect of RAD51D silencing on Olaparib sensitivity. CAL51 (a) or MCF7 (b) cells were transfected with siCONTROL, siRNA directed against RAD51D or siRNA directed against BRCA2 and then treated with olaparib for 7 days before assaying for cell viability. Wild-type CHO cells or CHO cells mutated in RAD51D were treated with olaparib for 7 days before assaying for cell viability (c).
Online Methods
Patients and Samples
Cases
We used lymphocyte DNA from 1648 families with breast-ovarian cancer or breast cancer-only. These were ascertained from 24 genetics centres in the UK via the Genetics of Familial Breast Cancer Study (FBCS), which recruits women ≥18 years who have had breast cancer and/or ovarian cancer and have a family history of breast cancer and/or ovarian cancer. At least 97% of families are of European ancestry. Index cases from each family were screened and negative for germline mutations, including large rearrangements, in BRCA1 and BRCA2. Informed consent was obtained from all participants and the research was approved by the London Multicentre Research Ethics Committee (MREC/01/2/18).
Breast-Ovarian Cancer Pedigrees
We included 911 unrelated index cases from breast-ovarian cancer pedigrees. The index cases were diagnosed with breast and/or ovarian cancer. Each family contained an individual with both breast and ovarian cancer or contained at least one case of breast cancer and at least one case of ovarian cancer with ≤1 intervening unaffected female relatives. Cases of ovarian cancer below the age of 20 were excluded from the analysis, as an appreciable proportion are likely to represent non-epithelial ovarian tumours, for example germ cell cancers. 271/911 probands had ovarian cancer (+/− breast cancer) and 617 probands had breast cancer only. The number of family members (including the probands) diagnosed with breast cancer and/or ovarian cancer, in the 911 breast-ovarian cancer pedigrees included in the analysis is illustrated in Supplementary Table 2.
Breast Cancer-only Pedigrees
We included 737 unrelated index cases from breast cancer-only pedigrees. The index case from each family was diagnosed with breast cancer, and had bilateral disease and/or a family history of breast cancer. There was no known case of ovarian cancer in any pedigree. The number of family members (including the probands) diagnosed with breast cancer, in the 737 breast cancer-only pedigrees included in the analysis is illustrated in Supplementary Table 2. The six cases of isolated breast cancer all had bilateral disease.
Samples and pathology information from mutation-positive Families
For families in which a mutation in RAD51D was detected, we sought DNA samples from relatives and all obtainable samples were genotyped for the family mutation. We also requested tumor material, pathology information, and receptor status in probands and affected relatives from the hospitals where they had been treated.
Controls
We used lymphocyte DNA from 1060 population-based controls obtained from the 1958 Birth Cohort Collection, an ongoing follow-up of persons born in Great Britain in one week in 1958. Biomedical assessment was undertaken during 2002-2004 at which blood samples and informed consent were obtained for creation of a genetic resource but phenotype data for these individuals is not available (http://www.cls.ioe.ac.uk/studies.asp?section=000100020003). At least 97% of the controls were of European ancestry.
Mutation analysis of RAD51D
We analysed genomic DNA extracted from lymphocytes for mutations by direct sequencing of the full coding sequence and intron/exon boundaries of RAD51D. Primer sequences and PCR conditions are given in Supplementary Table 1. The PCR reactions were performed in multiplex using the Qiagen Multiplex PCR Kit (Qiagen) according to the manufacturer’s instructions. Amplicons were unidirectionally sequenced using the BigDyeTerminator Cycle sequencing kit and an ABI3730 automated sequencer (ABI Perkin Elmer). Sequencing traces were analysed using Mutation Surveyor software (www.softgenetics.com) and by visual inspection. All mutations were confirmed by bidirectional sequencing from a fresh aliquot of the stock DNA. Samples from members of RAD51D mutation-positive families were tested for the family mutation by direct sequencing of the appropriate exon.
In silico analyses of identified variants
We computed the predicted effects of RAD51D missense variants on protein function using PolyPhen24 and SIFT25. All variants (intronic and coding) were analysed for their potential effect on splicing. In the first instance, variants were analysed using two splice prediction algorithms NNsplice26 and MaxEntScan27, via the Alamut software interface (Interactive Biosoftware). If both NNsplice and MaxEntScan scores were altered by >20% (i.e. a wildtype splice-site score decreases and/or a cryptic splice-site score increases) three further prediction algorithms were utilised; NetGene228, HumanSplicingFinder29, and Genscan30. A consensus decrease in a wildtype splice-site score and/or a consensus increase in a cryptic splicer-site score across all algorithms was considered indicative of disruption of normal splicing.
Tumor analysis
Representative tumor sections were stained with nuclear fast red and microdissected using a sterile needle and a stereomicroscope (Olympus SZ61, Tokyo, Japan) to ensure the proportion of tumour cells was >90%, as previously described31. DNA was extracted using the DNeasy kit (Qiagen) according to the manufacturer’s instructions. DNA concentration was measured using the PicoGreen assay (Invitrogen), according to the manufacturer’s instructions. RAD51D specific fragments encompassing the relevant mutations were PCR-amplified using the primers in Supplementary Table 1, and bidirectionally sequenced using the BigDyeTerminator Cycle sequencing kit and an ABI3730 automated sequencer (ABI Perkin Elmer). Sequence traces from tumor DNA were compared to sequence traces from lymphocyte DNA from the same individual.
Drug sensitivity
We used non-silencing BRCA2 and RAD51D siGENOME siRNAs (Dharmacon, Lafayette, Colorado, USA). CAL51 and MCF7 cells were grown in DMEM (Gibco,, Invitrogen) supplemented with 10% (v/v) FCS (Gibco, Invitrogen). CHO RAD51D WT (51D1.3 clone) and CHO RAD51 dysfunctional (51D1 clone) cells were grown in αMEM (Gibco,, Invitrogen) supplemented with 10% FCS (Gibco,, Invitrogen). Cells were siRNA transfected using RNAiMAX (Invitrogen), plated in 96 well microtitre plates and then exposed to a titration of olaparib for 7 days. Media and drug was replenished every 3 days. After 7 days continuous culture, cell viability was estimated using Cell TitreGlo reagent (Promega Madison, Wisconsin, USA) and surviving fractions calculated as previously described32.
Statistical methods
Statistical analyses were performed using STATA v11 software (StataCorp, College Station, Texas, USA). The frequency of mutations in cases and controls was compared using a two-sided Fisher’s exact test. We estimated the RAD51D combined mutation frequency, the breast cancer risk ratio and the ovarian cancer risk ratio relative to non-RAD51D mutation carriers simultaneously using modified segregation analysis implemented in the pedigree analysis software MENDEL33. The analysis was based on breast and ovarian cancer occurrence in the combined dataset of families and controls. All individuals were censored at age 80 years, the age of their first cancer or their age of death or last observation, whichever occurred first. Females who had had bilateral prophylactic mastectomy were censored for breast cancer, and those who had had bilateral prophylactic oophorectomy were censored for ovarian cancer. Thus, only information on the first cancer was included in the primary analysis. We assumed that the breast incidence depends on the underlying genotype through a model of the form: λ(t) = λ0(t)exp(βx) where λ0(t) is the baseline incidence at age t in non-mutation carriers, β is the log risk ratio associated with the mutation and x takes value 0 for non-mutation carriers and 1 for mutation carriers. A similar model was assumed for the ovarian cancer incidences. Breast and ovarian cancers were assumed to occur independently, conditional on the genotype22. The overall breast and ovarian cancer incidences were constrained to agree with the population incidences for England and Wales in the period of 1993-199723, as described previously34,35. The models were parameterised in terms of the mutation frequencies and log-risk ratios for breast and ovarian cancer. Parameters were estimated using maximum likelihood estimation. Since RAD51D mutation screening was carried out in all index cases and controls we were able to incorporate information from all controls and the full pedigrees from all cases (including those without a RAD51D mutation) together with the segregation information from the families in which a RAD51D mutation was detected and genotyping was possible in relatives of the index case. To adjust for ascertainment, we modelled the conditional likelihood of all family phenotypes and mutation status of the index family member and other tested family members, given the disease phenotypes of all family members. For the controls we modelled the likelihood of the mutation status given they were unaffected. The variances of the parameters were obtained by inverting the observed information matrix. Log risk ratios were assumed to be normally distributed. Because this model does not explicitly incorporate the effects of other susceptibility genes, it assumes implicitly that the effects of RAD51D and other potential susceptibility genes can be regarded as independent, as in a multiplicative model. Power calculations were based on two-sided association testing with a significance level of α=0.05. We assumed that the observed frequency of truncating mutations in cases from breast-ovarian cancer families (0.88%) and controls (0.094%) reflects the true underlying mutation frequencies in the population, and that the effect calculated from the segregation analysis (OR=6.30) represents the true risk of ovarian cancer in the population. We assumed that the same ratio of truncating mutations: missense variants (predicted deleterious) would be detected in isolated cases of ovarian cancer as cases from breast-ovarian cancer families. We assumed that in association testing of mutation frequencies across 25,000 genes that the Χ2 statistics will be normally distributed and we applied a Bonferroni correction for multiple testing.
Supplementary Material
Acknowledgments
We thank all the patients and families that participated in the research. We thank Anita Hall, Darshna Dudakia, Jessie Bull, Rachel Linger and Anna Zachariou for their assistance in recruitment, Bernadette Ebbs for assistance in DNA extraction and running the ABI sequencers, Larry Thompson for the provision of cell lines and Ann Strydom for assistance in preparing the manuscript. We also thank. We are very grateful to all the clinicians and counsellors in the Breast Cancer Susceptibility Collaboration UK (BCSC) that have contributed to the recruitment and collection of the FBCS samples. The full list of BCSC contributors is in the Supplementary Note. This work was funded by Cancer Research UK (C8620/A8372 and C8620/A8857); US Military Acquisition (ACQ) Activity, Era of Hope Award (W81XWH-05-1-0204), Breakthrough Breast Cancer and the Institute of Cancer Research (UK). We acknowledge NHS funding to the NIHR Biomedical Research Centre. C.T. is an MRC-funded Clinical Research Fellow. A.C.A. is a Cancer Research UK Senior Cancer Research Fellow (C12292/A11174). We acknowledge use of DNA from the British 1958 Birth Cohort collection, funded by the Medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/02.
Footnotes
Accession numbers
RAD51D mutation nomenclature corresponds to Ensembl Transcript ID ENST00000345365.
References
- 1.Meindl A, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–4. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 2.Heyer W-D, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44:113–39. doi: 10.1146/annurev-genet-051710-150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Futreal PA, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–83. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;9:321–45. doi: 10.1146/annurev.genom.9.081307.164339. [DOI] [PubMed] [Google Scholar]
- 5.Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52:678–701. [PMC free article] [PubMed] [Google Scholar]
- 6.Gayther SA, et al. The contribution of germline BRCA1 and BRCA2 mutations to familial ovarian cancer: no evidence for other ovarian cancer-susceptibility genes. Am J Hum Genet. 1999;65:1021–1029. doi: 10.1086/302583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramus SJ, et al. Contribution of BRCA1 and BRCA2 mutations to inherited ovarian cancer. Hum Mutat. 2007;28:1207–1215. doi: 10.1002/humu.20599. [DOI] [PubMed] [Google Scholar]
- 8.Antoniou AC, Gayther SA, Stratton JF, Ponder BA, Easton DF. Risk models for familial ovarian and breast cancer. Genet Epidemiol. 2000;18:173–190. doi: 10.1002/(SICI)1098-2272(200002)18:2<173::AID-GEPI6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara A, et al. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993;4:239–43. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- 10.Masson JY, et al. Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev. 2001;15:3296–307. doi: 10.1101/gad.947001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijers-Heijboer H, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–9. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 12.Renwick A, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–5. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 13.Rahman N, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39:165–7. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seal S, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006;38:1239–41. doi: 10.1038/ng1902. [DOI] [PubMed] [Google Scholar]
- 15.Howlett NG, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 16.Levitus M, et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005;37:934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 17.Reid S, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2007;39:162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 18.Vaz F, et al. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 2010;42:406–9. doi: 10.1038/ng.570. [DOI] [PubMed] [Google Scholar]
- 19.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 20.Hinz JM, et al. Repression of mutagenesis by Rad51D-mediated homologous recombination. Nucleic Acids Res. 2006;34:1358–68. doi: 10.1093/nar/gkl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–7. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoniou A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IARC Sci Publ . Cancer incidence in five continents. Volume VIII. 2002. pp. 1–781. ( IARC Sci Publ ). [PubMed] [Google Scholar]
- 24.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–74. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J Comput Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 27.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11:377–94. doi: 10.1089/1066527041410418. [DOI] [PubMed] [Google Scholar]
- 28.Brunak S, Engelbrecht J, Knudsen S. Prediction of human mRNA donor and acceptor sites from the DNA sequence. J Mol Biol. 1991;220:49–65. doi: 10.1016/0022-2836(91)90380-o. [DOI] [PubMed] [Google Scholar]
- 29.Desmet FO, et al. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 31.Geyer FC, et al. Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas. J Pathol. 2010;220:562–73. doi: 10.1002/path.2675. [DOI] [PubMed] [Google Scholar]
- 32.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 33.Lange K, Weeks D, Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5:471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- 34.Antoniou AC, Easton DF. Polygenic inheritance of breast cancer: Implications for design of association studies. Genet Epidemiol. 2003;25:190–202. doi: 10.1002/gepi.10261. [DOI] [PubMed] [Google Scholar]
- 35.Antoniou AC, et al. Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet Epidemiol. 2001;21:1–18. doi: 10.1002/gepi.1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.