Abstract
Background. Tuberculosis is a known occupational hazard for healthcare workers (HCWs), especially in countries with a high burden of tuberculosis. It is estimated that HCWs have a 2- to 3-fold increased risk of developing tuberculosis compared with the general population. The objective of this study was to identify occupational risk factors for tuberculosis among HCWs in 3 district hospitals with specialized multidrug-resistant tuberculosis wards in KwaZulu-Natal, South Africa.
Methods. We conducted a case-control study of HCWs diagnosed with tuberculosis between January 2006 and December 2010. Cases and controls were asked to complete a self-administered questionnaire regarding potential risk factors for tuberculosis.
Results. Of 307 subjects selected, 145 (47%) HCWs responded to the questionnaire; 54 (37%) tuberculosis cases and 91 (63%) controls. Cases occurred more frequently among clinical staff 46% (n = 25) and support staff 35% (n = 19). Thirty-two (26% [32/125]) HCWs were known to be infected with human immunodeficiency virus (HIV), including 45% (21/54) of cases. HCWs living with HIV (odds ratio [OR], 6.35; 95% confidence interval [CI], 3.54–11.37) and those who spent time working in areas with patients (OR, 2.24; 95% CI, 1.40–3.59) had significantly greater odds of developing tuberculosis, controlling for occupation, number of wards worked in, and household crowding.
Conclusions. HIV was the major independent risk factor for tuberculosis among HCWs in this sample. These findings support the need for HCWs to know their HIV status, and for HIV-infected HCWs to be offered antiretroviral therapy and isoniazid preventive therapy. Infection prevention and control should also be improved to prevent transmission of tuberculosis in healthcare settings to protect both HCWs and patients.
Keywords: tuberculosis, healthcare worker, occupational health, HIV, South Africa
Mycobacterium tuberculosis continues to cause significant morbidity and mortality in low- and middle-income countries [1–4]. The World Health Organization (WHO) estimates that one-third of the world's population is infected with tuberculosis [5], with approximately 9 million new tuberculosis cases and 1.4 million deaths worldwide each year [6]. Tuberculosis continues to be a major health issue in South Africa, where the estimated incidence in 2010 was 981 per 100 000 [6]. In the province of KwaZulu-Natal, the tuberculosis incidence was estimated to be 1142 per 100 000 that same year [7].
The resurgence of tuberculosis worldwide is due, in part, to the emergence of human immunodeficiency virus (HIV) [1, 8]. This is of particular concern in South Africa, which has a longstanding HIV epidemic with an estimated prevalence of 17%—among the highest in the world. It is estimated that in South Africa, up to 80% of individuals with tuberculosis are coinfected with HIV [9, 10]. The prevalence of HIV among healthcare workers (HCWs) is not well documented, but has been estimated to be between 11% and 16% among HCWs in South Africa [11, 12].
Tuberculosis has long been considered an occupational hazard for HCWs [13], and HCWs have an increased risk to develop tuberculosis and drug-resistant tuberculosis relative to the general population [4, 14–16]. Hospital-associated transmission of tuberculosis from patient to HCW is believed to be the most likely cause of transmission, and likely due to poor or nonexistent infection control measures, especially in low-resource settings [4, 14, 17]. Little is known about other specific occupational risk factors for tuberculosis among HCWs. Some researchers have identified HIV infection, time spent with patients, job designation, duration of service, work location, and failure to wear personal protective equipment to be significant risk factors for tuberculosis [18–20]. However, no risk factors were the same across these studies. The objective of this study was to identify occupational risk factors for tuberculosis among HCWs in KwaZulu-Natal, South Africa, in order to inform interventions to reduce tuberculosis transmission to HCWs.
METHODS
Study Setting and Population
We conducted a case-control study among HCWs who worked in any ward or area of the 3 district hospitals with a specialized multidrug-resistant (MDR) tuberculosis ward [21] in KwaZulu-Natal, South Africa, over a 5-year period (January 2006–December 2010). At the time of this study, there were 3 district hospitals in KwaZulu-Natal with a specialized MDR tuberculosis ward located on the hospital grounds. The size of the district hospitals varied from 170 to 280 beds and employed between 332 and 700 HCWs.
Patients were admitted to the specialized MDR tuberculosis wards once MDR tuberculosis was confirmed by drug susceptibility testing (DST). Patients presumed to have MDR tuberculosis were often admitted to the drug-susceptible tuberculosis ward or waited at home while awaiting DST results, and were isolated as best as possible. Patients suspected of having or confirmed to have extensively drug-resistant (XDR) tuberculosis were isolated as best as possible while awaiting to be transferred to the provincial referral hospital for drug-resistant tuberculosis.
Infection prevention and control measures varied by hospital and by ward within hospital. Almost all of the inpatient wards in these 3 hospitals were multibed open wards with limited facilities to isolate patients suspected of having tuberculosis. Ultraviolet germicidal irradiation and mechanical ventilation were not used in any ward or areas of the 3 hospitals. N95 respirators were not consistently available across all wards and areas of the hospitals, and were often only available in the MDR tuberculosis or tuberculosis ward.
Data Collection
We used the WHO definition of HCW, defined as any person whose main activities are aimed at enhancing health [22]. This includes clinical staff (doctors, nurses, etc), paramedical staff (radiographers, laboratory personnel, pharmacists, counselors, etc), support staff (cleaners, orderlies, porters, etc), and administrative staff.
A review of occupational health clinic employees’ medical records was conducted at each hospital to identify cases of tuberculosis among HCWs and is described in more detail elsewhere [16]. Cases were defined as any staff member working in a hospital who had a documented case of tuberculosis disease during the 5-year period of interest (1 January 2006 to 31 December 2010) and who was employed by the hospital at least 6 months prior to being diagnosed with tuberculosis. Controls were selected from HCWs identified as part of the records review without a history of tuberculosis during the study period and who had worked in the same hospital as cases for at least 6 months. Controls were frequency matched to cases (n = 83) on duration of employment at a ratio of 3 controls to each case and randomly selected. We randomly selected 224 controls from HCWs without a documented case of tuberculosis during the same 5-year period of interest. Three hundred seven questionnaires were distributed.
Cases and controls were contacted through the occupational health nurses at each site between July 2011 and January 2012. Study staff prepared questionnaires with an informed consent document placed in opaque envelopes. The occupational health nurses explained the study and asked staff to complete the 56-item self-administered questionnaire and return in the sealed opaque envelope. The informed consent attached to each questionnaire described the study and informed HCWs that by completing the questionnaire, they consented to participate in the study. HCWs were instructed to not write their name on the questionnaires. Questionnaires and informed consent were available in English and isiZulu. The informed consent and questionnaire were translated from English to isiZulu by an external translator and then back-translated by native speakers to ensure content and cultural validity.
Study data were collected on paper forms and entered into a secure password-protected database, the REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Johns Hopkins University on a secure server [23].
Definitions
Occupational Characteristics
Clinical staff were defined as medical doctors/medical officers and nursing staff (professional nurse, enrolled nurse, and nursing assistant); support staff included orderlies, cleaners, groundsmen, and maintenance, kitchen, laundry, and security staff; paramedical staff included lay counselors, radiographers, pharmacists, laboratory technicians, and mortuary attendants; and administrative staff were defined as those working in an administrative capacity such as finance, human resources, management, office clerks, data capturers, and ward clerks.
High-risk areas in the hospital were defined as areas where diagnosed or undiagnosed patients with tuberculosis were likely to be cared for and included tuberculosis wards, MDR tuberculosis wards, casualty, outpatient departments, primary healthcare centers, tuberculosis outpatient clinics, HIV/antiretroviral therapy (ART) clinics, general wards, and radiography. Intermediate-risk areas were defined as pediatric wards, maternity wards, laboratories, pharmacies, operating theaters, mortuaries, and working throughout the hospital. Low-risk areas were defined as areas of the hospital where there was a low chance of having contact with patients and included the following areas: administrative offices, kitchen, laundry, maintenance, and outdoors. Because many HCWs worked in >1 ward, they were classified as working in an area of greater risk if they worked in a lower-risk area (low-risk or intermediate-risk) and a high-risk area.
Individual Characteristics
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Household crowding was calculated by dividing the number of people living in a household by the total number of rooms in the home. Study participants self-reported their HIV status as HIV uninfected, HIV infected, or never tested; there was no independent verification of participants' HIV status.
Ethical Review
This study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions, the South African Medical Research Council's Ethics Committee, and the KwaZulu-Natal Provincial Department of Health's Research Committee. Letters of approval were also received from the hospital management of each site.
Statistical Analysis
Univariate analysis was first performed on all variables to screen for coding and entry errors and to check for normality and skewness. Bivariate analysis compared characteristics of cases and controls; differences were assessed with χ2 or Fisher exact test for categorical variables, as appropriate. Continuous variables were compared using Student t tests.
Multivariate logistic regression was performed to assess independent risk factors for tuberculosis. Variables were retained in the final model if the P value was ≤.20 in bivariate logistic regression. The final model included both hospital-associated exposures and non-hospital-associated risk factors for tuberculosis disease at an α < .05. This model was clustered by facility to account for correlation among HCWs attributable to working in the same hospital [24]. All analyses were performed using Stata software, version 11 [25]. The model was a good fit as assessed by the Hosmer–Lemeshow goodness-of-fit test (P = .39).
RESULTS
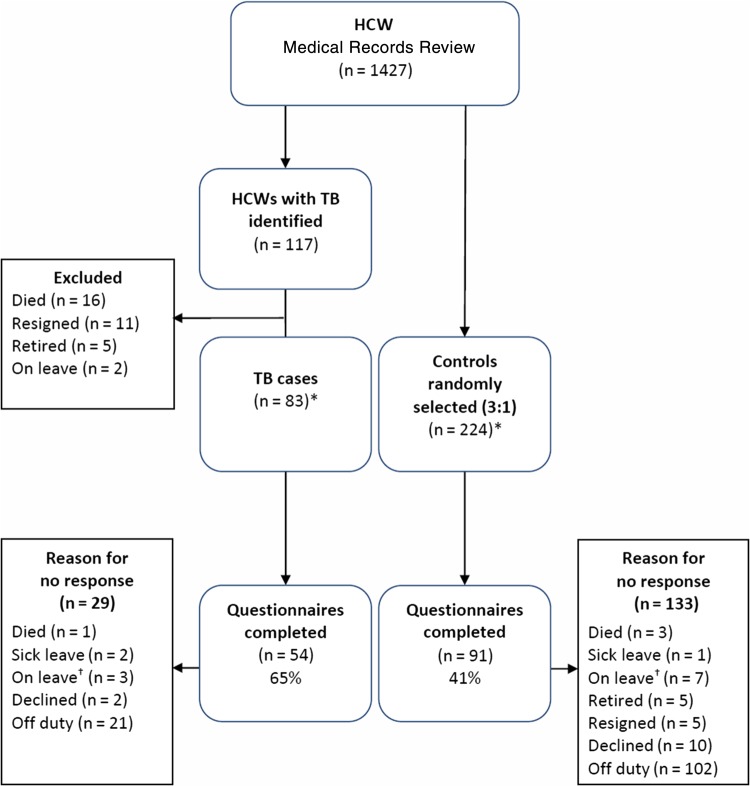
Of the 1427 occupational health employee medical records reviewed, 117 (8%) cases of tuberculosis during the period January 2006 to December 2010 were identified (Figure 1). Of these 117 cases, 83 were still alive and working in the hospitals at the time of this study (July 2011–January 2012). Of the 307 questionnaires distributed to 83 cases and 224 randomly selected controls without a documented case of tuberculosis during the same 5-year period of interest, 145 (47%) questionnaires were returned: 54 (37%) from cases and 91 (63%) from controls. Demographic characteristics of HCWs who responded and those who did not respond to the questionnaire were similar (Table 1). Cases were more likely to respond than controls (54/83 [65%] vs 91/224 [41%], respectively; P < .01). Responders had worked in a hospital longer than nonresponders (P = .02), and responders were more likely to be HIV infected than nonresponders (18/145 [12%] vs 11/162 [7%]), respectively (P = .04).
Figure 1.
Case-control profile for 3 district hospitals in KwaZulu-Natal, South Africa (January 2006–December 2010). *Misclassifications: 1 case misclassified as a control and 1 control misclassified as a case. †On leave refers to staff who were on extended leave not due to illness such as study leave. Abbreviations: HCW, healthcare worker; TB, tuberculosis.
Table 1.
Characteristics of Healthcare Workers Responding or Not Responding to Questionnaires From 3 District Hospitals in KwaZulu-Natal, South Africa (N = 307)
| Variable | Responders (n = 145) | Nonresponders (n = 162) | Total (N = 307) | P Value |
|---|---|---|---|---|
| Mean age (SD) (n = 298) | 42.94 (9.4) | 41.25 (10.1) | 42.07 (9.8) | .14a |
| Sex (n = 302) | ||||
| Female | 110 (78) | 133 (83) | 243 (81) | .22b |
| Race (n = 306) | ||||
| African/Black | 144 (99) | 158 (98) | 302 (99) | .15c |
| Asian | 0 | 3 (2) | 3 (1) | |
| White | 1 (1) | 0 | 1 (0.3) | |
| Occupation (n = 303)d | ||||
| Clinical | 81 (56) | 89 (56) | 170 (56) | .13b |
| Support | 40 (28) | 34 (22) | 74 (24) | |
| Paramedical | 14 (10) | 12 (8) | 26 (9) | |
| Administrative | 10 (7) | 23 (15) | 33 (11) | |
| Mean years of employment at hospital (n = 307) | 10.24 (7.7) | 8.29 (6.6) | 9.21 (7.2) | .02a |
| Case or control (n = 307) | ||||
| Case | 54 (37) | 29 (18) | 83 (27) | .00b |
| Control | 91 (63) | 133 (82) | 224 (73) | |
| HIV status (n = 307)e | ||||
| Uninfected | 32 (22) | 24 (15) | 56 (18) | .04b |
| Infected | 18 (12) | 11 (7) | 29 (10) | |
| Status unknown | 95 (66) | 127 (78) | 222 (72) | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
a Student t test.
b χ2 test.
c Fisher exact test.
d Clinical staff includes medical doctors/medical officers and nurses (professional nurse, enrolled nurse, and nursing assistant); support staff includes orderlies, cleaners, porters, groundsmen, and maintenance, kitchen, laundry, and security staff; paramedical staff includes lay counselors, radiographers, pharmacists, laboratory technicians, and mortuary attendants; and administrative staff includes administrative staff (finance, human resources, management), data capturers, and office/ward clerks.
e HIV status as documented in the occupational health medical record.
Of the 145 HCWs who responded to the questionnaire, demographic characteristics were similar for cases and controls (Table 2). Overall, 81 of 145 participants (56%) were clinical staff, and almost all worked in a nursing role. All 25 cases among clinical staff worked as nurses. The majority of cases and controls worked in general wards (51% [n = 74]), tuberculosis wards (37% [n = 53]), and pediatric wards (35% [n = 50]), but there was no significant difference between cases and controls. Due to small sample sizes in specific wards, we collapsed wards into risk areas (high, intermediate, and low), and there was no significant difference between cases and controls (P = .86). The majority of HCWs reported having worked in >1 ward 56% (n = 81) and ranged from 1 to 8 wards; this did not differ significantly between cases and controls (P = .53).
Table 2.
Characteristics of Healthcare Workers With or Without Tuberculosis in 3 District Hospitals, KwaZulu-Natal, South Africa (January 2006–December 2010) (N = 145)
| Variable | Cases: HCWs With TB (n = 54) |
Controls: HCWs Without TB (n = 91) |
Total (N = 145) |
P Value |
|---|---|---|---|---|
| Mean age, y (SD) (n = 139) | 42.14 (8.5) | 43.38 (9.9) | 42.92 (9.4) | .46a |
| Sex, female | 38 (70) | 72 (79) | 110 (76) | .23b |
| BMI, kg/m2 (n = 93) | ||||
| 18.5–24 | 7 (21) | 10 (17) | 17 (18) | .66b |
| 25–29 | 9 (27) | 21 (36) | 30 (32) | |
| ≥30 | 18 (53) | 28 (48) | 46 (50) | |
| Race | ||||
| African/Black | 54 (100) | 90 (99) | 144 (99) | .44c |
| White | 0 | 1 (1) | 1 (1) | |
| Occupation (n = 144)d | ||||
| Clinical | 25 (46) | 56 (62) | 81 (56) | .26c |
| Support | 19 (35) | 21 (23) | 40 (28) | |
| Paramedical | 6 (12) | 6 (7) | 12 (8) | |
| Administrative | 4 (7) | 7 (8) | 11 (8) | |
| HIV status (n = 125) | ||||
| Infected | 21 (45) | 11 (14) | 32 (26) | <.01c |
| Uninfected | 25 (53) | 64 (82) | 89 (71) | |
| Never tested | 1 (2) | 3 (4) | 4 (3) | |
| Mean years of employment at hospital (SD) (n = 143) | 12 (8.5) | 13 (8.5) | 12 (8.5) | .62a |
| Risk classification of area where HCW worked (n = 139) | ||||
| Low-risk | 6 (12) | 12 (14) | 18 (13) | .86b |
| Intermediate-risk | 8 (15) | 11 (13) | 19 (14) | |
| High-risk | 38 (73) | 64 (74) | 102 (73) | |
| No. of wards HCW worked in | ||||
| 1 | 22 (41) | 42 (46) | 64 (44) | .53b |
| >1 | 32 (59) | 49 (54) | 81 (56) | |
| HCW worked night shifts >50% of shifts (n = 137) | 20 (38) | 43 (51) | 63 (46) | .12b |
| Hours per day spent in area with TB patients (n = 129) | ||||
| 0 | 4 (8) | 14 (18) | 18 (14) | <.01b |
| 1–8 | 37 (71) | 33 (43) | 70 (54) | |
| >8 | 11 (21) | 30 (39) | 41 (32) | |
| Mean household crowding (SD) (n = 141) | 1.50 (1.3) | 1.11 (0.7) | 1.26 (1) | .02a |
| Household member with TB (n = 127) | 18 (36) | 22 (29) | 40 (32) | .38b |
| Ever smoked (n = 141) | 6 (11) | 6 (7) | 12 (9) | .38b |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: BMI, body mass index; HCW, healthcare worker; HIV, human immunodeficiency virus; SD, standard deviation; TB, tuberculosis.
a Student t test.
b χ2 test.
c Fisher exact test.
d Clinical staff includes medical doctors/medical officers and nurses (professional nurse, enrolled nurse, and nursing assistant); support staff includes orderlies, cleaners, porters, groundsmen, and maintenance, kitchen, laundry, and security staff; paramedical staff includes lay counselors, radiographers, pharmacists, laboratory technicians, and mortuary attendants; and administrative staff includes administrative staff (finance, human resources, management), data capturers, and office/ward clerks.
Eight of 11 (73%) cases who had drug-resistant tuberculosis and were still working in the hospitals responded to the questionnaire—7 with MDR tuberculosis and 1 with XDR tuberculosis. The majority of these cases (6/8 [75%]) were primary cases (ie, no prior history of tuberculosis or tuberculosis treatment). Three of 8 (38%) cases with drug-resistant tuberculosis self-reported they were HIV infected.
Cases were significantly more likely than controls to be HIV infected (21/54 [45%] vs 11/91 [14%], respectively; P < .01). Time spent in areas with tuberculosis patients varied significantly between cases and controls, with cases more likely than controls to spend ≤8 hours with tuberculosis patients (P < .01). Median household crowding was significantly greater among cases (1.2; interquartile range [IQR], 0.8–1.7) than controls (1.1; IQR, 0.7–1.4) (P = .02). A slightly higher proportion of cases than controls (18/54 [36%] vs 22/91 [29%]) had a household contact with tuberculosis; however, this was not statistically significant (P = .38).
Only 93 (64%) subjects provided information on weight and height, so BMI could not be calculated on all participants. We therefore used weight as a proxy indicator for BMI, as weight was available for 137 (95%) subjects. There was no significant association of weight with tuberculosis disease (P = .52).
The final multivariate logistic regression model included variables for HIV status, occupation (clinical vs nonclinical), hours per day spent with tuberculosis patients (0–8 hours vs >8 hours), household crowding, and number of wards an HCW worked in (1 vs >1) over the study period (Table 3). HIV (odds ratio [OR], 6.35; 95% CI, 3.54–11.37) and time spent working in areas with tuberculosis patients (OR, 0.45; 95% CI, .28–.71) were the only significant independent risk factors for tuberculosis in adjusted analysis. Household crowding was significant in unadjusted analysis (OR, 1.56; 95% CI, 1.02–2.38), but did not remain significant in the final model.
Table 3.
Risk Factors for Tuberculosis Among Healthcare Workers in 3 District Hospitals in KwaZulu-Natal, South Africa (January 2006–December 2010) (n = 106)
| Variable | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|
| HIV status | ||
| Uninfected | Referent | Referent |
| Infected | 4.92 (2.08–11.61) | 6.35 (3.54–11.37) |
| Occupation | ||
| Nonclinical | Referent | Referent |
| Clinical | 0.52 (.26–1.04) | 0.54 (.77–3.73) |
| Household crowdingb | 1.56 (1.02–2.38) | 1.55 (.30–8.03) |
| Hours per day spent with TB patients | ||
| 0–8 | Referent | Referent |
| >8 | 0.42 (.19–.94) | 0.45 (.28–.71) |
| No. of wards worked in | ||
| 1 | Referent | Referent |
| >1 | 1.24 (.62–2.50) | 0.99 (.67–1.46) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; TB, tuberculosis.
a This model was clustered by hospital to account for correlation among healthcare workers attributable to working in the same hospital [24].
b Continuous variable.
DISCUSSION
In this sample of hospital-based HCWs in KwaZulu-Natal, South Africa, HIV infection was the strongest independent risk factor for active tuberculosis disease. This finding supports previous findings from a sample of HCWs in Kenya [18]. HIV is a well-known risk factor for tuberculosis, especially in persons with latent tuberculosis or who have been newly infected with tuberculosis. Furthermore, it is estimated that the risk for tuberculosis is up to >20 times greater in those living with HIV, thus placing HCWs living with HIV at a much greater risk for active tuberculosis [26, 27]. Our study adds further evidence and supports the urgent implementation of measures to improve the occupational safety of HCWs—particularly for those living with HIV—such as HIV counseling and testing, ART, and isoniazid preventive therapy. Better infection prevention and control measures are also warranted to prevent transmission of tuberculosis in healthcare settings.
Three previous case-control studies have examined risk factors for tuberculosis among HCWs, one each in Kenya, Malaysia, and India [18–20], with only 2 common risk factors identified across these studies: time spent with patients and work location [18, 20]. We also identified time spent with tuberculosis patients as an independent risk factor for tuberculosis. However, unlike these other studies, we found that HCWs who spent ≤8 hours per day with tuberculosis patients had 2-fold odds of developing tuberculosis compared with HCWs who spent >8 hours with tuberculosis patients. The majority of those spending ≤8 hours per day with tuberculosis patients were clinical staff, so this increased risk may be due to closer or more frequent contact with patients. Additionally, the majority (68%) of respondents reported working ≤8 hours with patients; this may be due to working an 8-hour shift, which could explain why we did not find an increase in risk of tuberculosis with an increase in time spent with patients. Galgalo and colleagues found an increased risk for tuberculosis among HCWs spending ≥5 hours per day with patients in Kenya [18]. Additionally, a case-control study from India identified 2-fold odds of tuberculosis among HCWs with frequent contact with patients [20]. However, we did not find a significant difference between where HCWs worked and the risk for tuberculosis among our sample. This is likely due to the small sample size in our study and equal proportions of cases and controls working in high-risk areas.
Other investigators have found higher rates of tuberculosis among certain HCW occupations, but we were unable to demonstrate a difference by job category or profession. Mathew and colleagues from India found a >2-fold greater odds of tuberculosis among various levels of nursing cadre than among other occupations [20]. Although the majority of cases of active tuberculosis in our sample were among nursing staff (46%), their risk for tuberculosis did not differ significantly from other cadre of staff.
Due to the high tuberculosis incidence in the communities where this study took place, it is possible that HCWs may have been exposed to tuberculosis in the community, which we were unable to assess. However, under a separate aim of this study, we compared tuberculosis incidence rates in the larger sample with the general population and found that HCWs had approximately a 2-fold greater incidence of tuberculosis than the general population in KwaZulu-Natal and the districts where the study hospitals were located [16]. This supports findings from other studies that HCWs, despite high levels of tuberculosis in the community, have greater risk of developing tuberculosis than the general population [28]. These findings strongly suggest that infection prevention and control measures and occupational health services are not completely effective at preventing transmission and protecting HCWs in the workplace.
There are several limitations to this study. Responses to the questionnaire were self-reported, which may have led to socially desirable responses. However, it is unlikely to have happened to a different degree in cases and controls, reducing the possibility of bias. In addition, cases in this sample were significantly more likely to respond than controls, which may have resulted in selection bias (65% of cases vs 41% of controls). The overall response rate was low (47%); however, this is similar to the response rate (54%) from another case-control study conducted in Kenya [18]. Twenty (14%) HCWs did not report their HIV status, a main variable of interest in this study; as a result, we may have underestimated the prevalence of HIV infection among this sample. More than half of all HCWs (56%), especially clinical staff, reported working in >1 ward during the study period, thereby making it difficult to pinpoint where tuberculosis exposure occurred, although we controlled for this in the multivariate analysis. Finally, due to the relatively small sample size, there may be differences between cases and controls that we were unable to detect.
HIV infection and time spent with tuberculosis patients were the independent risk factors for tuberculosis among HCWs in this sample, as previously reported in the literature [18, 20]. These data support the need for improved occupational health services in healthcare settings, especially to provide HIV counseling and testing, ART, and isoniazid preventive therapy for HIV-infected HCWs, and to reassign HIV-infected HCWs to low-risk areas when possible. Further research and larger prospective studies are needed to evaluate occupational risk factors for tuberculosis among HCWs. Although we were not able to identify specific areas of the hospitals where HCWs were more likely to be exposed to tuberculosis, the implementation and maintenance of infection control measures and practices remain important in the protection of HCWs working in healthcare settings.
Notes
Acknowledgments. The authors thank the KwaZulu-Natal Department of Health. The authors also acknowledge the HCWs and occupational health nurses who participated in this study for their assistance, hard work, and enthusiasm.
Financial support. Funding for this study was provided by grants from the K-RITH (a collaboration of the Howard Hughes Medical Institute and the University of KwaZulu-Natal) Collaborative Grants Program; Sigma Theta Tau International Small Grants; and the South African Medical Research Council.
Supplement sponsorship. This article appears as part of the supplement “Healthcare Workers and Tuberculosis Prevention,” sponsored by Aeras.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Harries AD, Maher D, Nunn P. Practical and affordable measures for the protection of health care workers from tuberculosis in low-income countries. Bull World Health Organ 1997; 75:477–89. [PMC free article] [PubMed] [Google Scholar]
- 2.International Council of Nurses. Tuberculosis exposure in the health care setting: Prevention of occupational transmissions, 2009. Available at: http://www.icn.ch/images/stories/documents/publications/fact_sheets/4m_FS-TB_Exposure_HC_Setting.pdf. Accessed 9 February 2016.
- 3.World Health Organization. WHO Policy on TB infection control in health-care facilities, congregate settings and households, 2009. Available at: WHO/HTM/TB/2009.419: http://apps.who.int/iris/bitstream/10665/44148/1/9789241598323_eng.pdf. Accessed 9 February 2016. [PubMed]
- 4.Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 2007; 11:593–605. [PubMed] [Google Scholar]
- 5.World Health Organization. Tuberculosis: Fact sheet N°104, 2015. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/index.html. Accessed 9 February 2016.
- 6.World Health Organization. Global tuberculosis control 2011. Geneva, Switzerland, 2011. Available at: WHO/HTM/TB/2011.16. Accessed 17 February 2016.
- 7.Day C, Barron P, Massyn N, Padarath A, English R, editors. District health barometer 2010/2011. Durban, South Africa: Health Systems Trust; 2012. [Google Scholar]
- 8.Markowitz N, Hansen NI, Hopewell PC et al. . Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann Intern Med 1997; 126:123–32. [DOI] [PubMed] [Google Scholar]
- 9.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2010. Geneva, Switzerland, 2010 [cited February 2016]. Available at: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. Accessed 9 February 2016.
- 10.Gandhi NR, Moll A, Sturm AW et al. . Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006; 368:1575–80. [DOI] [PubMed] [Google Scholar]
- 11.Shisana O, Hall EJ, Maluleke R, Chauveau J, Schwabe C. HIV/AIDS prevalence among South African health workers. S Afr Med J 2004; 94:846–50. [PubMed] [Google Scholar]
- 12.Connelly D, Veriava Y, Roberts S et al. . Prevalence of HIV infection and median CD4 counts among health care workers in South Africa. S Afr Med J 2007; 97:115–20. [PubMed] [Google Scholar]
- 13.Sepkowitz KA. Tuberculosis and the health care worker: a historical perspective. Ann Intern Med 1994; 120:71–9. [DOI] [PubMed] [Google Scholar]
- 14.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis 2011; 17:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell MR, Jarand J, Loveday M et al. . High incidence of hospital admissions with multidrug-resistant and extensively drug-resistant tuberculosis among South African health care workers. Ann Intern Med 2010; 153:516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tudor C, Van der Walt M, Margot B et al. . Tuberculosis among health care workers in KwaZulu-Natal, South Africa: a retrospective cohort analysis. BMC Public Health 2014; 14:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med 2006; 3:e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galgalo T, Dalal S, Cain KP et al. . Tuberculosis risk among staff of a large public hospital in Kenya. Int J Tuberc Lung Dis 2008; 12:949–54. [PubMed] [Google Scholar]
- 19.Jelip J, Mathew GG, Yusin T et al. . Risk factors of tuberculosis among health care workers in Sabah, Malaysia. Tuberculosis (Edinb) 2004; 84:19–23. [DOI] [PubMed] [Google Scholar]
- 20.Mathew A, David T, Thomas K et al. . Risk factors for tuberculosis among health care workers in South India: a nested case-control study. J Clin Epidemiol 2013; 66:67–74. [DOI] [PubMed] [Google Scholar]
- 21.KwaZulu-Natal Province Department of Health. Provincial Hospital Contact Details, 2001. Available at: http://www.kznhealth.gov.za/hospitals.htm. Accessed 29 November 2015.
- 22.World Health Organization. The World Health Report 2006: Working together for health, 2006. Available at: http://www.who.int/whr/2006/whr06_en.pdf?ua=1. Accessed February 2016.
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000; 56:645–6. [DOI] [PubMed] [Google Scholar]
- 25.StataCorp LP. Stata statistical software: release 11. College Station, TX: StataCorp LP, 2009.
- 26.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 2010; 50(suppl 3):S201–7. [DOI] [PubMed] [Google Scholar]
- 27.Granich R, Akolo C, Gunneberg C, Getahun H, Williams P, Williams B. Prevention of tuberculosis in people living with HIV. Clin Infect Dis 2010; 50(suppl 3):S215–22. [DOI] [PubMed] [Google Scholar]
- 28.Naidoo S, Jinabhai CC. TB in health care workers in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis 2006; 10:676–82. [PubMed] [Google Scholar]