Abstract
IMPORTANCE
Treatments for symptomatic intracerebral hemorrhage (sICH) are based on expert opinion, with limited data available on efficacy.
OBJECTIVE
To better understand the natural history of thrombolysis-related sICH, with a focus on the efficacy of various treatments used.
DESIGN, SETTING, AND PARTICIPANTS
Multicenter retrospective study between January 1, 2009, and April 30, 2014, at 10 primary and comprehensive stroke centers across the United States. Participants were all patients with sICH, using the definition by the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST), which included a parenchymal hematoma type 2 and at least a 4-point increase in the National Institutes of Health Stroke Scale score.
MAIN OUTCOMES AND MEASURES
The primary outcome was in-hospital mortality, and the secondary outcome was hematoma expansion, defined as a 33% increase in the hematoma volume on follow-up imaging.
RESULTS
Of 3894 patients treated with intravenous recombinant tissue plasminogen activator (rtPA) within 4½ hours after symptom onset of ischemic stroke, 128 (3.3%) had sICH. The median time from initiation of rtPA therapy to sICH diagnosis was 470 minutes (range, 30–2572 minutes), and the median time from diagnosis to treatment of sICH was 112 minutes (range, 12–628 minutes). The in-hospital mortality rate was 52.3% (67 of 128), and 26.8% (22 of 82) had hematoma expansion. In the multivariable models, code status change to comfort measures after sICH diagnosis was the sole factor associated with increased in-hospital mortality (odds ratio, 3.6; 95% CI, 1.2–10.6). Severe hypofibrinogenemia (fibrinogen level, <150 mg/dL) was associated with hematoma expansion, occurring in 36.3% (8 of 22) of patients without hematoma expansion vs in 25.0% (15 of 60) of patients with hematoma expansion (P = .01), highlighting a role for cryoprecipitate in reversing rtPA coagulopathy.
CONCLUSIONS AND RELEVANCE
In this study, treatment of postthrombolysis sICH did not significantly reduce the likelihood of in-hospital mortality or hematoma expansion. Shortening the time to diagnosis and treatment may be a key variable in improving outcomes of patients with sICH.
Intravenous thrombolytic therapy with recombinant tissue plasminogen activator (rtPA) improves the outcome of patients with ischemic stroke treated within 4½ hours after symptom onset.1 The most serious complication is symptomatic intracerebral hemorrhage (sICH), which was reported in 6% of patients with acute ischemic stroke in the National Institute of Neurological Disorders and Stroke rtPA trials.2 Despite its infrequent occurrence, sICH is associated with significant morbidity and a high mortality rate approaching 50%.2 Recommendations by the American Heart Association and the American Stroke Association for the management of sICH are to replace coagulation factors with cryoprecipitate and to transfuse with platelets.1 While prior studies3–7 have investigated clinical and imaging risk factors for sICH, to our knowledge, the efficacy of these treatment recommendations has been examined in few investigations, which have been largely single-center studies.8 Prior evidence has shown substantial variability in treatment practices and limited success in halting sICH.8 These studies are limited by small sample sizes and incomplete accounting for confounders, such as code status change after sICH diagnosis. Therefore, we pooled contemporary data on treatment practices across several high-volume stroke centers in the United States with the objective to study the natural history of thrombolysis-related sICH, with a particular focus on the potential efficacy of various treatments. We also sought to identify key variables in the acute stroke treatment period that contributed to a high in-hospital mortality rate.
Methods
Study Population
This was a multicenter retrospective study of sICH after intravenous rtPA treatment at 10 US primary and comprehensive stroke centers (Columbia University Medical Center, Cleveland Clinic Foundation, Washington University in St Louis, University of Arkansas for Medical Sciences, Tulane University, The University of Alabama at Birmingham, UCLA [University of California, Los Angeles], Hospital of the University of Pennsylvania, University of Oklahoma, and Massachusetts General Hospital) (Figure 1). Patients with sICH based on the National Institute of Neurological Disorders and Stroke definition were identified via local Get With the Guidelines stroke databases at each center. We identified patients using the comprehensive National Institute of Neurological Disorders and Stroke definition and only included patients who met the criteria for the Safe Implementation of Thrombolysis in Stroke-Monitoring Study9 (SITS-MOST) definition. Confirmatory and additional data were obtained directly from hospital patient records, including hematoma characteristics, the time to detection and treatment, and in-hospital mortality. We reviewed the medical records of all patients identified and included only patients with sICH, using the definition by the SITS-MOST. This definition includes the presence of a parenchymal hematoma accounting for more than one-third of the infarct volume (parenchymal hematoma type 2) on head computed tomography (CT) and at least a 4-point increase from the baseline National Institutes of Health Stroke Scale (NIHSS) score. In prior investigations, the presence of a parenchymal hematoma type 2 has correlated well with mortality and poor outcome.10 The primary outcome herein was in-hospital mortality, and posthospitalization outcomes were not available at most centers. The secondary outcome was hematoma expansion, defined as a 33% increase in the hematoma volume on follow-up imaging.11 The primary predictor of interest was the receipt of any type of treatment for sICH.
Figure 1. Patient Flowchart.
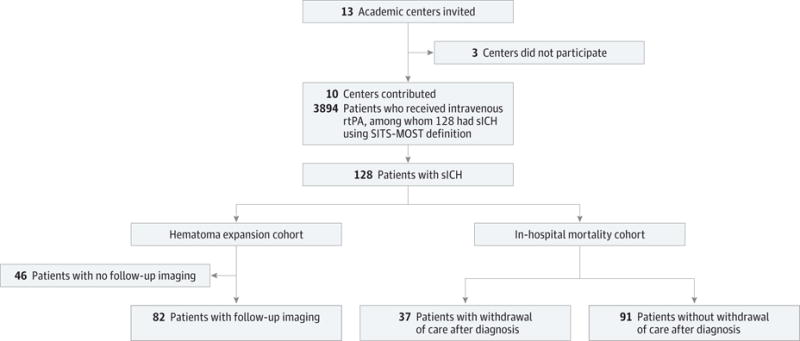
rtPA indicates recombinant tissue plasminogen activator; sICH, symptomatic intracerebral hemorrhage; and SITS-MOST, Safe Implementation of Thrombolysis in Stroke-Monitoring Study.
Additional variables were examined as predictors of in-hospital mortality and hematoma expansion. First were pretreatment factors, including demographics (age, race/ethnicity, and sex), stroke risk factors (hypertension, diabetes mellitus, hyperlipidemia, congestive heart failure, history of cancer, and renal disease), antiplatelet or anticoagulant use before thrombolysis, admission NIHSS score, and code status change to comfort care measures after sICH diagnosis. Second were laboratory measures, including pretreatment blood glucose level, coagulation laboratory values after sICH diagnosis (plasma thromboplastin time and international normalized ratio), and fibrinogen level after sICH diagnosis (<150 vs ≥150 mg/dL12) (to convert fibrinogen level to micromoles per liter, multiply by 0.0294). Third were imaging characteristics, including the hematoma volume at the time of sICH diagnosis, calculated using the (A × B × C)/2 method,13 and the time from initiation of rtPA therapy to sICH diagnosis (ie, the time to CT showing hemorrhage). Fourth was the type of treatment used, including fresh frozen plasma, cryoprecipitate, vitamin K, platelet transfusion, recombinant factor VIIa, aminocaproic acid, and surgical decompressive craniotomy or hematoma evacuation. Local institutional review board approval was obtained at each of the participating centers.
Statistical Analysis
Statistical software (SAS, version 9.3; SAS Institute Inc) was used in a univariable analysis to examine the association between any treatment for sICH vs none with in-hospital mortality and with hematoma expansion. Factors used in the multivariable models for predicting in-hospital mortality included predictors that were statistically significant or had a suggested increased mortality (P < .10) in the univariable analysis. Variables included in the multivariable models for predicting hematoma expansion included those that were statistically significant in the univariable analysis. Given the limited data available to inform an expected effect size, a power calculation was not prespecified before conducting this study. Analyses were considered statistically significant at P < .05.
Results
In this retrospective analysis, we identified 3894 patients who received intravenous rtPA between January 1, 2009, and April 30, 2014, within 4½ hours of symptom onset of ischemic stroke. Among them, 128 patients (3.3%) had sICH. Figure 1 shows the flow of patients in the study. Their baseline characteristics are summarized in Table 1. The median age was 74 years. In total, 38.2% (49 of 128) received any treatment for sICH, and 28.9% (37 of 128) had code status change to comfort care measures within the first 24 hours after sICH diagnosis. The most commonly used treatment was cryoprecipitate (31.3% [40 of 128]). The median time from initiation of rtPA therapy to sICH diagnosis was 470 minutes (range, 30–2572 minutes), and the median time from sICH diagnosis to treatment of sICH was 112 minutes (range, 12–628 minutes). Those patients diagnosed as having sICH within the first 3 hours after symptom onset of ischemic stroke had significantly lower mean admission NIHSS scores than those receiving the diagnosis after 3 hours (median score, 11 vs 17; P = .01). The sICH diagnosis was made more than 2 hours after initiation of intravenous rtPA therapy in 85.9% (110 of 128) of patients.
Table 1.
Baseline Demographics and Clinical Characteristics of the 128 Patients
| Variable | Value |
|---|---|
| Center, No. (%) | |
| Columbia University Medical Center | 18 (14.1) |
| UCLA | 9 (7.0) |
| Washington University in St Louis | 18 (14.1) |
| Massachusetts General Hospital | 27 (21.1) |
| University of Arkansas for Medical Sciences | 16 (12.5) |
| Cleveland Clinic Foundation | 9 (7.0) |
| Hospital of the University of Pennsylvania | 5 (3.9) |
| University of Oklahoma | 4 (3.1) |
| Tulane University | 16 (12.5) |
| The University of Alabama at Birmingham | 6 (4.7) |
| Age, median (range), y | 74 (23–99) |
| Male sex, No. (%) | 65 (50.8) |
| Race/ethnicity, No. (%) | |
| White | 81 (63.2) |
| African American | 38 (29.7) |
| Hispanic | 7 (5.5) |
| Other | 2 (1.6) |
| Hypertension, No. (%) | 106 (82.8) |
| Diabetes mellitus, No. (%) | 38 (29.7) |
| Hyperlipidemia, No. (%) | 68 (53.3) |
| Prior stroke, No. (%) | 39 (30.5) |
| NIHSS score, median (range) | 15 (0–33) |
| ASPECTS score, median (range) | 10 (5–10) |
| Neurointerventional procedure, No. (%) | 18 (14.1) |
| Stroke subtype, No. (%) | |
| Small-vessel disease | 5 (4.1) |
| Large-vessel disease | 11 (9.0) |
| Cardioembolic | 57 (46.7) |
| Cryptogenic | 13 (10.7) |
| Other | 12 (9.8) |
| Hematoma volume, median (range), mL | 25 (1–245) |
| Time from initiation of rtPA therapy to sICH diagnosis, median (range), min | 470 (30–2572) |
| Time from sICH diagnosis to treatment of sICH, median (range), min | 112 (12–628) |
| Type of treatment, No. (%) | |
| Any treatment | 49 (38.2) |
| Fresh frozen plasma | 26 (20.3) |
| Vitamin K | 7 (5.5) |
| Cryoprecipitate | 40 (31.3) |
| Platelet transfusion | 37 (28.9) |
| Aminocaproic acid | 2 (1.6) |
| Prothrombin complex concentrate | 3 (2.3) |
| Recombinant factor VIIa | 3 (2.3) |
| Surgical decompressive craniotomy or hematoma evacuation | 18 (14.6) |
| Code status change to comfort measures in the first 24 h, No. (%) | 37 (28.9) |
| Hematoma expansion after diagnosis, No. (%) | 22 (26.8) |
| In-hospital mortality, No. (%) | 67 (52.3) |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT [Computed Tomographic] Score; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; sICH, symptomatic intracerebral hemorrhage; UCLA, University of California, Los Angeles.
In-Hospital Mortality
There was a nonsignificant suggestion of lower in-hospital mortality in patients receiving any treatment for sICH vs no treatment (47.5% [29 of 61] vs 29.9% [20 of 67], P = .06). The receipt of an endovascular neurointerventional procedure (20.9% [14 of 67] vs 6.6% [4 of 61], P = .02), a higher median hematoma volume (29 vs 16 mL, P = .02), and code status change to comfort measures in the first 24 hours (44.7% [30 of 67] vs 11.5% [7 of 61], P < .001) were the only statistically significant factors associated with a higher in-hospital mortality rate in the univariable analysis. The association between lower in-hospital mortality and surgical treatment vs nonsurgical treatment was nonsignificant (18.6% [11 of 61] vs 10.5% [7 of 67], P = .10). Other variables did not differ between the 2 groups of those who died and those who survived and are summarized in Table 2. In the multivariable logistic regression analyses, the only variable associated with increased in-hospital mortality was code status change to comfort measures in the first 24 hours after sICH diagnosis (odds ratio, 3.6; 95% CI, 1.2–10.6). A sensitivity analysis was performed that excluded patients with code status change to comfort measures in the first 24 hours, and the results remained unchanged.
Table 2.
Univariable Analysis of Clinical, Radiological, and Laboratory Predictors of In-Hospital Mortality and Hematoma Expansion
| Variable | In-Hospital Mortality Cohort | Hematoma Expansion Cohort | ||||
|---|---|---|---|---|---|---|
| No Mortality (n = 61) | Mortality (n = 67) | P Value | No Hematoma Expansion (n = 60) | Hematoma Expansion (n = 22) | P Value | |
| Age, median (range), y | 74 (23–99) | 75 (42–94) | .87 | 75 (23–94) | 66 (36–85) | .05 |
| Male sex, No. (%) | 27 (44.3) | 36 (53.7) | .28 | 30 (50.0) | 7 (31.8) | .07 |
| Black race/ethnicity, No. (%) | 17 (27.9) | 21 (31.3) | .51 | 18 (30.0) | 6 (27.2) | .98 |
| Hypertension, No. (%) | 47 (77.0) | 59 (88.1) | .11 | 46 (76.7) | 19 (86.4) | .54 |
| Diabetes mellitus, No. (%) | 15 (24.6) | 23 (34.3) | .25 | 15 (25.0) | 7 (31.8) | .58 |
| Hyperlipidemia, No. (%) | 30 (49.1) | 38 (56.7) | .48 | 33 (55.0) | 11 (50.0) | .80 |
| Prior stroke, No. (%) | 16 (26.2) | 23 (34.3) | .34 | 16 (26.7) | 7 (31.8) | .78 |
| Warfarin sodium use, No. (%) | 1 (1.6) | 4 (5.9) | .18 | 1 (1.7) | 2 (9.1) | .16 |
| ASPECTS score, median (range) | 10 (6–10) | 10 (5–10) | .67 | 10 (5–10) | 10 (5–10) | .57 |
| Neurointerventional procedure, No. (%) | 4 (6.6) | 14 (20.9) | .01 | 8 (13.3) | 4 (18.2) | .23 |
| Type of treatment, No. (%) | ||||||
| Vitamin K | 3 (4.9) | 4 (5.9) | .29 | 3 (5.0) | 2 (9.1) | .29 |
| Cryoprecipitate | 22 (36.1) | 18 (26.9) | .26 | 17 (28.3) | 10 (45.4) | .07 |
| Aminocaproic acid | 2 (3.3) | 0 | .23 | 2 (3.3) | 0 | .53 |
| Fresh frozen plasma | 11 (18.0) | 15 (22.4) | .54 | 11 (18.3) | 6 (27.3) | .16 |
| Prothrombin complex concentrate | 1 (1.6) | 2 (2.9) | .40 | 3 (5.0) | 0 | .39 |
| Recombinant factor VIIa | 1 (1.6) | 2 (2.9) | .40 | 2 (3.3) | 1 (4.6) | .44 |
| Platelet transfusion | 20 (32.8) | 17 (25.4) | .36 | 13 (21.7) | 11 (50.0) | .01 |
| Surgical decompressive craniotomy or hematoma evacuation | 11 (18.6) | 7 (10.9) | .10 | 6 (10.0) | 4 (18.2) | .17 |
| Any treatment | 29 (47.5) | 20 (29.9) | .11 | 30 (50.0) | 13 (59.1) | .47 |
| Code status change to comfort measures in the first 24 h, No. (%) | 7 (11.5) | 30 (44.8) | <.001 | 13 (21.7) | 4 (18.2) | .23 |
| NIHSS score, median (range) | 14 (0–28) | 15 (0–33) | .17 | 14 (3–33) | 14 (1–28) | .43 |
| Hematoma volume, median (range), mL | 16 (1–166) | 28.5 (1–245) | .02 | 23 (1–150) | 26 (1–245) | .87 |
| Fibrinogen level <150 mg/dL, No. (%) | 11 (21.1) | 17 (34.0) | .35 | 15 (25.0) | 8 (36.4) | .01 |
| Time from initiation of rtPA therapy to sICH diagnosis, median (range), min | 563 (40–2166) | 420 (30–2572) | .21 | 720 (30–2572) | 395 (38–1482) | .10 |
| Time from sICH diagnosis to treatment of sICH, median (range), min | 97 (12–419) | 120 (30–628) | .80 | 120 (42–419) | 90 (12–354) | .16 |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT [Computed Tomographic] Score; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; sICH, symptomatic intracerebral hemorrhage.
SI conversion factor: To convert fibrinogen level to micromoles per liter, multiply by 0.0294.
Hematoma Expansion
Follow-up CT was performed in 82 of 128 patients (64.1%). The baseline characteristics were similar between patients with and without follow-up CT (eTable in the Supplement). Hematoma expansion was observed in 22 of 82 patients (26.8%). In the univariable analysis, the receipt of any medical treatment vs no treatment was not associated with hematoma expansion (50.0% [30 of 60] vs 59.0% [13 of 22], P = .20). Patients with hematoma expansion were more likely to receive platelet transfusion (50.0% [11 of 22] vs 21.6% [13 of 60], P = .01) and have severe hypofibrinogenemia (fibrinogen level, <150 mg/dL) (36.3% [8 of 22] vs 25.0% [15 of 60], P = .01). Other clinical and radiographic factors were not statistically significant and are summarized in Table 2. In the multivariable models, none of the factors were associated with hematoma expansion. Figure 2 shows the CT scan of a patient with sICH whose hematoma expansion continued despite treatment with cryoprecipitate.
Figure 2. Patient With Thrombolysis-Related Symptomatic Intracerebral Hemorrhage.
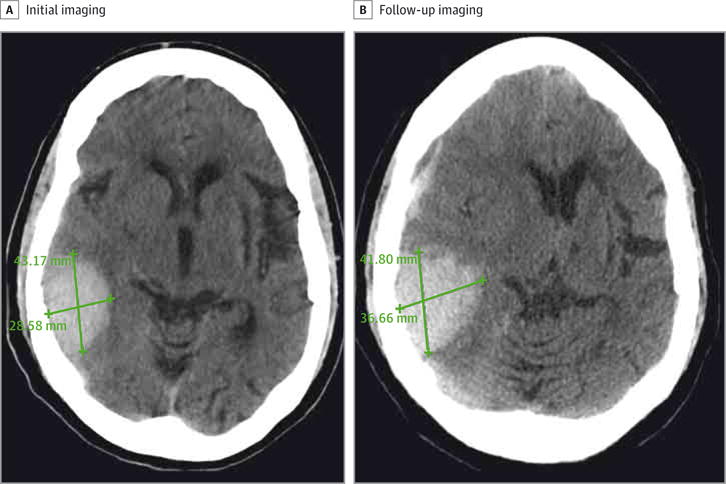
Hematoma expansion was noted on follow-up imaging despite treatment with cryoprecipitate.
Discussion
In this study, we report treatment and associated outcome of patients with thrombolysis-related hemorrhage across 10 stroke centers in the United States. As expected, most sICH occurred in patients with cardioembolic stroke. Various hemostatic agents were used to treat sICH. We found significant delays in the time to diagnosis and treatment for sICH, which could explain the lack of efficacy of these treatments.
The time from initiation of rtPA therapy to sICH diagnosis was long, particularly among patients with a high admission NIHSS score. This likely reflects a “ceiling effect” for the scale.14 Our results support the use of sICH prediction scores3–5 to screen patients with a high NIHSS score, which may help reduce the time to diagnosis and treatment. In our cohort, 85.9% (110 of 128) of patients were diagnosed as having sICH more than 2 hours after thrombolysis, the time after which standard postthrombolysis care protocols recommend less frequent neurological assessments.1 To detect sICH earlier, additional neurological assessments should be performed more frequently, up to 12 hours after rtPA administration. The time from sICH detection to treatment was also long (median, 1.9 hours), and hematoma expansion is expected to typically occur in the early hours after the onset of hemorrhage,15 which presents opportunities for protocols that administer hemostatic treatment earlier and for systematic measurement of these timelines as part of quality improvement initiatives at the hospital level. With earlier and aggressive treatment, reversal of coagulopathy might be achieved before hematoma expansion occurs, which may be in line with research findings in patients with anticoagulant-related intracerebral hemorrhage.16 The association herein between hypofibrinogenemia and hematoma expansion suggests a role for cryoprecipitate in reversing coagulopathy in patients with rtPA-related hemorrhage. Although rtPA has a short half-life, the coagulopathy it produces by lowering fibrinogen levels may be maintained up to 24 hours after infusion.14 A decrease in fibrinogen level by more than 200 mg/dL from baseline at 6 hours after rtPA infusion is associated with sICH.14 Given that 10 U of cryoprecipitate increases fibrinogen levels by 50 to 80 mg/dL, administration of this standard dose may have resulted in underdosing and could partially explain the lack of efficacy in preventing hematoma expansion in our sample. Therefore, it may be reasonable to initiate empirical treatment with cryoprecipitate once sICH is diagnosed, before obtaining fibrinogen levels, and additional cryoprecipitate treatment can be given as needed once the fibrinogen level is known.14
In our study, we found that diverse treatments were used, which may have contributed to our neutral results. Hemostatic agents used at the various centers included vitamin K, fresh frozen plasma, cryoprecipitate, prothrombin complex concentrate, aminocaproic acid, and recombinant factor VIIa. Surgery (decompressive craniotomy or hematoma evacuation) was the only treatment associated with reduced in-hospital mortality in our sICH cohort, although the association was nonsignificant. This finding was previously noted in an acute myocardial infarction study,17 in which among patients treated with thrombolytic therapy the outcome of patients with thrombolysis-related intracerebral hemorrhage improved after surgical treatment as opposed to nonsurgical treatment. Surgical intervention may be beneficial in a small proportion of patients with spontaneous intracerebral hemorrhage,18 and parallels could be drawn to sICH. However, the benefit of surgery may simply be a reduction in mortality, leaving survivors with substantial morbidity and disability.
Our study has several limitations, including its retrospective nature and wide variations in the management and treatment protocols used by participating centers. Also, because few patients received each of the sICH treatments, it is possible that the effects of therapy may be underestimated. However, this trial is the first multicenter study, to our knowledge, that examines real-world treatments used by comprehensive stroke centers and includes code status change to comfort measures in the analysis. While our analysis included several confounders of outcome (eg, admission NIHSS score, Alberta Stroke Program Early CT Score [ASPECTS] score, and code status change to comfort measures after sICH diagnosis), we lacked data on other potential confounders, such as adequate blood pressure control and certain neuroimaging findings.
Conclusions
There is significant room for improvement in reducing the time from initiation of rtPA therapy to sICH diagnosis and the time to treatment of sICH. Despite the potential thrombotic complications of more aggressive treatments, agents for reversal of coagulopathy with a high efficacy and short onset of action should be studied to help improve the mortality and morbidity of sICH. No current treatments factor in the contribution of a breakdown of the blood-brain barrier in acute ischemic stroke, an important gap in treatment considerations.19 Collaborative efforts across disciplines and institutions are needed to identify the reversal agents that can lead to improved outcome in patients with postthrombolysis hemorrhage. Larger multicenter prospective studies may help generate an algorithm to screen and diagnose high-risk patients earlier and more aggressively reverse the coagulopathy induced by rtPA when sICH occurs.
Supplementary Material
Footnotes
Supplemental content at jamaneurology.com
Author Contributions: Dr Yaghi and Ms Boehme had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acquisition, analysis, or interpretation of data: Boehme, Dibu, Leon Guerrero, Ali, Martin-Schild, Sands, Noorian, Blum, Chaudhary.
Drafting of the manuscript: Yaghi.
Critical revision of the manuscript for important intellectual content: Dibu, Leon Guerrero, Ali, Martin-Schild, Sands, Noorian, Blum, Chaudhary, Schwamm, Liebeskind, Marshall, Willey.
Administrative, technical, or material support: Yaghi.
Conflict of Interest Disclosures: Dr Yaghi reported receiving funding from the Stroke Trials Network (StrokeNet) of the National Institute of Neurological Disorders and Stroke. Dr Liebeskind reported being a consultant for Stryker and Covidien. Dr Willey reported being a consultant for HeartWare Inc. No other disclosures were reported.
Additional Contributions: Salah G. Keyrouz, MD (Washington University in St Louis), Archana Hinduja, MD (University of Arkansas for Medical Sciences), Nicolas Bianchi, MD (University of Arkansas for Medical Sciences), Brett Cucchiara, MD (Hospital of the University of Pennsylvania), and Joao Gomes, MD (Cleveland Clinic Foundation) (all in the Department of Neurology at their respective institutions) collaborated in the study.
References
- 1.Jauch EC, Saver JL, Adams HP, Jr, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17(6):331–333. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Lou M, Safdar A, Mehdiratta M, et al. The HAT score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71(18):1417–1423. doi: 10.1212/01.wnl.0000330297.58334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazya M, Egido JA, Ford GA, et al. SITS Investigators Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43(6):1524–1531. doi: 10.1161/STROKEAHA.111.644815. [DOI] [PubMed] [Google Scholar]
- 6.McMeekin P, Flynn D, Ford GA, Rodgers H, Thomson RG. Validating the stroke-thrombolytic predictive instrument in a population in the United kingdom. Stroke. 2012;43(12):3378–3381. doi: 10.1161/STROKEAHA.112.671073. [DOI] [PubMed] [Google Scholar]
- 7.Strbian D, Engelter S, Michel P, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71(5):634–641. doi: 10.1002/ana.23546. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JN, Marrero M, Masrur S, et al. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch Neurol. 2010;67(8):965–969. doi: 10.1001/archneurol.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 10.Gumbinger C, Gruschka P, Böttinger M, et al. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke. 2012;43(1):240–242. doi: 10.1161/STROKEAHA.111.623033. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CS, Huang Y, Wang JG, et al. INTERACT Investigators Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol. 2014;71(9):1181–1185. doi: 10.1001/jamaneurol.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313(8):824–836. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 17.Mahaffey KW, Granger CB, Sloan MA, et al. GUSTO Investigators Neurosurgical evacuation of intracranial hemorrhage after thrombolytic therapy for acute myocardial infarction: experience from the GUSTO-I trial. Am Heart J. 1999;138(3, pt 1):493–499. doi: 10.1016/s0002-8703(99)70152-3. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill JC, III, Greenberg SM, Anderson CS, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Fan W, Cai P, et al. Recombinant ADAMTS13 reduces tissue plasminogen activator-induced hemorrhage after stroke in mice. Ann Neurol. 2013;73(2):189–198. doi: 10.1002/ana.23762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


