Abstract
Importance
The long-term impact of axillary pathologic complete response (pCR) on survival among women treated with primary systemic chemotherapy (PST) is unknown.
Objective
To assess the long-term impact of axillary pCR on relapse-free and overall survival.
Design
We retrospectively analyzed the impact of axillary pCR on 10-year overall (OS) and relapse-free survival (RFS) among women treated with PST at one institution. Women were stratified by post-PST axillary status, and survival outcomes were estimated and compared according to response in the breast and axilla.
Setting
A retrospective analysis of women who received neoadjuvant chemotherapy in a large U.S. comprehensive cancer center.
Participants
All women (N=1600) diagnosed with breast cancer stages II to III with cytologically confirmed axillary metastases between 1989 and 2007 who received PST at our institution were included.
Main Outcome Measure
Outcomes of interest were relapse-free and overall survival.
Results
Of 1600 women treated, 454 (28.4%) achieved axillary pCR. These patients were more likely to have HER2-positive and triple-negative disease, high-grade tumors, and lower clinical and pathologic T stage. Ten-year OS rates were 84% and 57% (P<.001) and 10-year RFS rates 79% and 50% (P<.001) for patients with axillary pCR and residual axillary disease, respectively. For patients with axillary pCR, 10-year OS rates were 90% for those with breast pCR and 72% for those with residual breast disease (P<.001). For patients with residual axillary disease, 10-year OS rates were 66% for patients with and 56% for patients without breast pCR (P=.02). Of patients receiving HER2-targeted therapy for HER2-positive disease, 67.1% (100/149) achieved axillary pCR; 10-year OS rates were 92% and 57% (P=.006) and 10-year RFS rates 89% and 44% (P<.001) for those with axillary pCR and residual axillary disease, respectively.
Conclusion and Relevance
Axillary pCR is associated with improved 10-year OS and RFS. Patients with axillary and breast pCR after PST have superior long-term survival outcomes. Patients undergoing HER2-targeted therapy for HER2-positive disease had high rates of axillary pCR, and those with axillary pCR had excellent 10-year overall survival.
Background
For patients with large operable and locally advanced breast cancer, primary systemic chemotherapy (PST) is currently the standard of care. PST has been shown to be equivalent to adjuvant chemotherapy in terms of overall survival (OS) and relapse-free survival (RFS),1,2 and use of PST also increases the rates of breast conservation surgery. PST has an additional advantage in that it allows response to be assessed clinically in vivo throughout treatment and pathologically after surgery. Several studies have shown that patients treated with PST who achieve a pathologic complete response (pCR) in the breast and axilla have superior long-term outcomes.2–4
The presence of axillary lymph node metastases at diagnosis is an important prognostic factor in breast cancer. A prior study from our institution examined 403 patients with cytologically confirmed axillary metastases, 89 of whom achieved an axillary pCR after systemic therapy. Patients who achieved an axillary pCR had significantly improved 5-year OS and RFS relative to patients who did not.4
Over the past several years, more data have emerged regarding the rates of pCR in the breast and axilla in different breast cancer subtypes after PST with different chemotherapy backbones. Of particular interest is the response to HER2-targeted therapy in HER2-positive breast cancers. There have been several reports of high pCR rates, ranging from 40% to 74%, with HER2-targeted therapy plus cytotoxic chemotherapy.5–7
Previous studies have shown that pCR in the axillary nodes has a greater impact on survival than the response to chemotherapy in the primary breast tumor.4,7,8 A prior study from our group showed that among patients with axillary pCR, 5-year RFS did not differ between patients with breast pCR and those with residual disease in the breast, with the hypothesis that residual tumor cells in the breast do not have metastatic potential.4
In the study reported here, we sought to determine the impact of axillary pCR on 10-year OS and RFS in patients with breast cancer and cytologically confirmed axillary metastases at diagnosis. This study is an expansion of the prior study from our group4 and includes all patients with stage II-III breast cancer with cytologically confirmed axillary metastases treated with PST at one institution.
Methods
We performed a retrospective review of the Breast Medical Oncology Institutional database at The University of Texas MD Anderson Cancer Center. All patients diagnosed between 1989 and 2007 with clinical stage II–III breast cancer, histologically confirmed axillary metastases, and who underwent PST, were included in the analysis. Patients were categorized as having axillary pCR or residual axillary disease at the time of surgery. Data on relevant prognostic factors were abstracted, including age at diagnosis, race/ethnic group (determined by self-report on patient questionnaire), number of lymph nodes removed at surgery, breast cancer subtype (by hormone receptor [HR] and HER2 status), tumor and lymph node clinical and pathologic staging (according to the American Joint Committee on Cancer [AJCC]/International Union Against Cancer [UICC] TNM staging classification) nuclear grade, adjuvant therapies (additional chemotherapy, radiation, and endocrine therapy), and class of PST utilized (anthracycline-based only, taxane-based only, or containing both an anthracycline and a taxane). Breast pCR (T0 disease) was defined as no residual invasive carcinoma; residual intraductal carcinoma alone was classified as T0. Race and ethnicity were collected by self-report by questionnaire at the time of new patient visit and are included to demonstrate the diversity of patients seen at our institution. The institutional review board of The University of Texas MD Anderson Cancer Center approved this study.
Patient characteristics were summarized, and the axillary pCR and residual axillary disease groups were compared using chi-square test or t-test, as appropriate. OS was measured from the date of definitive surgery (after the completion of PST) to the date of death or last follow-up. RFS was measured from the date of definitive surgery (after the completion of PST) to the date of first documented local or distant recurrence or last follow-up, or to death, whichever came first. The Kaplan-Meier product-limit method was used to estimate the survival outcomes of patients by groups; groups were compared using the log-rank statistic. Subset analyses were carried out within the group of patients with HER2-positive breast cancers who received HER2-targeted therapy.
Cox proportional hazards models were fit to determine the association of axillary pCR or residual axillary tumor with survival outcomes after adjustment for other patient and disease characteristics. Variables that were significant on univariate log-rank test were candidates for the multivariable models. A backward selection was used to determine variables retained in the final models. Multivariate analysis was also performed within the subset of patients with HER2-positive tumors. Results are expressed in hazard ratios and 95% CIs. P values less than .05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.2 (SAS Institute, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc.).
Results
Patient Characteristics and Associations with pCR
A total of 1600 women with stage II–III breast cancer diagnosed between 1989 and 2007 were identified; 77% of the patients were treated between 2000 and 2007. Patient characteristics are summarized in eTable 1 in the Supplement. Four hundred fifty-four women (28.4%) achieved an axillary pCR and 1146 (71.6%) had residual axillary disease after PST. Of the clinicopathologic parameters examined, the factors associated with axillary pCR were triple-negative and HER2-positive breast cancer (P < .001), lower clinical and pathologic T stage (P = .002), pCR in the breast (P < .001), and higher grade (P < .001). Patients with clinical N3 disease had higher rates of axillary pCR than patients with N1 or N2 disease (P = 0.003). Two hundred eighty-five patients (18.6%) achieved a pCR in both the breast and axilla.
Nearly three-quarters of the 1600 patients in this analysis received PST that included both a taxane and an anthracycline; for these patients, the rate of axillary pCR was 32.0%. Axillary pCR rates were lower for patients treated with a taxane without an anthracycline (23.7%) or an anthracycline without a taxane (19%). Most patients (85%) underwent adjuvant radiation, and 57% of patients received adjuvant endocrine therapy.
HR and/or HER2 status was unknown for 254 patients. Among the remaining patients, rates of axillary pCR were 16.4% for HR-positive/HER2-negative tumors, 40.8% for HR-positive/HER2-positive tumors, 40.8% for HR-negative/HER2-negative tumors, and 55.2% for HR-negative/HER2-positive tumors. Rates of pCR in both breast and axilla were 7.3% for HR-positive/HER2-negative tumors, 28.8% for HR-positive/HER2-positive tumors, 28.7% for HR-negative/HER2-negative tumors, and 40.9% for HR-negative/HER2-positive tumors.
Three hundred thirty-eight patients (25.1%) had HER2-positive tumors; treatment regimen was known for 336 patients. Many of these patients were treated before the approval of trastuzumab in the adjuvant setting; only 149 patients (44.3%) received HER2-targeted therapy (predominantly trastuzumab; 2 patients received lapatinib on protocol). Among patients with HER2-positive tumors, 67.1% of patients who received HER2-targeted therapy achieved an axillary pCR, compared to 32.6% of those who did not receive HER2-targeted therapy (P < .001).
Survival Estimates
Median follow-up time among all women was 79 months (range, 5–277 months). At the time of this analysis, 480 women (30%) had died, and 546(34.1%) had experienced a recurrence. Of the 546 patients with a recurrence, 37 (6.8%) had locoregional recurrence only, 360 (65.9%) had distant relapse only, and 149 (27.3%) developed both locoregional and distant relapse.
Five and ten-year OS and RFS rates by patient characteristics are available in eTable 2 in the Supplement. For the overall cohort, the 5-year OS and RFS rates were 79% and 67%, respectively; the 10-year OS and RFS rates were 64% and 58%, respectively.
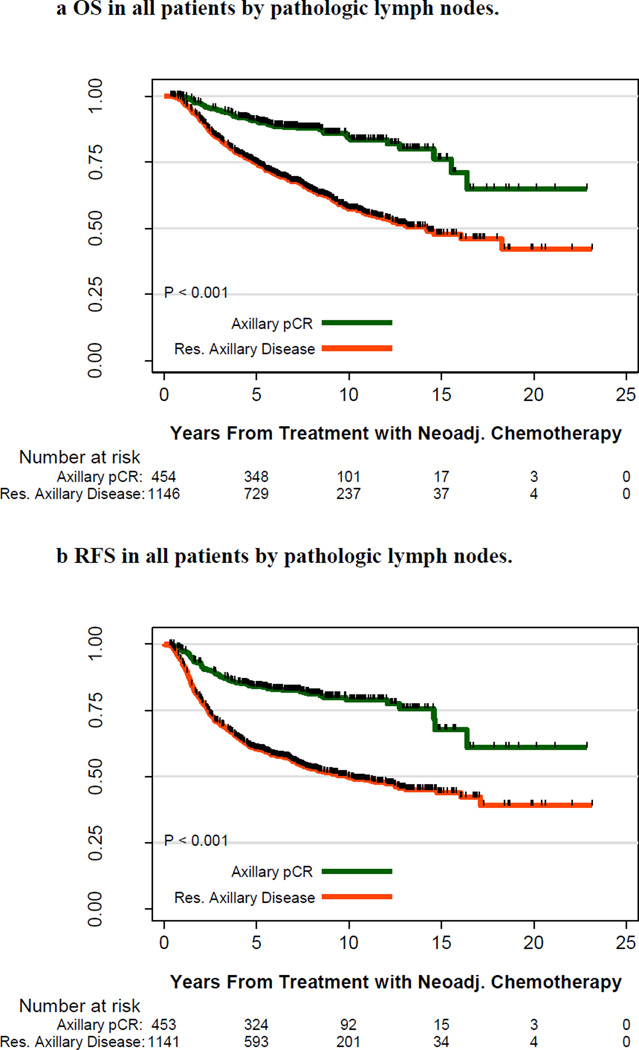
Kaplan–Meier OS and RFS curves by the disease status of the axilla after PST are shown in Figure 1. Five-year OS rates were 91% and 75%, respectively, for patients with axillary pCR and residual axillary disease; 10-year OS rates were 84% and 57%, respectively (P < .001). Five-year RFS rates were 84% and 60%, respectively, for patients with axillary pCR and residual axillary disease; 10-year RFS rates were 79% and 50%, respectively (P < .001).
Figure 1.
A. Overall Survival in all patients by pathologic lymph node status.
Overall survival in all patients stratified by response to primary systemic therapy (PST) in the axilla. Pathologic complete response (pCR) in the axilla is in green, and residual axillary disease is in red. The number of patients at risk is indicated under the x-axis.
B. Relapse Free Survival in all patients by pathologic lymph node status.
Relapse-free survival in all patients stratified by response to PST in the axilla. pCR in the axilla is in green, and residual axillary disease is in red. The number of patients at risk is indicated under the x-axis.
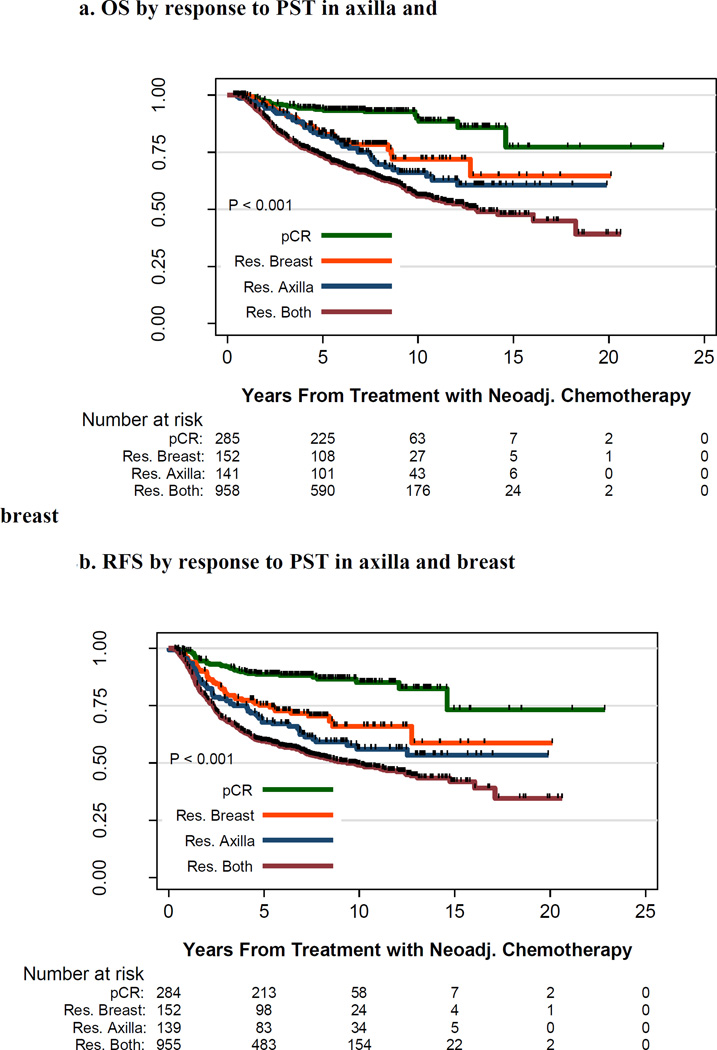
Kaplan–Meier OS and RFS curves by disease status of the axilla and the breast after PST are shown in Figure 2. Ten-year OS rates were 90% and 72%, respectively, for patients with axillary and breast pCR and patients with axillary pCR but residual breast disease (P < .001); 10-year RFS rates were 85% and 66%, respectively (P < .001). Ten-year OS rates were 66% and 56%, respectively, for patients with residual axillary disease but breast pCR and patients with residual axillary and breast disease (P < .02); 10-year RFS rates were 56% and 49%, respectively (P = .04).
Figure 2.
A. Overall Survival by pathologic response to PST in axilla and breast.
Overall survival in patients stratified by response to PST in the axilla and the breast. pCR in both the axilla and breast is in green, pCR in the axilla but residual disease in the breast is in red, residual disease in the axilla but pCR in the breast is in blue, and residual disease in both the breast and axilla is in black. The number of patients at risk is indicated under the x-axis.
B. Relapse Free Survival by pathologic response to PST in axilla and breast.
Relapse-free survival in patients stratified by response to PST in the axilla and the breast. pCR in both the axilla and breast is in green, pCR in the axilla but residual disease in the breast is in red, residual disease in the axilla but pCR in the breast is in blue, and residual disease in both the breast and axilla is in black. The number of patients at risk is indicated under the x-axis.
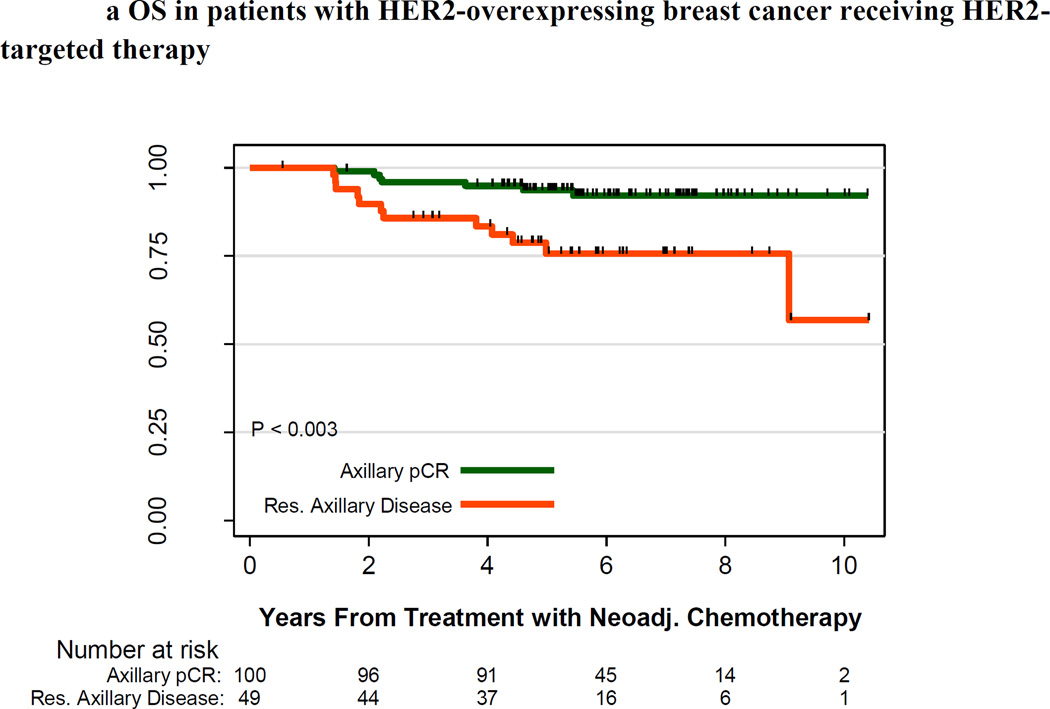
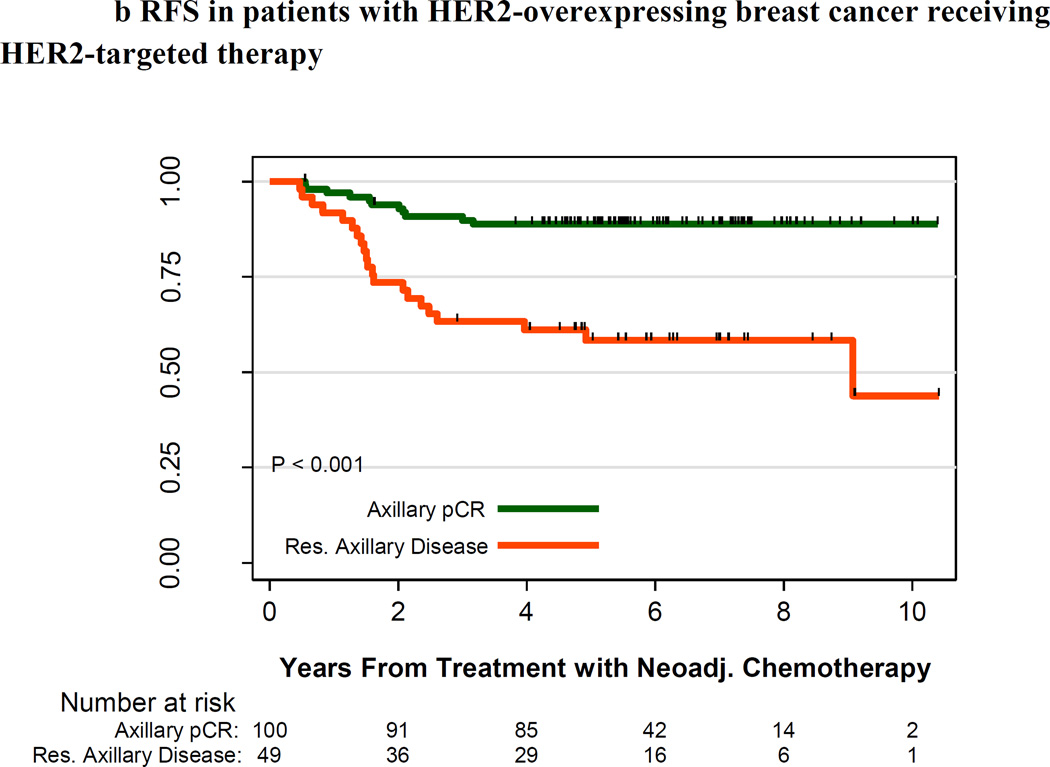
For the subset of all patients with HER2-positive tumors (n=339), 10-year survival estimates were slightly better to those of the overall cohort: 10-year OS and RFS rates were 68.1% and 61.3%, respectively. Among the 336 patients with HER2-positive tumors for whom treatment regimen was known, patients who received HER2-targeted therapy had higher survival rates than patients who did not receive HER2-targeted therapy: 10-year OS rates were 77% and 62%, respectively (P < .001), and 10-year RFS rates were 70% and 53%, respectively (P < .001). Among patients with HER2-positive tumors who received HER2-targeted therapy, those who achieved an axillary pCR had higher survival rates than those with residual axillary disease: the 10-year OS estimates were 92% and 57%, respectively (P = .003), and the 10-year RFS estimates were 89% and 44%, respectively (P < .001) (Figure 3).
Figure 3.
A. Overall Survival by pathologic lymph node status for HER2-positive breast cancer receiving HER2-targeted therapy
Overall survival in patients with HER2-positive breast cancer receiving HER2-targeted therapy. pCR in the axilla is in green, and residual axillary disease is in red. The number of patients at risk is indicated under the x-axis.
B. Relapse Free Survival by pathologic lymph node status for HER2-positive breast cancer receiving HER2-targeted therapy
Relapse-free survival in patients with HER2-positive breast cancer receiving HER2-targeted therapy. pCR in the axilla is in green, and residual axillary disease is in red. The number of patients at risk is indicated under the x-axis.
Multivariable Modeling
Table 1 summarizes the multivariable Cox proportional hazards models for OS and RFS, adjusted for axillary pCR vs. residual axillary disease, age, race, breast cancer subtype, clinical T stage, breast pCR vs. residual breast disease, grade, and adjuvant radiation and endocrine therapy in all patients. Compared with women who achieved an axillary pCR, those with residual axillary disease had a 3-fold increase in the risk of death (hazard ratio = 3.02; 95% CI, 2.14–4.27; P < .001) and a 3.1-fold increase in the risk of recurrence (hazard ratio = 3.10; 95% CI, 2.31 – 4.15; P < .001). With respect to OS, the following factors were associated with an increased risk of death: higher clinical T stage; residual disease in the breast; and grade 3. Receiving adjuvant radiation and endocrine therapy were protective (P < .001). Similar results were obtained for RFS.
Table 1.
Cox Proportional Hazards Models
| Overall Survival | Recurrence-Free Survival | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio |
95% CI | P | Hazard Ratio |
95% CI | P | |
| All Patients | ||||||
| Axillary Lymph Node Disease | ||||||
| Axillary pCR | Ref | Ref | ||||
| Axillary Residual Disease | 3.02 | (2.14, 4.27) | <0.001 | 3.10 | (2.31, 4.15) | <0.001 |
| Age | ||||||
| ≤ 50 | Ref | Ref | ||||
| > 50 | 1.16 | (0.95, 1.43) | 0.15 | 0.92 | (0.76, 1.10) | 0.35 |
| Race | ||||||
| Other | Ref | Ref | ||||
| African American | 1.3 | (0.98, 1.72) | 0.07 | 1.18 | (0.92, 1.52) | 0.19 |
| Breast Cancer Subtype | ||||||
| HR+/HER2− | Ref | Ref | ||||
| HR+/HER2+ | 1.07 | (0.76, 1.50) | 0.70 | 1.09 | (0.81, 1.47) | 0.57 |
| HR−/HER2− | 1.25 | (0.86, 1.81) | 0.23 | 1 | (0.72, 1.40) | 0.99 |
| HR−/HER2+ | 0.7 | (0.44, 1.10) | 0.12 | 0.82 | (0.55, 1.21) | 0.31 |
| Clinical T Stage | ||||||
| T0–T2 | Ref | Ref | ||||
| T3–T4 | 1.72 | (1.39, 2.12) | <0.001 | 1.65 | (1.37, 1.99) | <0.001 |
| Pathological T Stage | ||||||
| T0 | Ref | Ref | ||||
| T1–T4 | 2.15 | (1.53, 3.02) | <0.001 | 1.91 | (1.43, 2.55) | <0.001 |
| Nuclear grade | ||||||
| I or II | Ref | Ref | ||||
| III | 1.63 | (1.25, 2.11) | <0.001 | 1.47 | (1.18, 1.83) | 0.001 |
| Adjuvant Treatment | ||||||
| No Adj XRT/No Adj ET | Ref | Ref | ||||
| Adj XRT/No Adj ET | 0.43 | (0.31, 0.61) | <0.001 | 0.37 | (0.28, 0.51) | <0.001 |
| No Adj XRT/Adj ET | 0.30 | (0.17, 0.53) | <0.001 | 0.25 | (0.15, 0.41) | <0.001 |
| Adj XRT/Adj ET | 0.22 | (0.15, 0.32) | <0.001 | 0.18 | (0.12, 0.24) | <0.001 |
| Patients with HER2-Overexpressing Breast Cancer | ||||||
| Axillary Lymph Node Disease | ||||||
| Axillary pCR | Ref | Ref | ||||
| Residual Axillary Disease | 3.49 | (1.98, 6.16) | <0.001 | 4.51 | (2.75, 7.39) | <0.001 |
| Clinical T Stage | ||||||
| T0–T2 | Ref | Ref | ||||
| T3–T4 | 1.71 | (1.12, 2.62) | 0.01 | 1.42 | (0.98, 2.06) | 0.06 |
| HER2-Targeted Therapy | ||||||
| No | Ref | Ref | ||||
| Yes | 0.58 | (0.33, 1.00) | 0.05 | 0.72 | (0.47, 1.12) | 0.15 |
| Patients with HER2-Overexpressing Breast Cancer Receiving HER2-Targeted Therapy | ||||||
| Axillary Lymph Node Disease | ||||||
| Axillary pCR | Ref | Ref | ||||
| Residual Axillary Disease | 1.24 | (0.39, 3.99) | 0.70 | 2.34 | (0.96, 5.71) | 0.06 |
| Clinical T Stage | ||||||
| T0–T2 | Ref | Ref | ||||
| T3–T4 | 4.32 | (1.22, 15.29) | 0.02 | 1.33 | (0.63, 2.81) | 0.45 |
| Pathological T Stage | ||||||
| T0 | Ref | Ref | ||||
| T1–T4 | 6.61 | (1.58, 27.66) | 0.01 | 3.82 | (1.42, 10.31) | 0.008 |
| HR Status | ||||||
| Negative | Ref | Ref | ||||
| Positive | 0.37 | (0.13, 1.07) | 0.07 | 0.30 | (0.13, 0.68) | 0.004 |
Adj = Adjuvant
XRT = Radiation Therapy
ET = Endocrine Therapy
Within the subset of all patients with HER2-positive tumors, compared with patients with an axillary pCR, patients with residual axillary disease had a 3.49fold increase in the risk of death (hazard ratio = 3.49; 95% CI, 1.98–6.16; P < .001) and a 4.51-fold increase in the risk of recurrence (hazard ratio = 4.51; 95% CI, 2.75–7.39; P < .001). Patients with larger clinical tumor size had increased risk of death and increased risk of recurrence. Patients who received HER2-targeted therapy had a 42% decreased risk of death of borderline significance (hazard ratio = 0.58; 95% CI, 0.33–1.00; P = .052), but there was no significant impact on the risk of recurrence.
Within the subset of patients with HER2-positive tumors who received HER2-targeted therapy, patients with residual axillary disease did not have a significantly increased risk of death (hazard ratio = 1.24; 95% CI, 0.39–3.99; P = 0.70) but did have a 2.34-fold increased risk of recurrence of borderline significance (hazard ratio = 2.34; 95% CI, 0.96–5.71; P = .06) after adjustment for clinical T stage, pathologic T stage, and HR status.
Discussion
We report on a large cohort of patients with cytologically confirmed axillary lymph node metastases who underwent PST. We found that associations between axillary pCR and triple-negative and HER2-positive disease, high grade, and lower clinical and pathologic T stage. Patients with axillary pCR had better OS and RFS than patients with residual axillary disease, both overall and in the subset of patients with HER2-positive disease treated with HER2-targeted therapy. Furthermore, among patients with axillary pCR, those with breast pCR had better OS and RFS than those with residual breast disease.
Our finding that 18.6% of patients had no residual invasive carcinoma in the breast or axilla after PST is similar to those from other studies.9 Our finding that 28.4% of patients had an axillary pCR is also consistent with prior analyses. Hennessy et al. reported an axillary pCR rate of 22% in patients treated with PST,4 and earlier studies showed axillary pCR rates after PST of 23% to 40%.2,4,10–12 Many recent studies have examined rates of pCR in both the breast and axilla after PST in patients with HER2-positive tumors; in this population, pCR rates of 39% to 60.6% have been reported.5–7,13,14 In our study, the axillary pCR rate in patients who received HER2-targeted PST was 67.1%, consistent with other reported axillary pCR rates in this subset.6,7
In our study, high rates of axillary pCR after PST were seen despite poor primary tumor-related prognostic features, such as high grade and negative HR status. The survival differences observed between patients in whom PST eradicated axillary lymph node metastases and patients with residual axillary disease support the use of axillary pCR as a marker for long-term outcome. The definition of “overall pCR” should include axillary nodal pCR, in keeping with recent international group recommendations.15
The addition of trastuzumab for treatment of patients with HER2-positive disease has had a profound positive impact on the rates of axillary pCR and the long-term outcomes for these patients.16,17 Further benefit is seen when trastuzumab is combined with other HER2-targeting agents, such as lapatinib or pertuzumab.18,19 Continued advances in the development and study of targeted agents with better toxicity profiles than those of traditional cytotoxic chemotherapy drugs may further improve pCR rates in all subtypes of breast cancer. Genomic and proteomic profiling may assist in predicting which patients may achieve a pCR with only a taxane or an anthracycline so as to avoid overtreatment and its cost and toxic effects.
We found that patients with a pCR in the axilla but residual disease in the breast had better survival than patients with a pCR in the breast but residual disease in the axilla, in agreement with findings from previous studies by our group and others.4,8 This finding suggests that after PST, clearance of axillary metastases is more important than clearance of disease in the breast. This finding may relate to the inherent biology and increased metastatic potential of axillary metastases. However, in contrast to our prior analysis,4 our current analysis showed that among patients with axillary pCR, patients with a breast pCR had better survival than those with residual disease in the breast. This difference in findings between our previous analysis and our current analysis is likely due to an increased number of patients with discordant pathologic findings in the breast and axilla and therefore increased power to detect survival differences related to the status of residual disease in the breast.
A higher proportion of patients in the current study (73.5%) than in a prior analysis (35.7%) received third-generation chemotherapy;4 in this study, patients who received both an anthracycline and a taxane were more likely to achieve an axillary pCR than were patients who received only one. However, nearly one-quarter of patients who received a taxane without an anthracycline achieved an axillary pCR, suggesting that treatment without an anthracycline might be sufficient for a subset of patients. Such treatment would spare patients the toxic effects of anthracyclines.
In our current study, the risk of relapse after 5 years was small for patients who achieved an axillary pCR: the difference between the 5-year and 10-year RFS rates was 5 percentage points (84% vs. 79%). Patients who did not have an axillary pCR had a higher risk of relapse even after 5 years (5-year RFS rate, 60%; 10-year RFS rate, 50%).
If clearance of disease in the axillary nodes with PST is associated with a better prognosis, then it may be desirable to determine which patients do not require axillary lymph node dissection after PST. Two recent trials, SENTINA18 and ACOSOG Z1071,12 both evaluated the feasibility and accuracy of sentinel lymph node biopsy after PST. Unfortunately, both reported false-negative rates for sentinel lymph node surgery of greater than 10% after PST.12,18 Further studies are needed to determine what clinical and radiologic tools might enable patients who achieve an axillary pCR after PST to avoid axillary dissection with its complications and morbidity. At our institution, we currently place a marker in the affected axillary node prior to PST to facilitate targeting of the sentinel node after chemotherapy and thereby mitigate the risk of false-negative findings on sentinel lymph node biopsy.
All of the patients included in this analysis had cytologic confirmation of axillary lymph node metastases prior to PST, and pathologic examination of the axillary nodes after surgery was performed at our institution. However, our study does have some limitations. This is a retrospective analysis of patients treated at a single center, which limits generalizability. While the chemotherapeutic agents used were recorded, the precise dosages and numbers of cycles were not. Some of the patients included in the analysis were treated on protocol, whereas others were treated according to standards for our institution or were treated at other institutions. Stratification based on the chemotherapy backbone used (i.e. anthracycline and/or taxane) was possible, but further analysis comparing specific regimens was not possible. Additionally, missing data such as data on HR and HER2 status could have impacted our findings. Finally, the patients in this study were diagnosed over a long time period, although most patients were treated in 2000 or later. Treatment modalities and strategies have changed over the time frame under study, which may have affected our results.
In summary, our findings indicate that patients who achieve a pCR in the axilla after PST have better long-term clinical outcomes than patients with residual disease in the axilla. Molecular-based correlates in future protocols may enhance our understanding of the biologic differences between the primary tumor in the breast and axillary lymph node metastases. Further studies are needed to determine if patients with residual disease after PST, particularly in the axillary lymph nodes, may benefit from further therapy after surgery to reduce the risk of relapse and improve survival.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Ms. Stephanie Deming, Senior Scientific Editor and The University of Texas MD Anderson Cancer Center, for her editorial assistance. No compensation was received for her contribution. This work was supported by the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group and the Clinical Trials Support Resource. The funder of this research played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Mike Hernadez and Xiudong Lei, both of The University of Texas MD Anderson Cancer Center, conducted the data analysis, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
All information and materials in the manuscript are original.
Footnotes
None of the authors has any conflicts of interest to report.
This study was presented at the ASCO Annual Meeting, June 2013.
Contributor Information
Sarah S. Mougalian, Email: sarah.mougalian@yale.edu, Department of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX.
Mike Hernandez, Email: mhernandez@mdanderson.org, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX.
Xiudong Lei, Email: xiulei@mdanderson.org, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX.
Siobhan Lynch, Email: slynch@txcc.com, The Center for Cancer and Blood Disorders, Arlington, TX.
Henry M. Kuerer, Email: hkuerer@mdanderson.org, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
William F. Symmans, Email: fsymmans@mdanderson.org, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Richard L. Theriault, Email: richard.theriault@yahoo.com, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Bruno D. Fornage, Email: bfornage@mdanderson.org, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Limin Hsu, Email: lhsu@mdanderson.org, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Thomas A. Buchholz, Email: tbuchhol@mdanderson.org, Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Aysegul A. Sahin, Email: asahin@mdanderson.org, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Kelly K. Hunt, Email: khunt@mdanderson.org, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Wei Tse Yang, Email: wyang@mdanderson.org, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Gabriel N. Hortobagyi, Email: ghortoba@mdanderson.org, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
Vicente Valero, Email: vvalero@mdanderson.org, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX.
References
- 1.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 3.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 4.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23(36):9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 5.Bayraktar S, Gonzalez-Angulo AM, Lei X, et al. Efficacy of neoadjuvant therapy with trastuzumab concurrent with anthracycline- and nonanthracycline-based regimens for HER2-positive breast cancer. Cancer. 2012;118(9):2385–2393. doi: 10.1002/cncr.26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 7.Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116(12):2884–2889. doi: 10.1002/cncr.25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouzier R, Extra JM, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20(5):1304–1310. doi: 10.1200/JCO.2002.20.5.1304. [DOI] [PubMed] [Google Scholar]
- 9.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 10.McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989;124(1):21–25. doi: 10.1001/archsurg.1989.01410010027005. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GF, Birchansky CA, Komarnicky LT, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73(2):362–369. doi: 10.1002/1097-0142(19940115)73:2<362::aid-cncr2820730221>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Boughey J, Suman V, Mittendorf E, et al. The role of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0–T4, N1–2) who receive neoadjuvant chemotherapy - results from the ACOSOG Z1071 trial. Cancer Research. 2012;72(24 Supplement):S2–S1. [Google Scholar]
- 13.Buzdar A, Suman VJ, Meric-Bernstam F, et al. ACOSOG Z1041 (Alliance): Definitive analysis of randomized neoadjuvant trial comparing FEC followed by paclitaxel plus trastuzumab (FEC −>P+T) with paclitaxel plus trastuzumab followed by FEC plus trastuzumab (P+T −>FEC+T) in HER2+ operable breast cancer. ASCO Meeting Abstracts. 2013;31(15_suppl):502. [Google Scholar]
- 14.Ohzawa H, Sakatani T, Niki T, Yasuda Y, Hozumi Y. Pathological responses and survival of patients with human epidermal growth factor receptor 2-positive breast cancer who received neoadjuvant chemotherapy including trastuzumab. Breast Cancer. 2012 doi: 10.1007/s12282-012-0424-4. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 16.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 17.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 18.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.