Abstract
The fragile X-associated tremor ataxia syndrome (FXTAS) is caused by the premutation in FMR1 gene. Recent reports of environmental toxins appear to worsen the progression of FXTAS. Here we present a case of male adult with FXTAS and a long history of methadone use. The patient shows a faster progression in both symptoms of disease and MRI changes compared to what is typically seen in FXTAS. There has been no research regarding the role of narcotics in onset, progression, and severity of FXTAS symptoms. However, research has shown that narcotics can have a negative impact on several neurodegenerative diseases, and we hypothesize that in this particular case, methadone may have contributed to a faster progression of FXTAS as well as exacerbating white matter disease through RNA toxicity seen in premutation carriers.
Keywords: premutation, FMR1 gene, narcotics, methadone, white matter, FXTAS
INTRODUCTION
The FMR1 premutation is caused by a trinucleotide repeat expansion from 55 to 200 CGGs in the 5’ UTR region of FMR1 gene. Fragile X-associated tremor/ataxia syndrome (FXTAS) (OMIM: 300623) is an FMR1 premutation-driven neurodegenerative disorder. CGG repeats expansions greater than 200 cause methylation which blocks transcription so that little or no mRNA and FMR1 protein (FMRP) is produced. The lack of FMRP leads to the full mutation and fragile X syndrome (FXS) (OMIM: 300624), a very different disorder than FXTAS which is caused by up-regulation of the FMR1 mRNA [Hagerman et al., 2013].
The major radiological findings in FXTAS include white matter hyperintensities (WMH) in the middle cerebellar peduncles (MCP), brain stem, and corpus callosum in addition to generalized brain atrophy [Hagerman et al., 2013]. In other neurodegenerative diseases, white matter disease (WMD) is associated with cognitive impairment [Prins et al., 2005; Gouw et al., 2006], physical decline [Zheng et al., 2012] and depressive symptoms in the elderly [de Groot et al., 2000]. Furthermore, WMD occurs in schizophrenia [Voineskos et al., 2013] and bipolar disorder [Ahn et al., 2004; Lagopoulos et al., 2013], and is correlated with mortality in patients with and without dementia or stroke [Bokura et al., 2006; Kerber et al., 2006; Kuller et al., 2007; Ikram et al., 2009; Oksala et al., 2009].
Narcotics have been extensively studied and reported to trigger white matter changes in chronic substance users [Lyoo et al., 2004; Bae et al., 2006; Schlaepfer et al., 2006; Bava et al., 2009; Yucel et al., 2010; Bora et al., 2012; Lin et al., 2012; Shen et al., 2012; Bava et al., 2013]. Furthermore, chronic exposure to opioids can lead to cell death [Boronat et al., 2001].
Methadone is a long-acting opioid agonist that is prescribed as a treatment for opioid dependence. Many studies report that methadone has adverse effects such as memory dysfunction and depression symptoms [Mintzer et al., 2002; Prosser et al., 2006; Peles et al., 2007]. Conflicting with the previous studies, cognitive deficits are improved in patients stabilized on long-term Methadone Maintenance Treatment (MMT) [Wang et al., 2014]. However, enrollment in MMT has also positive effects induced by lifestyle changes that could be confounders in the study. Thus, whether or not methadone itself induces cognitive impairment remains unclear.
Substance abuse has been reported to be increased in premutation carriers compared to controls perhaps related to self- treatment of anxiety or depression which are also common in carriers [Kogan et al., 2008; Hagerman et al., 2013].
We present a case of a patient with FXTAS who has a history of narcotics use (Methadone), which may have exacerbated or accelerated his neurological symptoms of FXTAS.
METHODS
A male patient was evaluated at the Fragile X Research and Treatment Center located at the UC Davis MIND Institute. The patient signed an IRB approved consent form for this research when he was seen. Data were acquired from the medical history obtained during study visits.
Genotyping
CGG repeatsize and methylationstatus (Activation Ratio, AR) were measured by Southern Blot and PCR analysis as previously described [Tassone et al., 2008; Filipovic-Sadic et al., 2010].
Imaging
Two different structural Brain MRI protocols were used for the radiological evaluations of the subject: Time 1: MRI acquired with a 1.5 Tesla GE Signa Horizon LX Echospeed. Sequences were as follows: 3D spoiled gradient recalled echo (IR-prepped SPGR) acquisition (oblique axial plane), T1-weighted sequence (sagittal plane), T2-weighted high resolution fluid-attenuated inversion recovery (FLAIR) sequence (oblique axial plane), and a T2-weighted sequence (oblique axial plane). Time 2: The subject had two separate visits to the MIND Institute (separated by five years), during which an MRI was done. The second MRI was acquired with a 3 Tesla Siemens Magnetom TrioTim syngo MR B15. Sequences were as follows: T1 magnetization prepared rapid acquisition gradient echo (MPRAGE) 3D sequence, T2-FLAIR (sagittal plane), and a T2-turbo spin echo (TSE) (oblique axial plane) acquisition. MRI outcome measures are cerebral and cerebellar volume, thickness of the body of the corpus callosum and white matter abnormalities in the MCP, pons, corpus callosum body (3T MRI only), and splenium.
CLINICAL REPORT
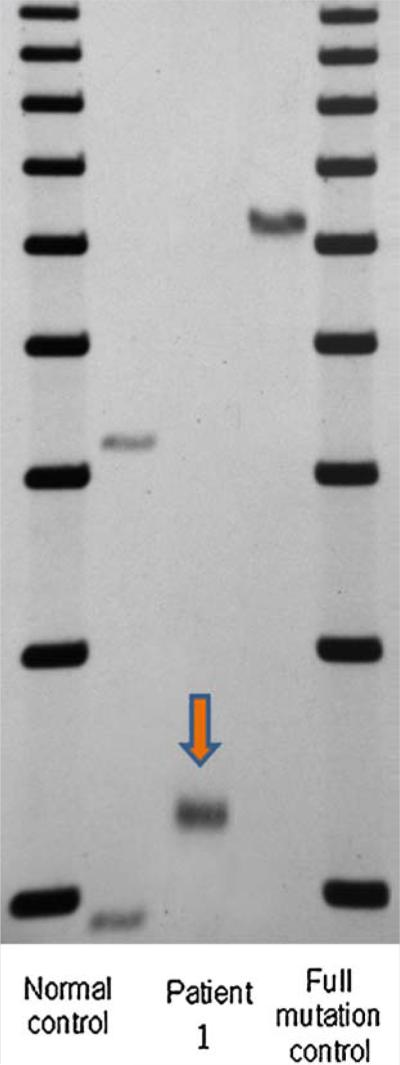
A 64-year-old Caucasian man with 44 years of methadone treatment came to our clinic for a follow up in 2013. He was previously seen in 2008 and at that time was diagnosed with probable FXTAS, at age 59. He has 127 CGG repeats (Fig. 1). After a short bout of heroin use right after his military service he was placed on methadone maintenance, started at 20 years of age and left on it for many years.
FIG. 1.
Southern blot results of a patient with FXTAS with methadone use. Lane 1 and 5 (left to right): 1 Kb ladder size marker. Lane 2 and 4: normal female and full mutation male control, respectively. Lane 3 showing the presence of a premutation allele in patient 1. CGG repeat size was measured by PCR analysis.
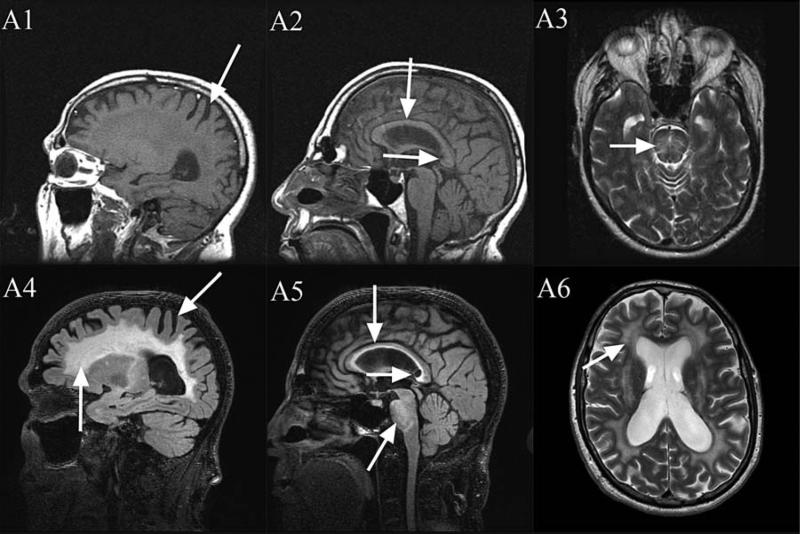
The onset of his tremor and ataxia was at age 57. At age 59, the Wechsler Adult Intelligence Scale III (WAIS-III) showed a full scale IQ of 89. His Wechsler memory scale III (WMS-III) showed an auditory immediate memory of 94, an auditory delayed memory of 99, an auditory receptive memory of 95, and a working memory of 71. He had executive function deficits documented by a Behavior Dyscontrol Scale II(BDS-II) score of 9. On MRI, he had avery subtle middle cerebellar peduncle sign, and massive white matter disease in the pons and cerebrum consistent with FXTAS (Fig. 2). Recommendations for tapering and discontinuing methadone were made but not carried out.
FIG. 2.
1.5 Tesla MRI: T1 (A1, A2), T2 (A3). 3 Tesla MRI: T2-FLAIR (A4, A5), T2-TSE (A6). Moderate cerebral volume loss (A1, A4), severe increased T2 signal intensity in both the truncus and the splenium (A5), severe diffuse increased T2 signal intensity in the deep white matter of the cerebrum (A4 and A6), moderately thin truncus of the corpus callosum (A2, A5) and severe increased T2 signal intensity in the pons (A3, A5). Slight increases in severity from age 59 (A1–A3) to age 64 (A4–A6), with largest changes in the splenium of the corpus callosum (A2 compared to A5).
At age 60, he was started on a higher dose of methadone of 180 mg per day and his ataxia and falling became much worse. At age 61, he started using a cane. His tremor had become worse and it was interfering with dressing and carrying things like plates. He dropped things from his hand more frequently, but was able to eat on his own without significant spilling. At age 63, his handwriting became problematic and he also developed swallowing and choking problems. At age 64, he started to taper the methadone maintenance dosage to approximately 24 mg per day. However, at that time he had worsening of his cognitive function and memory. He was beginning to nap more frequently during the day. He was also complaining of numbness and tingling in his feet and severe left ankle pain. His ataxia led to frequent falling, specifically six times within the previousyear and ahalf. As he was tapered down from the methadone, his falling decreased. He has not fallen in the last four months. However, with the taper of the methadone, he did notice more anxiety and sleep problems. On the assessments at age 64, his WAIS-IV full-scale IQ was 74. On the WMS scale, his visual memory is 65, auditory memory is 58, visual working memory is 73, immediate memory is 60, and delayed memory is 58. His BDS-II score of only 2, demonstrated severe executive function deficits.
Overall he developed significant deterioration in IQ, memory and executive function compared to his first visit. His MRI abnormalities were somewhat increased from his visit at age 59 and showed moderate cerebral volume loss, mild cerebellar volume loss, severe increased T2 signal intensity in both the truncus and the splenium of the corpus callosum, severe diffuse increased T2 signal intensity in the deep white matter of the cerebrum, a moderately thin truncus of the corpus callosum, and severe increased T2 signal intensity in the pons (see Fig. 2).
DISCUSSION
Leehey et al., studied a cohort of male premutation carriers with FXTAS and reported median tremor onset at ~60 years of age. After onset of tremor, median onset of ataxia was 2 years later; onset of falls was 6 years later; dependence on a walking aid at 15 years later; and death after 21 years of onset [Leehey et al., 2007]. In our patient, onset of tremor and ataxia was at age 57, falling episodes started at age 58, and dependence on a walking aid is at age 61. The progression of FXTAS in the patient was more rapid as compared to the cohort study.
Methadone-induced brain toxic leukoencephalopathy is reported in several cases with decreased level of consciousness [Salgado et al., 2010; Cerase et al., 2011; Bileviciute-Ljungar et al., 2014]. In addition, there is a cerebellar and basal ganglia involvement in methadone overdose as well [Corre et al., 2013]. Euphoria, slurred speech and ataxia were the most common initial symptoms of severe methadone poisoning [Caplehorn,1998]. Thus far, methadone toxicity occurred in acute setting but there is no study reporting the toxicity in chronic use particularly in patients with FXTAS.
Fragile X Mental Retardation Protein (FMRP) can be a translational repressor at synapse. Unfortunately, we do not have FMRP data on this patient, but generally for large premutation alleles (~80–200 CGG repeats) FMRP levels are mild to moderately reduced due to translational inefficiency [Primerano et al., 2002].
Fibromyalgia and chronic pain are common in premutation carriers [Coffey et al., 2008; Leehey et al., 2011; Winarni et al., 2012]. We often see substance abuse with FXTAS patients, particularly when pain symptoms become problematic. Methadone recently has been proposed for the treatment of moderate-to-severe pain. In our case, tapering off methadone made his chronic pain become worse. We think that long term use of methadone helped with his pain issue, but may haveexacerbated his white matter disease and FXTAS progression. Opiate addiction is associated with decreases in white matter integrity [Bora et al., 2012]. Furthermore, white matter changes are correlated with memory deficits and depression in heroin users under MMT [Lin et al., 2012]. WMH is described as patchy or diffuse white matter changes identified on T2-weighted MRI in the elderly population [Awad et al., 1986]. It affects approximately 11–21% of adults at aged 64, and up to 94% at age 82 in the general population [Garde et al., 2000]. WMH has been associated with age-associated disease and cognitive decline [Carmichael et al., 2010; Debette et al., 2010]. WMH can predict progression from mild cognitive impairment to Alzheimer's disease [Prasad et al., 2011]. Furthermore, there is a significantly negative correlation between WMH volume and verbal IQ scores [Garde et al., 2005].
The premutation produces excessive levels of FMR1 mRNA leading to a toxic RNA gain-of-function effect in neurons [Tassone et al., 2000; Hagerman et al., 2004; Amiri et al., 2008; Garcia-Arocena et al., 2010]. Elevated FMR1 mRNA leads to sequestration of various proteins, including Sam68, DROSHA and DGCR8, important for neuronal function and miRNA maturation and this is likely the basis of RNA toxicity [Garcia-Arocena et al., 2010; Sellier et al., 2013]. Intriguingly, there is also a clear negative correlation between FMR1 mRNA levels and cell viability [Hoem et al., 2011] leading to early death of cultured premutation neurons compared to neurons without the premutation [Chen et al., 2010]. In addition, chronic exposure to opioids can lead to cell death [Boronat et al., 2001] so the premutation neurons may die even earlier with exposure to opioids over many years. Basically the premutation may be more vulnerable to environmental toxicity including chronic methadone use.
Some other factors might also contribute to progression of WMD, such as depression [Fields 2008; Godin et al., 2008], hypertension [Godin et al., 2011; Hamlin et al., 2012], and chronic pain [Buckalew et al., 2013], all of which this patient experienced.
For the presented case, we hypothesize that long term use of methadone may have exacerbated the neurological symptoms of FXTAS, including tremor, ataxia and cognitive decline as well as more rapid exacerbation of WMD.
CONCLUSIONS
In premutation carriers with narcotic dependence, long-term methadone use must be evaluated carefully to avoid possible exacerbation of symptoms of FXTAS including WMH and to avoid dependency of methadone to alleviate pain symptoms. Alternative treatments for pain include acupuncture, gabapentin, pregabalin, duloxetine, venlafaxine, tricyclics, omega 3 s and psychological approaches with cognitive behavioral therapy. In addition, supplementation of vitamin B12 might have a positive impact since there is a significant association between vitamin B12 status and severity of WMD [de Lau et al., 2009]. Proper management of depression, hypertension, chronic pain and preventing substance abuse and dependency may slow the progression of WMD in those with FXTAS. More comprehensive study is needed to explain the effect of methadone on WMD and progression of FXTAS in a large sample of patients
ACKNOWLEDGEMENT
This work was supported by grants from the National Fragile X Foundation, NIH HD036071, NIH UL1DE019583, the National Institute on Aging of the National Institutes of Health (P30AG043097) and NIH RL1AG032115. We thank the families who participated in this study. We also thank Kylee Cook for the clinical data.
Footnotes
Conflict of Interest: Dr Hagerman's work has been funded by the NIH. Other funding has been received for clinical trials from Roche, Alcobra, Neuren and Novartis and she has served on advisory boards to Roche/Genentech and Novartis.
REFERENCES
- Ahn KH, Lyoo IK, Lee HK, Song IC, Oh JS, Hwang J, Kwon J, Kim MJ, Kim M, Renshaw PF. White matter hyperintensities in subjects with bipolar disorder. Psychiatry Clin Neurosci. 2004;58:516–521. doi: 10.1111/j.1440-1819.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- Amiri K, Hagerman RJ, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome: An aging face of the fragile X gene. Arch Neurol. 2008;65:19–25. doi: 10.1001/archneurol.2007.30. [DOI] [PubMed] [Google Scholar]
- Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, Kim DJ, Hwang J, Kim SJ, Renshaw PF. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal Changes in White Matter Integrity Among Adolescent Substance Users. Alcohol-Clin Exp Res. 2013;37:E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I, Haglund V, Carlsson J, von Heijne A. Clinical and radiological findings in methadone-induced delayed leukoencephalopathy. J Rehabil Med. 2014;46:828–830. doi: 10.2340/16501977-1820. [DOI] [PubMed] [Google Scholar]
- Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, Oguro H, Takahashi K. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: A prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. Addict Biol. 2012;17:141–148. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- Boronat MA, Garcia-Fuster MJ, Garcia-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down- regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001;134:1263–1270. doi: 10.1038/sj.bjp.0704364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew N, Haut MW, Aizenstein H, Rosano C, Edelman KD, Perera S, Marrow L, Tadic S, Venkatraman V, Weiner D. White matter hyperintensity burden and disability in older adults: Is chronic pain a contributor?. PM R. 2013;5:471–480. doi: 10.1016/j.pmrj.2013.03.004. quiz 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplehorn JR. Deaths in the first two weeks of maintenance treatment in NSW in 1994: Identifying cases of iatrogenic methadone toxicity. Drug Alcohol Rev. 1998;17:9–17. doi: 10.1080/09595239800187551. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr, Weiner M, DeCarli C. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerase A, Leonini S, Bellini M, Chianese G, Venturi C. Methadone-induced toxic leukoencephalopathy: Diagnosis and follow-up by magnetic resonance imaging including diffusion-weighted imaging and apparent diffusion coefficient maps. J Neuroimaging. 2011;21:283–286. doi: 10.1111/j.1552-6569.2010.00530.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing FMR1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, Pillot J, Hilbert G. Methadone-induced toxic brain damage. Case Rep Radiol. 2013;2013:602981. doi: 10.1155/2013/602981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Smith AD, Refsum H, Johnston C, Breteler MM. Plasma vitamin B12 status and cerebral white-matter lesions. J Neurol Neurosurg Psychiatry. 2009;80:149–157. doi: 10.1136/jnnp.2008.149286. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, Hagerman PJ, Stenzel TT, Hadd AG, Latham GJ, Tassone F. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56:399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HBW. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet. 2000;356:628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- Garde E, Mortensen EL, Rostrup E, Paulson OB. Decline in intelligence is associated with progression in white matter hyperintensity volume. J Neuro Neurosurg Psychiatry. 2005;76:1289–1291. doi: 10.1136/jnnp.2004.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin O, Dufouil C, Maillard P, Delcroix N, Mazoyer B, Crivello F, Alp erovitch A, Tzourio C. White matter lesions as a predictor of depression in the elderly: The 3C-Dijon study. Biol Psychiatry. 2008;63:663–669. doi: 10.1016/j.biopsych.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: The Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123:266–273. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Van der Flier WM, van Straaten EC, Barkhof F, Ferro JM, Baezner H, Pantoni L, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Fazekas F, Scheltens P, LADIS Study Group Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: The LADIS study. J Neurol. 2006;253:1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: A maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AA, Sukharev D, Campos L, Mu Y, Tassone F, Hessl D, Nguyen DV, Loesch D, Hagerman RJ. Hypertension in FMR1 premutation males with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet A. 2012;158A:1304–1309. doi: 10.1002/ajmg.a.35323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoem G, Raske CR, Garcia-Arocena D, Tassone F, Sanchez E, Ludwig AL, Iwahashi CK, Kumar M, Yang JE, Hagerman PJ. CGG-repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Hum Mol Genet. 2011;20:2161–2170. doi: 10.1093/hmg/ddr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30:450–456. doi: 10.1016/j.neurobiolaging.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kerber KA, Whitman GT, Brown DL, Baloh RW. Increased risk of death in community-dwelling older people with white matter hyper-intensities on MRI. J Neurol Sci. 2006;250:33–38. doi: 10.1016/j.jns.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated Tremor/Ataxia syndrome: A controlled study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Arnold AM, Longstreth WT, Jr, Manolio TA, O'Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiology of Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Hermens DF, Hatton SN, Tobias-Webb J, Griffiths K, Naismith SL, Scott EM, Hickie IB. Microstructural white matter changes in the corpus callosum of young people with Bipolar Disorder: A diffusion tensor imaging study. PLoS ONE. 2013;8:e59108. doi: 10.1371/journal.pone.0059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Min SJ, Hall DA, Rice CD, Zhang L, Grigsby J, Greco CM, Reynolds A, Lara R, Cogswell J, Jacquemont S, Hessl DR, Tassone F, Hagerman R, Hagerman PJ. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22:203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Legg W, Tassone F, Hagerman R. Fibromyalgia in fragile X mental retardation 1 gene premutation carriers. Rheumatology (Oxford) 2011;50:2233–2236. doi: 10.1093/rheumatology/ker273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WC, Chou KH, Chen CC, Huang CC, Chen HL, Lu CH, Li SH, Wang YL, Cheng YF, Lin CP. White matter abnormalities correlating with memory and depression in heroin users under methadone maintenance treatment. PLoS ONE. 2012;7:e33809. doi: 10.1371/journal.pone.0033809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Streeter CC, Ahn KH, Lee HK, Pollack MH, Silveri MM, Nassar L, Levin JM, Sarid-Segal O, Ciraulo DA, Renshaw PF, Kaufman MJ. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131:135–145. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Oksala NK, Oksala A, Pohjasvaara T, Vataja R, Kaste M, Karhunen PJ, Erkinjuntti T. Agerelatedwhite matterchanges predictstroke death in long term follow-up. J Neurol Neurosurg Psychiatry. 2009;80:762–766. doi: 10.1136/jnnp.2008.154104. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Naumovsky Y, Adelson M. Depression in methadone maintenance treatment patients: Rate and risk factors. J Affect Disord. 2007;99:213–220. doi: 10.1016/j.jad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Prasad K, Wiryasaputra L, Ng A, Kandiah N. White matter disease independently predicts progression from mild cognitive impairment to Alzheimer's disease in a clinic cohort. Dement Geriatr Cogn Disord. 2011;31:431–434. doi: 10.1159/000330019. [DOI] [PubMed] [Google Scholar]
- Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8:1482–1488. [PMC free article] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- Prosser J, Cohen LJ, Steinfeld M, Eisenberg D, London ED, Galynker II. Neuropsychological functioning in opiate-dependent subjects receiving and following methadone maintenance treatment. Drug Alcohol Depend. 2006;84:240–247. doi: 10.1016/j.drugalcdep.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado RA, Jorens PG, Baar I, Cras P, Hans G, Parizel PM. Methadone-induced toxic leukoencephalopathy: MR imaging and MR proton spectroscopy findings. AJNR Am J Neuroradiol. 2010;31:565–566. doi: 10.3174/ajnr.A1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Lancaster E, Heidbreder R, Strain EC, Kosel M, Fisch HU, Pearlson GD. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9:147–153. doi: 10.1017/S1461145705005705. [DOI] [PubMed] [Google Scholar]
- Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, Alunni V, Moine H, Thibault C, Page A, Tassone F, Willemsen R, Disney MD, Hagerman PJ, Todd PK, Charlet-Berguerand N. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013;3:869–880. doi: 10.1016/j.celrep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wang E, Wang X, Lou M. Disrupted Integrity of White Matter in Heroin-addicted Subjects at Different Abstinent Time. J Addict Med. 2012;6:172–176. doi: 10.1097/ADM.0b013e318252db94. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet. 2000;94:232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile x (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70:472–480. doi: 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- Wang GY, Wouldes TA, Kydd R, Jensen M, Russell BR. Neuropsychological performance of methadone-maintained opiate users. J Psychopharmacol. 2014;28:789–799. doi: 10.1177/0269881114538541. [DOI] [PubMed] [Google Scholar]
- Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SM, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012;158A:2473–2481. doi: 10.1002/ajmg.a.35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Zalesky A, Takagi MJ, Bora E, Fornito A, Ditchfield M, Egan GF, Pantelis C, Lubman DI. White-matter abnormalities in adolescents with long-term inhalant and cannabis use: A diffusion magnetic resonance imaging study. J Psychiatry Neurosci. 2010;35:409–412. doi: 10.1503/jpn.090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JJ, Delbaere K, Close JC, Sachdev P, Wen W, Brodaty H, Lord SR. White matter hyperintensities are an independent predictor of physical decline in community-dwelling older people. Gerontology. 2012;58:398–406. doi: 10.1159/000337815. [DOI] [PubMed] [Google Scholar]