ABSTRACT
Nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells are located in the bulge area of the follicle. Previous studies have shown that HAP stem cells can differentiate to neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro. HAP stem cells effected nerve and spinal cord regeneration in mouse models. Recently, we demonstrated that HAP stem cells differentiated to beating cardiac muscle cells. The differentiation potential to cardiac muscle cells was greatest in the upper part of the follicle. The beat rate of the cardiac muscle cells was stimulated by isoproterenol. In the present study, we observed that isoproterenol directs HAP stem cells to differentiate to cardiac muscle cells in large numbers in culture compared to HAP stem cells not supplemented with isoproterenol. The addition of activin A, bone morphogenetic protein 4, and basic fibroblast growth factor, along with isoproternal, induced the cardiac muscle cells to form tissue sheets of beating heart muscle cells. These results demonstrate that HAP stem cells have great potential to form beating cardiac muscle cells in tissue sheets.
KEYWORDS: activin A, basic fiboblast growth factor, bone morphogenetic protein 4, cardiac muscle cell sheets, differentiation, GFP, hair follicle, isoproterenol, nestin, pluripotent, stem cells
Introduction
Nestin-expressing hair follicle cells were discovered in transgenic mice with nestin-driven green fluorescent protein (ND-GFP).1-4 We have termed these cells hair follicle-associated pluripotent (HAP) stem cells.5
The HAP stem cells can differentiate into neurons, glial cells, smooth muscle cells, keratinocytes and other cell types.3 HAP stem cells originate in the bulge area (BA) of the hair follicle and migrate to the dermal papilla (DP). HAP stem cells from the DP and BA cells differentiated into neuronal and glial cells after transplantation to the injured spinal cord of mice and enhanced injury repair and locomotor recovery within 4 weeks.6-8
HAP stem cells from mouse whisker follicles formed nerve-like structures contained β-III tubulin-positive fibers9 in Gelfoam® histoculture. The growing fibers had growth cones on their tips expressing F-actin, indicating they were growing axons. These results suggest a major function of HAP stem cells is for growth of the hair follicle sensory nerve.9
Recently, we demonstrated that HAP stem cells can differentiate to beating cardiac muscle cells. The beat rate of the cardiac muscle cells was stimulated by isoproterenol and inhibited by propranolol.10
In the present study, we demonstrate that isoproterenol directs HAP stem cells to differentiate to cardiac muscle cells, and that the further addition of activin A, bone morphogenetic protein 4 (BMP4), and basic fibroblast growth factor (bFGF) resulted in the formation of beating cardiac-muscle tissue sheets.
Results and discussion
Isoproterenol directs the differentiation of HAP cells into cardiac muscle cells
The mouse vibrissa hair follicle was separated into 3 parts (upper, middle, lower). The upper part of the follicle was placed on Matrigel™ and cultured for 14 days with 10% FBS in DMEM supplemented with isoproterenol alone, or the combination of isoproterenol, activin A, BMP4 and bFGF once every four days, or no supplement (Fig. 1A). Cardiac muscle cells were observed in all conditions after 14 days culture. Immunostaining demonstrated that hair follicles cultured in inducing conditions supplemented with isoproterenol alone, or the combination of isoproterenol, activin A, BMP4 and bFGF, had increased differentiation to cardiac muscle cells compared with non-supplemented conditions (Fig. 1B).
Figure 1.
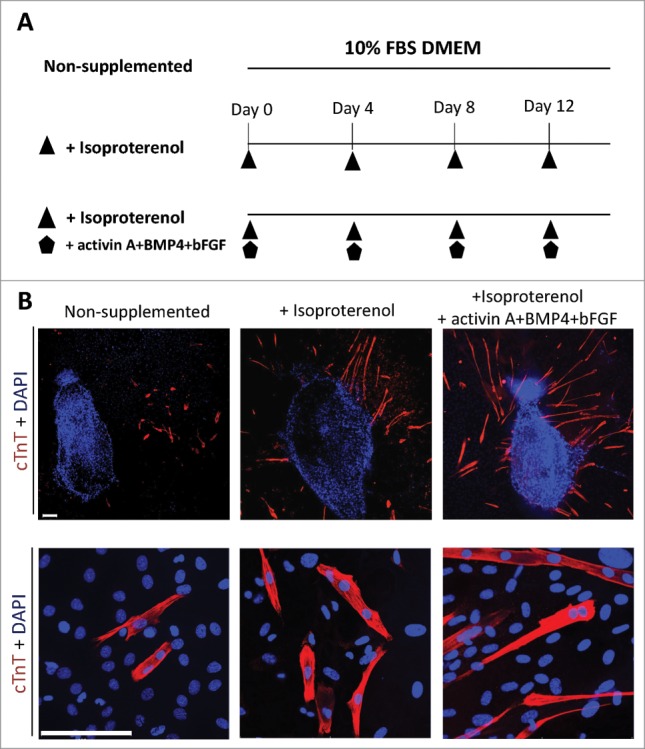
Isoproterenol directs the differentiation of HAP cells into cardiac muscle cells. (A) Schematic diagram of inducing conditions for cardiac muscle cell differentiation from the upper part of vibrissa hair follicles. Hair follicles were cultured for 14 days in 3 conditions in DMEM medium with 10% fetal bovine serum (FBS): unsupplemented; supplemented with isoproterenol; or supplemented with the combination of isoproterenol+activin A+BMP4+bFGF; once every four days. (B) Immunostaining of cardiac muscle cells differentiated from the upper part of vibrissa hair follicles in culture medium that was non-supplemented (left panel); isoproterenol-supplemented (middle panel); and supplemented with the combination of isoproterenol+activin A+BMP4+bFGF (right panel). Cardiac muscle cells were observed after 14 days culture. Red = cTnT, Blue = DAPI. Bars = 100 µm.
Flow cytometry analysis showed that the cardiac muscle cells were formed in non-supplemented medium at 8.78 ± 3.60%; formed in medium supplemented with isoproterenol alone at 19.71 ± 2.20%), and formed in medium supplemented with the combination of isoproterenol, activin A, BMP4 and bFGF at 16.84 ± 1.67%. The number of differentiated cTnT-positive cardiac muscle cells increased in the hair follicle cultures supplemented with isoproterenol alone or isoproterenol, activin A, BMP4 and bFGF compared with non-supplemented medium. There was no significant difference in the extent of differentiation of cardiac muscle cells between medium supplemented with isoproterenol alone and medium supplemented with the combination of isoproterenol, activin A, BMP4 and bFGF (Fig. 2B).
Figure 2.
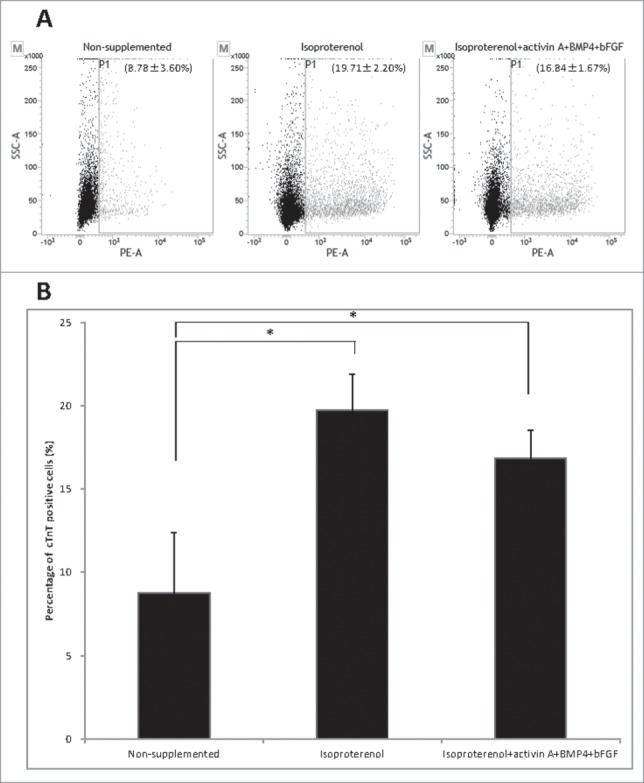
Flow cytometry to determine cTnT expression in HAP stem cell-derived cardiac muscle cells. (A) Flow cytometry analysis of cTnT-positive cardiac muscle cells that differentiated from the upper part of hair follicle cultured in medium that was non-supplemented at 8.78 ± 3.60% (left panel); in medium supplemented with isoproterenol alone at 19.71 ± 2.20% (middle panel); in medium supplemented with the combination isoproterenol, activin A, BMP4 and bFGF at 16.84 ± 1.67% (right panel). (B). The number of differentiated cTnT-positive cardiac muscle cells was significantly increased in the hair follicle cultured in medium supplemented with isoproterenol alone or the combination of isoproterenol, activin A, BMP4 and bFGF compared with unsupplemented medium.
The combination of isoproterenol, activin A, BMP4, and bFGF induced HAP stem cells to form beating cardiac-muscle tissue sheets
In order to investigate establishment of cardiac-muscle tissue sheets, the upper parts of 5 hair follicles from GFP-expressing mice were placed on Matrigel™ in glass bottom culture dishes and cultured for 14 days with 10% FBS in DMEM, supplemented with isoproterenol, activin A, BMP4 and bFGF once every four days (Fig. 3A). Cells grew from hair follicles, proliferated and adhered to the culture dish bottom. Then, the proliferating cells overlaid the hair follicles. Some of the cells started beating. The cardiac muscle cells spread around the hair follicles (Fig. 3A, Supplemental Video 1). On the 10th day of culture, we observed beating sheets layered over the hair follicles (Fig. 3A, Supplemental video 2). Immunostaining of the beating cardiac-muscle tissue sheets showed that the cardiac-muscle cells were distributed within the sheets (Fig. 3B).
Figure 3.
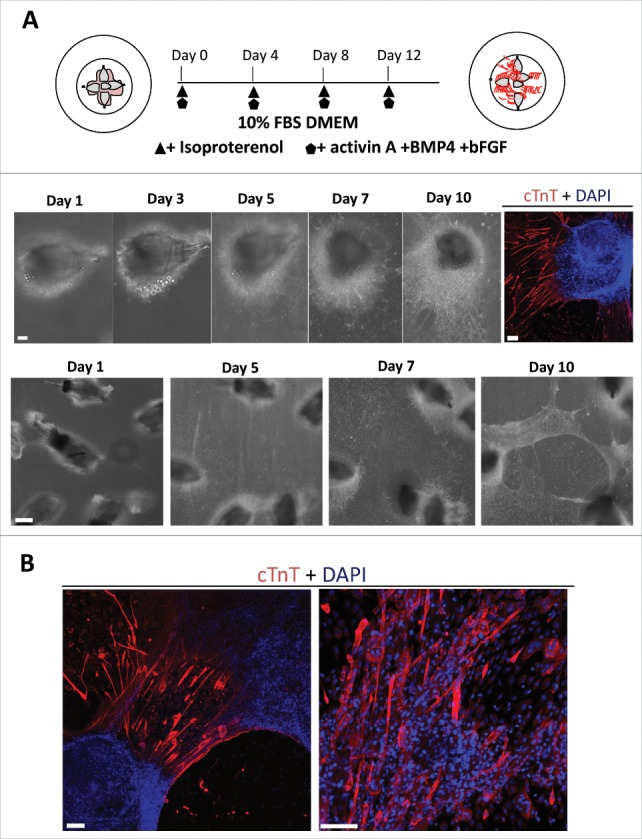
The combination of isoproterenol, activin A, BMP4, and bFGF induced HAP stem cells in vibrissa hair follicles to form beating cardiac-muscle tissue sheets. (A) Schematic diagram of upper parts of five hair follicles forming cardiac-muscle tissue sheets (upper panel). Bright-field image of upper part of hair follicle differentiatimg to cardiac muscle cells and immunostaining (middle panel). Red = cTnT, Blue = DAPI, Bars = 100 µm. Cardiac-muscle tissue sheet formation process (lower panel). Bar = 500 µm. (B) Immunostaining of beating cardiac-muscle tissue sheets. cTnT-positive cardiac muscle cells were extensively distributed within the sheets. Red = cTnT, Blue = DAPI. Bars = 100 µm.
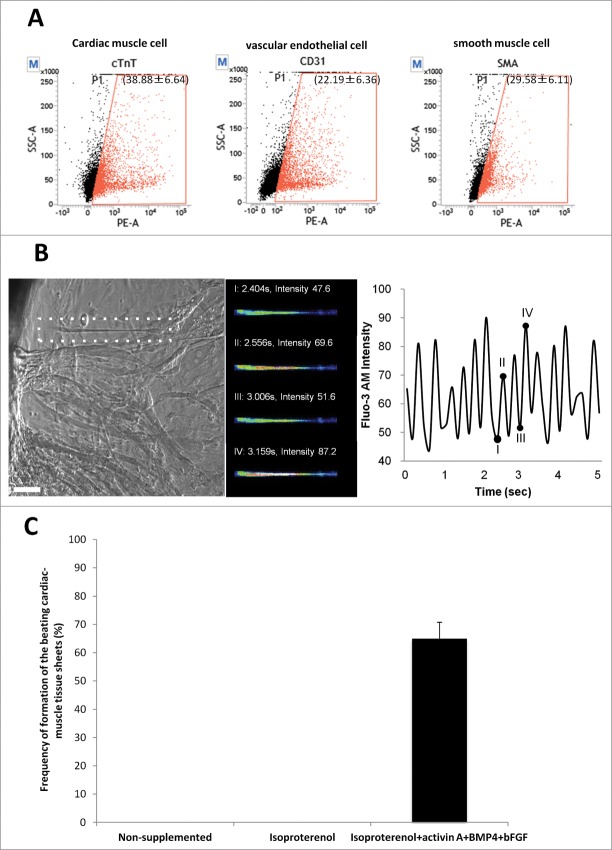
Flow cytometry analysis of the harvested cardiac-muscle tissue sheets showed that sheets contained 38.88 ± 6.64% cTnT-positive cardiac muscle cells; 22.19 ± 6.39% CD31-positive vascular endothelial cells; and 29.58 ± 6.11% smooth muscle actin-positive cells (Fig. 4A). Intercellular Ca2+ was observed in the beating cardiac-muscle tissue sheets using Fluo-3 (Fig. 4B).
Figure 4.
Flow cytometry analysis of beating cardiac-muscle tissue sheets. (A) Flow cytometry analysis of harvested cardiac-muscle tissue sheets showed that the sheets contain 38.88 ± 6.64% cTnT-positive cells (left panel); 22.19 ± 6.36% CD31-positive cells (middle panel); and 29.58 ± 6.11% SMA-positive cells (right panel). (B) Intercellular Ca2+ imaging of cardiac-muscle tissue sheets. Phase contrast image of cardiac muscle cells loaded with Flou-3 (white dashed area). Images were obtained every 150 msec (left panel). Bar =100 µm. Fluo-3 image at 4 points (I, II, III, IV) showing time course of Fluo-3 intensity change (middle and left panel). (C) High rate of formation of the beating cardiac-muscle tissue sheets from HAP stem cells. Hair follicles were cultured in medium supplemented with isoproterenol, activin A, BMP4 and bFGF, where cardiac-muscle tissue sheets formed at 65.0 ± 5.77%. No cardiac tissue sheets formed in non-supplemented medium or medium supplemented with isoproterenol alone.
To increase cardiac-muscle sheet formation, 10 cultures of 5 upper parts of hair follicles were placed on the bottom of glass culture dishes that were supplemented with isoproterenol alone, the combination isoproterenol, activin A, BMP4 and bFGF, or not supplemented. After 14 days of culture, the hair-follicle cultures supplemented with the combination of isoproterenol, activin A, BMP4 and bFGF formed beating cardiac-muscle tissue sheets at 65.0 ± 5.77%. In contrast, cardiac-muscle tissue sheets were not formed in the non-supplemented cultures or in cultures supplemented with isoproterenol alone (Fig. 4C).
Wang et al.11 have shown that transplantation of mesenchymal stem cells significantly increased local recruitment of macrophages to facilitate cardiac muscle repair. Wada et al.12 reported that induced cardiomyocyte-like cells (iCMs) can be directly generated from mouse cardiac fibroblasts in vitro and in vivo by transduction of 3 transcription factors: Gata4, Mef2c, and Tbx5, collectively termed GMT. Berry et al.13 reported that nestin-expressing interstitial cells can differentiate to cardiomyocytes, which could be used for regeneration of the dystrophic heart. Sawa et al.14 reported that the transplantation of neonatal cardiomyocyte or myoblast sheets could improve cardiac function in heart failure in animal models. Kawamura et al.15 reported that the transplantation of human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs) or hiPS-CM sheets could improve cardiac function in a porcine ischemic cardiomyopathy model. The transplantation of hiPS-CMs sheets along with a pedicled omental flap resulted in a new myocardium.16
In the present study, we demonstrate that HAP stem cell differentiation to beating cardiac muscle cells was directed by isoproterenol and that further additions of activin A, BMP4, and bFGF resulted in the formation of beating cardiac-muscle tissue sheets. The method described here is appropriate for future use of human hair follicles to grow nestin-expressing HAP stem cells in sufficient quantities to differentiate into heart muscle sheets to be used for heart muscle regeneration in the clinic.
Materials and methods
GFP transgenic mice
C57BL/6-EGFP mice (GFP mice) were used to isolate the vibrissa hair follicles.17 All animal experiments were conducted according to the Guidelines for Animal Experimentation at Kitasato University.
Isolation of vibrissa hair follicles and differentiation to cardiac muscle cells
The vibrissa hair follicles from GFP mice were isolated as described previously.10 For differentiation to cardiac muscle cells, the upper part of hair follicles were layered onto Matrigel™(BD Biosciences, Bedford, MA) in 35 mm glass-bottom culture dish (MatTeK corporation, Ashland, MA). The follicles were cultured in DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS), 50 µg/ml gentamycin, 2 mM L-glutamine (GIBCO, Grand Island, NY), 10 mM Hepes (MP Biomedicals, Santa Ana, CA), supplemented with isoproterenol (3 µM) (Sigma-Aldrich) alone, or in combination with bone morphogenetic protein 4 (BMP4) (10 ng /ml ) (HumanZyme, Chicago, IL); activin A (10 ng /ml) (HumanZyme); basic fibroblast growth factor (bFGF) (5 ng /ml) (Millipore, Temecula, CA). Beating cardiac muscle cells and cardiac-muscle tissue sheets were visualized and recorded with a BioStation IM-Q (Nikon, Tokyo, Japan) and video microscope camera (ScopPad-500, GelleX, Tokyo, Japan).
Immunofluorescence staining
The cells were incubated with anti-cardiac toroponin T (cTnT) mouse monoclonal antibody (1:250, Gene Tex, Hsinchu City, Taiwan) in blocking buffer (4% BSA, 0.5% Triton X100, 0.04% NaN3 in PBS) at room temperature for 3 hours. The cells were then incubated with goat anti-mouse IgG conjugated with Alexa Flour 568® (1:400, Molecular Probes, Eugene, Oregon) and 4′, 6-diamino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes) in blocking buffer for 2h at room temperature. The images were visualized using a LSM 710 microscope (Carl Zeiss, Germany).
Flow cytometry
The cells were incubated with anti-cTnT mouse monoclonal antibody (1:200) and anti-smooth muscle actin (SMA) mouse monoclonal antibody (1:200; Lab Vision, Fremont, CA) as the primary antibody and phycoerythrin (PE)-anti-mouse CD31 rat monoclonal antibody (1:200, BD science). Secondary antibodies were goat anti-mouse IgG H&L phycoerythrin (1:500; Abcam, Cambridge, UK). The cells were analyzed by FACS Verse (BD Bioscience), were analyzed by FACS suite™ software (BD Bioscience). Flow cytometry analyses were repeated in triplicate.
Intracellular calcium imaging
The cells were washed with HEPES Ringer solution at 37°C, and were loaded with Fluo-3AM (Molecular Probes) for 20 min at 37°C. Fluo-3-AM fluorescence (excitation at 508nm and emission at 527 nm) of beating cardiac sheets was measured every 150 msec with the LSM 710 microscope.
Statistical analysis
The experimental data are expressed as the mean ± SD. Statistical analyses were performed with the unpaired Student's t-test with a P-value of <0.05 considered significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Grant-in-Aid for Scientific Research (C) 23591653 from the Ministry of Education, Science, Sports, and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan Government (Assistance for Strategic Creation of Research Basis, 2014–2016), and the Terumo Life Science Foundation (to Y. Amoh).” This study was also supported by the US National Institute of Neurological Disorders and Stroke grant NS086217.
References
- [1].Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM.. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 2003; 100:9958-61; PMID:12904579; http://dx.doi.org/ 10.1073/pnas.1733025100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM.. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA 2004; 101:13291-95; PMID:15331785; http://dx.doi.org/ 10.1073/pnas.0405250101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM.. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA 2005; 102:5530-34; PMID:15802470; http://dx.doi.org/ 10.1073/pnas.0501263102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amoh Y, Li L, Katsuoka K, Hoffman RM.. Chemotherapy targets the hair-follicle vascular network but not the stem cells. J Invest Dermatol 2007; 127:11-15; PMID:16841031; http://dx.doi.org/ 10.1038/sj.jid.5700486 [DOI] [PubMed] [Google Scholar]
- [5].Hoffman RM. Nestin-expressing hair follicle-accessible pluripotent (HAP) stem cells for nerve and spinal cord repair. Cells Tissues Organs 2014; 200:42-47; PMID:25766743; http://dx.doi.org/ 10.1159/000366098 [DOI] [PubMed] [Google Scholar]
- [6].Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, et al.. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 2011; 10:830-39; PMID:21330787; http://dx.doi.org/ 10.4161/cc.10.5.14969 [DOI] [PubMed] [Google Scholar]
- [7].Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM.. Implanted hair follicle stem cells form Schwann cells which support repair of severed peripheral nerves. Proc Natl Acad Sci USA 2005; 102:17734-38; PMID:16314569; http://dx.doi.org/ 10.1073/pnas.0508440102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amoh Y, Li L, Katsuoka K, Hoffman RM.. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008; 7:1865-69; PMID:18583926; http://dx.doi.org/ 10.4161/cc.7.12.6056 [DOI] [PubMed] [Google Scholar]
- [9].Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y, Saito N, Katsuoka K, Hoffman RM.. The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 2013; 114:1674-84; PMID:23444061; http://dx.doi.org/ 10.1002/jcb.24509 [DOI] [PubMed] [Google Scholar]
- [10].Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y.. From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 2015; 14:2362-66; PMID:25970547; http://dx.doi.org/ 10.1080/15384101.2015.1042633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang M, Zhang G, Wang Y, Liu T, Zhang Y, An Y, Li Y.. Crosstalk of mesenchymal stem cells and macrophages promotes cardiac muscle repair. Int J Biochem Cell Biol 2015; 58:53-61; PMID:25462160; http://dx.doi.org/ 10.1016/j.biocel.2014.11.003 [DOI] [PubMed] [Google Scholar]
- [12].Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, et al.. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA 2013; 110:12667-72; PMID:23861494; http://dx.doi.org/ 10.1073/pnas.1304053110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berry SE, Andruszkiewicz P, Chun JL, Hong J.. Nestin expression in end-stage disease in dystrophin-deficient heart: implications for regeneration from endogenous cardiac stem cells. Stem Cells Transl Med 2013; 2:848-61; PMID:24068741; http://dx.doi.org/ 10.5966/sctm.2012-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sawa Y, Miyagawa S.. Present and future perspectives on cell sheet-based myocardial regeneration therapy. Biomed Res Int 2013; 2013:583912; PMID:24369013; http://dx.doi.org/ 10.1155/2013/583912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, et al.. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012; 126:S29-37; PMID:22965990; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.111.084343 [DOI] [PubMed] [Google Scholar]
- [16].Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, Sougawa N, Kawamura T, Daimon T, Shimizu T, et al.. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 2013; 128:S87-94; PMID:24030425; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.112.000366 [DOI] [PubMed] [Google Scholar]
- [17].Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y.. ‘Green mice’ as a source of ubiquitous green cells. FEBS Ltrs 1997; 407:313-9; PMID:9175875; http://dx.doi.org/ 10.1016/S0014-5793(97)00313-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.