Abstract
Evidence from both clinical and experimental studies indicates that Di-peptidyl peptidase-IV (DPP-4) inhibition may mediate favorable effects on the cardiovascular system. The objective of this study was to examine the acute effects of DPP-4 inhibition on vascular responses and to study the underlying mechanisms of alteration in tone. Aortic segments from C57BL/6 mice were treated with vasoconstrictors and exposed to various doses of alogliptin, a selective DPP-4 inhibitor. Vasodilator responses were evaluated using pathway specific antagonists to elucidate mechanisms of response. In parallel experiments, cultured human umbilical vein endothelial cells (HUVEC) were exposed to varying concentrations of alogliptin to evaluate the effects on candidate vasodilator pathways.
Alogliptin relaxed phenylephrine and U46619 pre-constricted aortic segments in a dose dependent manner. Relaxation responses were not affected by the glucagon-like peptide-1 (GLP-1) receptor antagonist, exendin fragment 9–39 (88±6 vs. 91±2, p<0.001). Vascular relaxation to alogliptin was significantly decreased by endothelial denudation, L-NG-monomethyl-arginine citrate (L-NMMA) and by the soluble guanylate cyclase inhibitor ODQ. DPP-4 inhibition induced relaxation was completely abolished by a combination of L-NMMA, charybdotoxin and apamin. Incubation of HUVECs with alogliptin resulted in eNOS and Akt phosphorylation (Ser1177 and Ser473 respectively) paralleled by a rapid increase in nitric oxide. Inhibition of Src kinase decreased eNOS and Akt phosphorylation, in contrast to a lack of any effect on insulin mediated activation of the eNOS-Akt, suggesting that alogliptin mediates vasodilation through Src kinase mediated effects on eNOS-Akt.
DPP-4 inhibition by alogliptin mediates rapid vascular relaxation via GLP-1 independent, Src-Akt-eNOS mediated NO release and the activation of vascular potassium channels.
Keywords: DPP-4, Alogliptin, Inhibition, Vascular, Cardiovascular
1. Introduction
Dipeptidyl Peptidase-4 (DPP-4) is a widely expressed glycoprotein peptidase that exhibits complex biological roles, including cell membrane associated activation of intracellular signal transduction pathways, cell-to-cell interaction, and enzymatic activity, exhibited by both membrane-anchored and soluble forms of the enzyme (Drucker, 2006; 2007). Inhibition of the DPP-4 system represents a new approach in the treatment of Type-2 diabetes by virtue of its effects on prolonging the half-life of incretins such as glucagon-like-peptide-1 (GLP-1) and glucagon induced peptide (GIP). Elevation in the levels of these incretin hormones results in favorable post-prandial glycemic profile and results in the lowering of surrogate measures of glycemia control (Drucker, 2007; Baggio & Drucker, 2007). GLP-1 is well known to exert important effects on multiple pathways including regulation of PI3-kinase and Akt through ligation of the GLP-1 receptor (Ban et al., 2008; Zhao et al., 2006). Previous studies have demonstrated important beneficial effects of GLP-1 in conditions such as cardiac remodeling and in the regulation of endothelial function (Zhao et al., 2006; Nikolaidis et al., 2004; Basu et al., 2007; Green et al., 2008). The effects of DPP-4 inhibition on cardiovascular function have thus been typically attributed to the obligatory elevation in GLP-1 levels that also leads to improvement in fasting and post-prandial glycemia indices. In contrast to the known effects of DPP-4 inhibition on GLP-1 mediated phenomena, much less is known about the direct cardiovascular effects of DPP-4 enzyme inhibition. DPP-4 is widely expressed in the cardiovascular system and is abundantly expressed in endothelial cells (Drucker, 2006; McIntosh, 2008; Moritoh et al., 2008). DPP-4 by virtue of its protease activity has been implicated in the metabolism of kinins, such as substance P and bradykinin (Ahmad et al., 1992; Byrd et al., 2007). Thus we hypothesized that DPP-4 inhibition may have important effects on vascular tone control which may be independent of the elevation of GLP-1/GIP. Alogliptin is a highly specific inhibitor of DPP-4 and has been shown by previous studies to improve glycemic indices in patients with Type II diabetes mellitus without changes in weight (Moritoh et al., 2008; Neumiller et al., 2010; Moritoh et al., 2009). Accordingly, the aim of our study was to examine the acute effects of DPP-4 inhibition using alogliptin on vascular function and its role in modulating vasodilator pathways.
2. Materials and methods
All experiments were performed in accordance with the guidelines set forth by the University Laboratory Animal Accreditation Committee at The Ohio State University.
2.1. Animals and materials
Male C57BL/6 (12 week old, n=40) were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed for at least 2 weeks before experimentation. Alogliptin (chemical name 2-({6-[(3R)-3-aminopiperidinyl-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2 H)-yl}methyl)benzonitrile monobenzoate) was provided by Takeda Pharmaceuticals, Oak Grove, IL. All other chemicals were obtained from Sigma Chemicals (St. Louis, MO).
2.2. Myograph experiments
Mice were euthanized by cervical dislocation. Thoracic aortas were dissected from the animals and immediately immersed in a physiological salt solution (PSS) buffer (sodium chloride, 130 mEq/L; potassium chloride, 4.7 mEq/L, calcium dichloride, 1.6 mEq/L; magnesium sulfate, 1.17 mEq/L; potassium diphosphate, 1.18 mEq/L; sodium bicarbonate, 14.9 mEq/L; EDTA, 0.026 mEq/L; and glucose, 99.1 mg/dL [5.5 mmol/L]; pH, 7.4) at room temperature. The aortas were than cleaned of adherent fat/connective tissue and were cut into rings of 2 mm to 3 mm length under a microscope. Vessel rings were mounted in a standard 5 ml organ bath (filled with PSS buffer). The bath medium was maintained at 37 °C with a pH of 7.4 and aerated continuously with 95% oxygen and 5% carbon dioxide. Extra care was taken to ensure that the endothelium was not damaged during the whole process of tissue preparation and mounting.
Briefly, the aortic rings were allowed to equilibrate for 90 min at a resting tension of 700 mg, with the PSS buffer changed every 15–25 min. All preparations were contracted with isotonic, high-potassium physiological saline solution (KPSS 120 mmol/L) to achieve maximum tension. Endothelial integrity was assessed with the single addition of acetylcholine (10 μmol/L) which caused relaxation of the aortic segments, sub maximally precontracted with phenylephrine (10–100 nmol/L). The vessels were then washed thoroughly and allowed to equilibrate for 1 h before beginning experiments. In some experiments, the rings were exposed to one or a combination of inhibitors for 30 min before the addition of phenylephrine.
2.3. Nitric oxide EPR measurements
Cultured endothelial cells (HUVECs passage 6–9) were serum-starved overnight.
The endogenous (Moritoh et al., 2009) N-L-arginine was then exchanged for (Shinobu et al., 1984) N-L-arginine by incubatingcells with Tyrode's solution supplemented with 84 mg/L (Shinobu et al., 1984) N-L-arginine (Sigma) for 30 min at 37 °C. After incubation, cells were washed with PBS and then incubated for 15 min with PBS containing CaCl2 and MgCl2 (Sigma), calcium ionophore A21387 (10 μM, Alexis Biochemicals), FeSO4 (3.5 mM), ammonium N-methyl-d-glucamine dithiocarbamate (NH4MGD) (20.8 mM synthesized according to the procedure developed by Shinobu et al. (1984)), and alogliptin (100 μM) at a final volume of 500 μL. Supernatants were then collected and stored at −87 °C until EPR measurements were carried out. Control experiments involved solutions in the absence of alogliptin, or in the presence of the nitric oxide donor, S-nitroso-N-acetyl-DL-penicillamine (SNAP, 50 μM, World Precision Instruments). EPR measurements of the samples were performed using quartz flat cell at room temperature on a Bruker EMX X-Band. Instrument parameters were as follows: modulation frequency; 100 kHz; modulation amplitude, 7.35 G; microwave power, 20 mW; scan time, 83.88 s; number of scans, 10.
2.4. Western blotting analysis
The cells were briefly washed with ice cold phosphate-buffered saline. Cells were then scraped in an extraction lysis buffer containing 25 mM bicine buffer (M-PER Mammalian Protein Extraction; Thermo Scientific). Cell debris was removed by microcentrifugation. The protein concentration of cell lysates was determined using Bio-Rad DC protein assay according to the manufacturer's instructions. The supernatants were then boiled with a Laemmli sample buffer for 5 min. Samples (25 μg of protein per lane) were separated on 8% sodium dodecyl sulfate-polyacrylamide gels at 30 mA for 3 h. Separated proteins were transferred electrophoretically into polyvinylidene difluoride membrane (Immuno-Blot; Bio-Rad) at 160 mA for 130 min. Membranes were blocked with a blocking buffer [phosphate-buffered saline and 0.1% Tween 20 (Acros Organics) containing 5% skim milk at 4 °C overnight.] For the detection of phosphorylated proteins, membranes were incubated with the respective primary antibody (β-Actin Santa Cruz, p-Akt Ser473 Cell Signaling and p-eNos Ser 1177 BD Transduction Laboratories, dilution 1:20,000, 1:1,000 and 1:500 respectively) overnight at 4 °C. After washing, the membranes were incubated with the secondary antibody (peroxidase-labeled anti-goat, anti-rabbit and anti-mouse immunoglobulin G, dilution of 1:20,000, 1:1,000, and 1:1,000 (Santa Cruz and Cell Signaling)) respectively at room temperature for 90 min. Membranes were stripped and reblotted with anti-eNOS and anti-Akt antibody, 1:1,000 and 1:200 (Cell Signaling and Santa Cruz) respectively to verify the total Akt and eNOS protein amount in each lane. Prestained markers (Precision Plus Protein; Bio-Rad) were used for molecular mass determinations. Immunoreactive bands were detected by enhanced chemiluminescence (Super Signal West Pico; Thermo Scientific).
2.5. Cell culture and nitric oxide release assays
Human umbilical vein endothelial cells (HUVECs) were maintained in medium 200 (Invitrogen) supplemented with a growth extract (Invitrogen) and Fetal Bovine Serum. All experiments were conducted with confluent cultures of cells used before their tenth passage. HUVECs were serum-starved overnight and then incubated for 30 min in absence or presence of various inhibitors prior to alogliptin and/or insulin treatment, as described in the figure legends. The total nitrate and nitrite in the media was estimated using R&D Systems Nitric Oxide Analysis kit (R&D Systems, INC, McKinley Place NE). To summarize, HUVECs were cultured in 6-welled plates until fully confluent. The wells were treated with different concentrations of alogliptin to measure the dose response effect. Also, cells were treated with 50 μM of alogliptin for different time periods to obtain a temporal response curve. After respective treatments, the media was collected and centrifuged in 10,000 MW filter tubes. The nitric oxide content present in the medium was determined following the instructions provided by R&D Systems using the standard solutions included in the kit.
2.6. Data analysis
All data are expressed as mean±SE unless otherwise specified. Differences among the groups were tested by one-way ANOVA and Boneferroni's post-hoc test. In addition, the interaction between the drug and angiotensin II was analyzed by two-way ANOVA using Graphpad Prism software (Version 4). A p value of 0.05 was considered statistically significant.
3. Results
3.1. Alogliptin induces vascular relaxation via NO and EDHF-mediated mechanisms
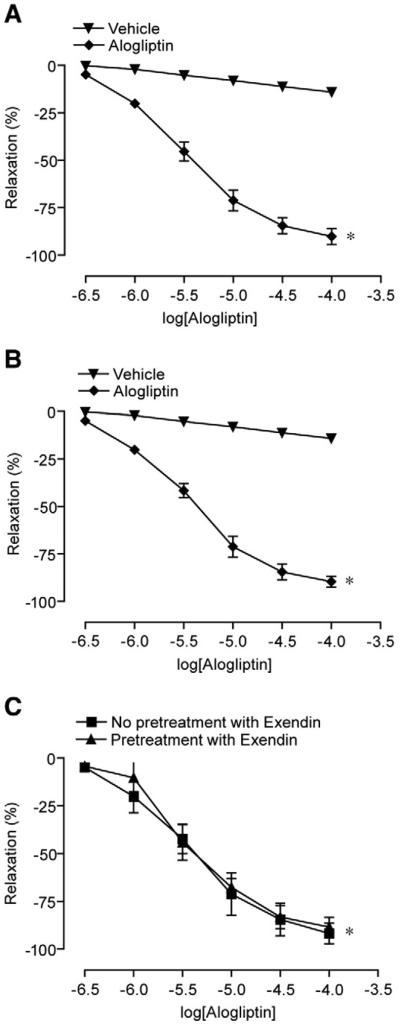
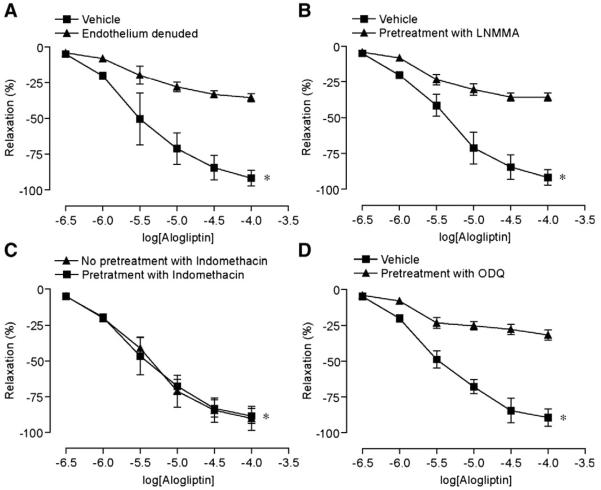
Fig. 1 depicts vascular responses to alogliptin after preconstriction with phenylephrine and U46619. Alogliptin caused dose-dependent relaxation of aortic rings irrespective of the agonist used (Fig. 1A, B). Relaxations due to alogliptin were unaffected by pre-incubating the artery rings with the specific GLP-1 receptor antagonist exendin (9–39) fragment (1 mM) suggesting that these effects were independent of GLP-1 (Fig. 1C). Relaxation effects of alogliptin were reduced by endothelial denudation and the NO synthase inhibitor, LNMMA (1 mM) (Fig. 2A, B). The cyclooxygenase inhibitor, indomethacin (10 μM), had no effect on relaxation (Fig. 2C). To evaluate the possibility that alogliptin induced relaxations involve soluble guanylyl cyclase, the endothelium-intact aortic rings were pre-incubated with selective guanylyl cyclase inhibitor ODQ (5 μM). As shown in Fig. 2D, ODQ causes significant inhibition of vasorelaxation evoked by alogliptin. Supplemental Table 1 provides the absolute tensions accomplished with the various interventions.
Fig. 1.
Effects of alogliptin on vascular relaxation in pre-constricted aortic segments. A. Phenylephrine pre-constricted segments. B. U46619 pre-constricted segments. C. Effect in the presence of a non-active GLP-1 fragment (pre-incubation with Exendin fragment 9–39 (1 mM) for 15 min). n=4-5/ intervention. *p<0.05 vs. control in all experiments for peak relaxation. Results are presented as mean±SE.
Fig. 2.
Endothelial dependence of alogliptin mediated vascular relaxation. A. Response of pre-constricted aortic rings to alogliptin after endothelial denudation B. Response following pre-incubation with LNMMA (1 mM) and C. Response following pre-incubation with Indomethacin (10 μM). D. Relaxation response following pre-incubation with ODQ (5 μM). Results are presented as mean±SE; n=5–6/intervention. * p<0.01 versus control for peak relaxation.
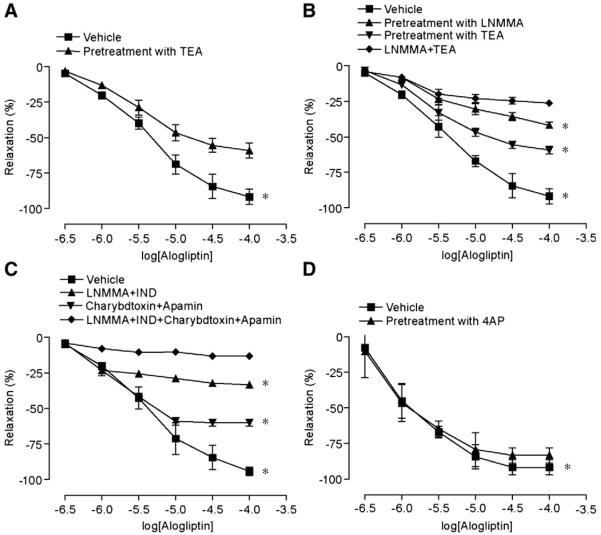
Fig. 3A depicts responses to alogliptin in the presence of tetraethylammonium chloride (TEA) (6 μM). As shown, TEA partially inhibited vasodilation by alogliptin. We then assessed the effects of charybdotoxin (100nM) plus apamin (100nM) and the effects of combined eNOS inhibition with the inhibition of EDHF mediated responses (Fig. 3B, C). Charybdotoxin is an inhibitor of intermediate conductance Ca++ dependent potassium (K+) channels while apamin is an inhibitor of small conductance Ca++ activated K+ channels. When used in combination, charybdotoxin and apamin represent a potent strategy to block Ca++ dependent K+ channels. (McGuire et al., 2001; Novakovic et al., 2006) Inhibition of eNOS in conjunction with total blockade of Ca++ dependent K channels resulted in near complete abolition of alogliptin mediated vasodilation. Pre-incubation of aortic rings with 4-Aminopyridine (4AP) (3 μM) (a selective inhibitor of voltage gated potassium channels) had no effect on alogliptin induced relaxation (Fig. 3D). These findings indicate that alogliptin induced endothelium-dependent relaxations of mice aortic rings, which include both NO and EDHF-mediated components. Moreover, the EDHF component of alogliptin-mediated relaxation involves calcium dependent potassium channels.
Fig. 3.
Role of potassium channels in alogliptin induced vasorelaxation. Mean cumulative relaxation of aortic rings in response to alogliptin after pre-incubation with A. TEA (6 μM), B. LNMMA, TEA and combined LNMMA and TEA, C. Charybdotoxin (100 nM), Apamin (100 nM), and combination of LNMMA, Charybdotoxin, and Apamin D. 4AP (1 mM). Results are presented as mean±SE; n=4–5/intervention; p<0.05 versus control for peak relaxation.
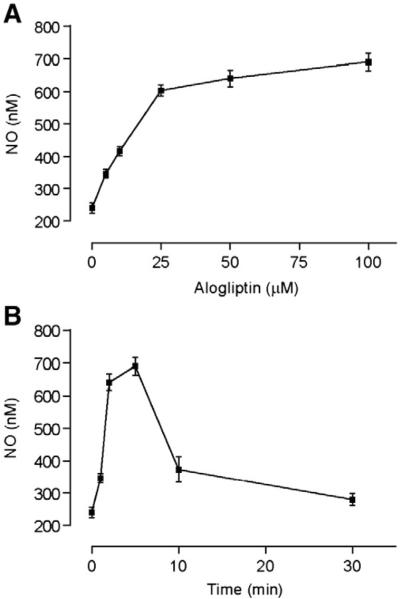
3.2. Release of NO by alogliptin
In order to calculate the dose and time responses for NO release, HUVECs were treated with a fixed dose of alogliptin (50 μM) for varying periods of time, as well as various doses of alogliptin for 5 min. Alogliptin caused rapid release of NO peaking within 5 min, with a maximum effect on NO production noted at 50 μM (Fig. 4A, B).
Fig. 4.
Alogliptin causes rapid NO release from HUVECs in dose and time dependent fashion. A. Dose dependent increase in the NO level after incubating HUVECs with various doses of alogliptin for 5 min. B. Time dependent NO release after incubating HUVECs with 50 μM alogliptin. All experiments were conducted 3 times and values represent mean±SD.
3.3. Alogliptin induces endothelium-dependent relaxations through Src and PI3-kinase/Akt pathways
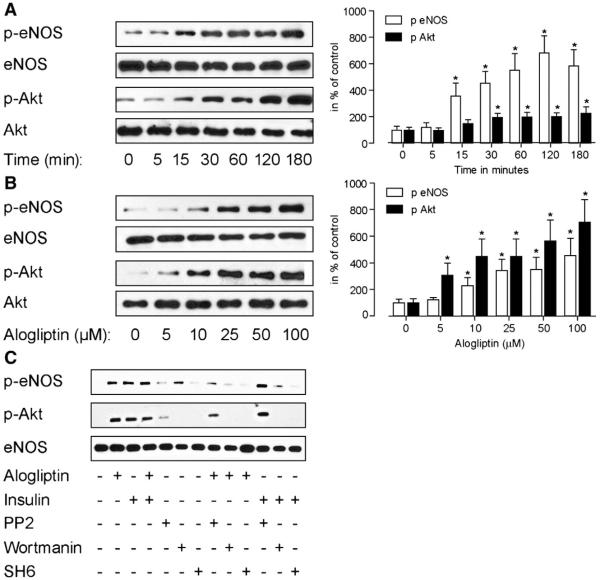
To further characterize the pathway involved in alogliptin mediated endothelial dependent vasodilation, we investigated its effects on HUVECs. Alogliptin induced rapid phosphorylation of both Akt and eNOS in a dose and time dependent fashion (Fig. 5A, B). No effects were noted on steady state levels of eNOS. Interestingly, phosphorylation of both Akt and eNOS was inhibited in presence of wortmannin (100 nM) and SH6 (1 μM), confirming the dependence of these effects on the PI3K/Akt pathway (Fig. 5C). Presence of the Src-kinase inhibitor PP2 (10 μM) reduced both Akt and eNOS phosphorylation in response to alogliptin but did not inhibit their phosphorylation with insulin (Fig. 5C). The addition of alogliptin to insulin did not further potentiate the PI3K/Akt pathway.
Fig. 5.
Alogliptin stimulates phosphorylation of eNOS in a time and dose dependent manner that requires the activation of Src kinase and PI-3 kinase. HUVECs were serum starved for 12 h prior to alogliptin treatment. A. Time-dependent phosphorylation of eNOS and Akt after treatment of cells with alogliptin (50 μM). B. Dose-dependent response of eNOS and Akt phosphorylation to alogliptin. C. Effect on phosphorylation of eNOS and Akt after the pretreatment of cells with Wortmanin (100 nM), PP2 (10 μM) and SH6 (1 μM) 30 min prior to treatment with alogliptin or insulin. A representative plot is demonstrated. n=3 separate experiments. *p<0.05 vs. control.
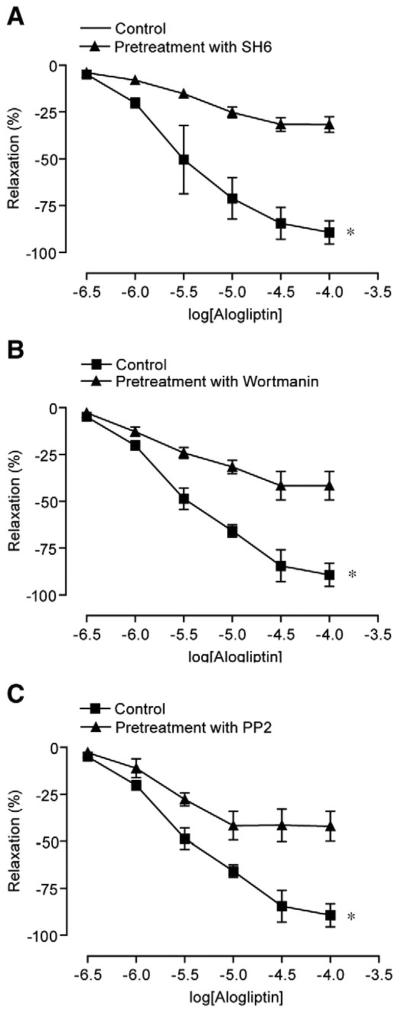
Myograph experiments were performed to confirm a physiologic role for Src-kinase and PI3-kinase/Akt pathways in response to alogliptin. Pre-incubation of vessel rings with PP2 (50 μM), wortmannin (200 nM), and SH6 (10 μM) reduced the relaxation caused by alogliptin to the extent noted with endothelial denudation, suggesting an integral role for these pathways in vasodilation by alogliptin. (Fig. 6A, B, C). In additional experiments, to exclude the possibility that DPP-IV inhibition with alogliptin may be mediating NO/EDHF mediated dilation by increase in bradykinin levels, we performed experiments with alogliptin (100 μM) in presence of the B2 receptor antagonist (HOE-140). HOE-140 had no effects on alogliptin mediated dilation (Supplemental Fig. 1).
Fig. 6.
Alogliptin induced relaxation involves SRC, P13K, AKT, eNOS and cGMP pathways. Mean cumulative relaxation of aortic rings in response to alogliptin after pre-incubation with A. SH6 (10 μM), B. Wortmanin (200nM) and C. PP2 (50 μM Results are presented as mean±SEM of 4–5 experiments; *p<0.05 vs. vehicle in all experiments for peak relaxation.
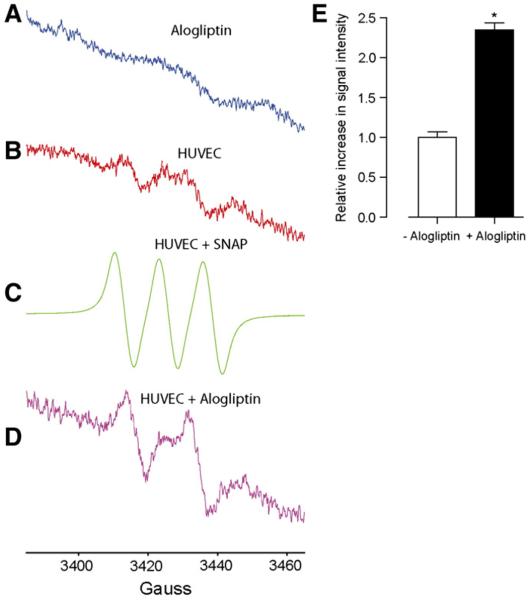
To exclude the possibility that the nitrile moiety in alogliptin may release NO, spin trapping using electron paramagnetic resonance spectroscopy (EPR) was employed to address the ability of alogliptin to donate nitric oxide (NO) (Xia & Zweier, 1997). Fig. 7 shows the EPR spectra of alogliptin treated and untreated cells. Alogliptin alone in the absence of cells did not show the distinctive triplet spectra for NO suggesting that alogliptin is not an NO donor by itself (Fig. 7A) while serum-starved BAECs in the absence of alogliptin only showed a very low flux of NO (Fig. 7B). The treatment of cells with an authentic NO-donor, SNAP, gave a distinctive (Moritoh et al., 2009) NO-Fe(MGD)2 triplet signal (Fig. 7C). However, (Shinobu et al., 1984) N-L-arginine substituted BAECs treated with alogliptin for 10 min gave a distinctive (Shinobu et al., 1984) NO-Fe(MGD)2 doublet signal, whose intensity is relatively higher compared to alogliptin-untreated cells, suggesting that NO production is endogenously derived.
Fig. 7.
X-band EPR spectra of NO trapping by Fe(MGD)2. (A) In the presence of physiological saline solution (PSS) and alogliptin but in the absence of HUVECs. (B) In serum-starved HUVECs in the absence of alogliptin. (C) In (Shinobu et al., 1984) N-L-arginine substituted HUVECs treated with the NO donor, SNAP (50 μM). (D) In (Shinobu et al., 1984) N-L-arginine substituted HUVECs treated with alogliptin (100 μM). (E) Bar graph shows the change in the EPR signal intensity following the treatment of cells with alogliptin compared to control.
4. Discussion
The main findings of this work are that alogliptin causes acute vascular relaxation in a non-diabetic model through GLP-1 independent pathways that are both nitric oxide and EDHF dependent. NO release occurs via PI3K-Akt dependent phosphorylation of eNOS with Src-kinase playing a proximal role in these changes.
Our findings may have important implications for the treatment of Type II diabetes. A substantial portion of diabetics are hypertensive, with these patients often requiring multiple drugs for effective control (Patel et al., 2007; Hansson et al., 1998; Cushman et al.; Jackson et al., 2008; Mistry et al., 2008). Strategies that lower blood glucose in addition to favorable effects on vascular tone would be intrinsically advantageous in this population of patients. Prior studies have suggested important effects of PPAR. agents in controlling blood pressure with randomized controlled trials suggesting a 2–4 mm reduction in systolic blood pressure (Dormandy et al., 2005). The incretin system (GLP-1 receptors, GIP receptors and DPP-4) is widely expressed in the cardiovascular system with prior work suggesting that ligation of the GLP-1 receptor by GLP-1 in the cardiomyocyte and the endothelial cells may result in beneficial effects, including up-regulation of cell survival pathways and endothelial function (Ban et al., 2008; Basu et al., 2007; Green et al., 2008; Sauve et al.; Grieve et al., 2009; Nystrom et al., 2005). Thus increases in GLP-1 as a consequence of DPP-4 inhibition may modulate favorable effects on endothelial function and cardiovascular remodeling in response to ischemia reperfusion (Basu et al., 2007; Bose et al., 2005) (Zhao et al., 2006; Nikolaidis et al., 2004). Additionally a number of lines of evidence suggest GLP-1R independent vascular effects of GLP-1 and additional effects mediated by truncated peptides of GLP-1 such as GLP-1 (9-36) (Ban et al., 2009). The vasodilatory effects of both GLP-1 and GLP-1 (9–36) correlate with an increase in cyclic guanosine monophosphate (cGMP) release and are typically attenuated by preincubation of vessels with nitric oxide synthase (NOS) inhibitors (Ban et al., 2008), suggesting that at least part of their vasodilatory mechanism is nitric oxide (NO)/cGMP-dependent. Thus the effects of GLP-1 and related peptides are believed widely to mediate the effects of DPP-4 inhibition. Recent studies have also suggested independent vascular protectant effects of DPP-4 inhibition although it is unclear from these studies as to what extent these effects are related to direct effects of DPP-4 versus its effects on GLP-1 elevation (Fadini et al.). We therefore were interested in understanding the effects of the DPP-4 inhibition in the absence of systemic elevations of GLP-1/GIP. Performance of the experiments in an organ chamber prevented the confounding of systemic effects of circulating GLP-1. The GLP-1 receptor ligation with the exendin fragment 9–39, did not antagonize the effects of the drug, signifying that the mechanism of vascular relaxation was independent of GLP-1. It is worth mentioning that some of the in-vivo effects of DPP-4 inhibition may also be modulated by GIP at least in animal models. Our data are suggestive of complex effects of DPP-4 inhibition that mediate relaxation through predominantly endothelium dependent but via both NO dependent and EDHF mediated pathways. The NO dependent effects were more dominant (when assessed as the magnitude of inhibition with L-NMMA or endothelial denudation) compared to the EDHF effects, which prompted us to investigate this pathway in detail. These results are in contrast to previous experiments by Green et al. (2008) who have shown that relaxant effects in the rat aorta were mediated via specific activation of ATP-sensitive potassium channels. The dichotomization of effects as NO and EDHF dependent may not account for NO itself exerting hyperpolarizing effects. The time course of the effects on PI3K and Akt activation by alogliptin corresponded to the NO release and vascular relaxation, suggesting that this is indeed a physiologically relevant pathway. Our results further indicate that Src-kinase is a proximal mediator of these effects. In this regard, the signaling cascade differs from the classic activation of PI3K-Akt by Insulin (Zeng et al., 2000). The effects of DPP-4 inhibition on PI3K-Akt phosphorylation were not incremental to those induced by insulin. However this may reflect maximal activation of these enzymatic pathways under these experimental conditions. In pathophysiologic states, such as Type II diabetes, there is decreased IRS phosphorylation (tyrosine) and increased serine phosphorylation that results in reduced PI3K-Akt signaling (Zeng et al., 2000; Montagnani et al., 2001). The activation of an alternate signaling pathway mediated by Src-kinase would be beneficial in obviating the proximal defect in insulin-insulin receptor substrate signaling and may be have favorable effects on the endothelial responses. Whether this will occur in-vivo, in the setting of insulin resistance remains to be determined. Another important aspect to consider is whether these effects are truly mediated by DPP-4 inhibition versus a direct effect of the compound tested. Local inhibition of DPP-4 may modulate vascular tone directly via prevention of kinin degradation, such as bradykinin and substance P as DPP-4 is a di-peptidyl-peptidase similar to other neutral endopeptidases found in the vessel wall (Drucker, 2006). We did not assess for DPP-4 activity on the vessel wall as it would have made our study very lengthy. However there is adequate scientific evidence supporting DPP-4 activity in the vessels, especially in endothelial cells (Pala et al.; Takasawa et al.). Our results with HOE-140, a potent inhibitor of the B2 receptor, imply alogliptin mediated vasodilation does not involve bradykinin mediated mechanisms (Supplemental Fig. 1). Dose ranging studies with Alogliptin have been performed that have tested a range of concentration in humans (Covington et al., 2008; Christopher et al., 2008). Using a dose of 25 mg, prior studies have demonstrated peak levels of >100 ng/ml in humans. Higher doses of 100 mg attain plasma levels of >1 μg/ml. The concentration range tested by us in organ chamber experiments corresponds to 23 ng/ml to 231 μg/ml with responses seen clearly at the lower dose range placing the concentrations used in our study well within the range of concentrations achieved with clinical doses of alogliptin used.
In conclusion, we have demonstrated the important effects of the DPP-4 inhibitor alogliptin in modulating vascular tone through NO and EDHF dependent pathways. Our findings would have important implications for the control of blood pressure with these agents.
Supplementary Material
Acknowledgements
This study was supported by an educational grant from Takeda Pharmaceuticals North America to Dr. Rajagopalan. Alogliptin was provided by Takeda Pharmaceuticals. Dr. Rajagopalan was also partially supported by RO1 ES015146, R01ES017290 and R21 DK088522.
Footnotes
See the accompanying review by P. Fadini and A. Avogaro, Cardiovascular effects of DPP-4 inhibition: Beyond GLP-1, doi:10.1016/j.vph.2011.05.001 (this issue).
Supplementary materials related to this article can be found online at doi:10.1016/j.vph.2011.03.001.
References
- Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidase IV and aminopeptidase M metabolize circulating substance P in vivo. J. Pharmacol. Exp. Ther. 1992;260(3):1257–1261. [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Ban K, Hui S, Drucker DJ, Husain M. Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin-based therapies. J. Am. Soc. Hypertens. 2009;3(4):245–259. doi: 10.1016/j.jash.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am. J. Physiol. Endocrinol. Metab. 2007;293(5):E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54(1):146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Byrd JB, Shreevatsa A, Putlur P, Foretia D, McAlexander L, Sinha T, Does MD, Brown NJ. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J. Allergy Clin. Immunol. 2007;120(2):403–408. doi: 10.1016/j.jaci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Christopher R, Covington P, Davenport M, Fleck P, Mekki QA, Wann ER, Karim A. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin. Ther. 2008;30(3):513–527. doi: 10.1016/j.clinthera.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER, Karim A. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin. Ther. 2008;30(3):499–512. doi: 10.1016/j.clinthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Cushman WC, Evans GW, Byington RP, Goff DC, Jr., Grimm RH, Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33(7):1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch. Biochem. Biophys. 2008;478(2):136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br. J. Pharmacol. 2009;157(8):1340–1351. doi: 10.1111/j.1476-5381.2009.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase IV inhibition on arterial blood pressure. Clin. Exp. Pharmacol. Physiol. 2008;35(1):29–34. doi: 10.1111/j.1440-1681.2007.04737.x. [DOI] [PubMed] [Google Scholar]
- McIntosh CH. Dipeptidyl peptidase IV inhibitors and diabetes therapy. Front. Biosci. 2008;13:1753–1773. doi: 10.2741/2797. [DOI] [PubMed] [Google Scholar]
- McGuire JJ, Ding H, Triggle CR. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79(6):443–470. [PubMed] [Google Scholar]
- Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J. Clin. Pharmacol. 2008;48(5):592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J. Biol. Chem. 2001;276(32):30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Chronic administration of alogliptin, a novel, potent, and highly selective dipeptidyl peptidase-4 inhibitor, improves glycemic control and beta-cell function in obese diabetic ob/ob mice. Eur. J. Pharmacol. 2008;588(2–3):325–332. doi: 10.1016/j.ejphar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Combining a dipeptidyl peptidase-4 inhibitor, alogliptin, with pioglitazone improves glycaemic control, lipid profiles and beta-cell function in db/db mice. Br. J. Pharmacol. 2009;157(3):415–426. doi: 10.1111/j.1476-5381.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2010;30(5):463–484. doi: 10.1592/phco.30.5.463. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110(8):955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- Novakovic A, Bukarica LG, Kanjuh V, Heinle H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin. Pharmacol. Toxicol. 2006;99(5):360–364. doi: 10.1111/j.1742-7843.2006.pto_531.x. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 2005;125(1–3):173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Pala L, Ciani S, Dicembrini I, Bardini G, Cresci B, Pezzatini A, Giannini S, Mannucci E, Rotella CM. Relationship between GLP-1 levels and dipeptidyl peptidase-4 activity in different glucose tolerance conditions. Diabet. Med. 2010;27(6):691–695. doi: 10.1111/j.1464-5491.2010.03010.x. [DOI] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- Sauve M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59(4):1063–1073. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinobu LA, Jones SG, Jones MM. Sodium N-methyl-D-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol. Toxicol. (Copenh) 1984;54(3):189–194. doi: 10.1111/j.1600-0773.1984.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Takasawa W, Ohnuma K, Hatano R, Endo Y, Dang NH, Morimoto C. Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem. Biophys. Res. Commun. 2010;401(1):7–12. doi: 10.1016/j.bbrc.2010.08.112. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zweier JL. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc. Natl Acad. Sci. U.S.A. 1997;94(23):12705–12710. doi: 10.1073/pnas.94.23.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101(13):1539–1545. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 2006;317(3):1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.