Abstract
Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) is the most common treatable chronic autoimmune neuropathy. Multiple diagnostic criteria have been established, with the primary goal of identifying neurophysiologic hallmarks of acquired demyelination. Treatment modalities have expanded to include numerous immuno-modulatory therapies, although the best evidence continues to be for corticosteroids, plasma exchange, and intravenous immunoglobulins (IVIg). This review describes the pathology, epidemiology, pathogenesis, diagnosis, and treatment of CIDP.
Keywords: CIDP, demyelination, inflammatory neuropathy
Introduction
Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) is the most common automimmune polyneuropathy in adults. The term CIDP was coined in 1975 by Peter Dyck and colleagues (1), although similar remitting disorders were described by Eichhorst in 1890 and Henrikson in 1956 (2) and Austin in 1958 (3). The key features, weakness (both proximal and distal), sub-acute to chronic onset (greater than 8 weeks), and areflexia were associated with electrodiagnostic features of conduction block and asymmetric conduction velocity slowing and cyto-albuminologic association (eleveated CSF protein without a pleocytosis)(1). Since that time, CIDP has been broadened to include multiple variants including distal acquired demyelinating symmetric (DADS)(4), multifocal acquired demyelinating sensory and motor neuropathy (MADSAM or Lewis-Sumner syndrome)(5), and sensory predominant CIDP(6), in addition to recognition of similar but pathologically distinct disorders of multifocal motor neuropathy (MMN)(7) and CIDP associated with monoclonal gammopathy(8;9). In this article, we will review the salient features, current evidence of pathogenesis, diagnostic testing, and treatment options, focusing on typical CIDP.
Pathogenesis
The pathologic features in CIDP described by Dyck (1) were “onion bulb” formations, perivascular inflammatory infiltrates and segmental demyelination in teased fibers. These have led to two assumptions: 1) that CIDP is a primarily demyelinating disorder, and 2) that inflammation or autoimmunity is a key feature of the pathogenesis. The exact cause of CIDP is still unknown. Humoral immune factors have been presumed to be involved given the response of most patients to corticosteroids, intravenous immunoglobulins (IVIg) or plasma exchange.
Segmental demyelination and remyelination are hallmarks of CIDP and repetitively over time lead to onion bulb formations by proliferation of Schwann cell processes. Thinly myelinated large axons are also frequently observed in nerve biopsy sections(10). Myelin itself is thought to be the source of antigenic epitopes, as immunization of animals with peripheral nerve myelin proteins and glycolipids can produce experimental autoimmune neuritis (EAN) which has similar physical and pathologic features to CIDP (11;12). Antibodies to peripheral nerve components such as protein zero, peripheral myelin protein 22, sulfated glucuronyl paragloboside (SGPG), LM1, GM1, and GD1a have also been found (13). However, none of these antibodies have been found in a majority of patients, suggesting a heterogenous cause of CIDP unlike myasthenia gravis where the vast majority of patients display acetylcholine receptor antibodies.
Cellular immune mechanisms are also a key feature of CIDP. Perivascular inflammation and infiltrates in nerves of macrophages and T cells suggest a cell-mediated mechanism of damage which may cause the actual demyelination. Elevated T helper cells have been found in the CSF of CIDP patients (14). EAN can also be induced by infusing auto-reactive T cells into naïve animals(15). Cytokines produced by auto-reactive T cells have been shown to be elevated in serum from CIDP patients (16-18). Elevated serum IL-2 and tumor necrosis factor (TNF)-α have been demonstrated in CIDP patients and correlate with longer distal latencies although this observation has not been reproduced (19). However, in patients’ biopsies, T cells infiltrates are much less prevalent than in macrophages (20). Because of the similarity to multiple sclerosis, a CNS demyelinating disease, investigation into activation of T cells and induction of macrophages also show B7/CD28 pathway activation, which is involved in co-stimulation of antigen presenting cells (macrophages) in CIDP (21). Schwann cells may also be involved in the process by upregulating CD58 , an adhesion molecule which interacts with T cells and natural killer cells (22). Upregulation of B7-1 and B7-2 molecules has been demonstrated in Schwann cells from CIDP patients and treatment with an antiCD28 monoclonal antibody improves the disease course of EAN (23).
Presentation/Symptomatology
CIDP is distinguished from acute inflammatory demyelinating polyradiculoneuropathy (AIDP), the most common form of Guillain-Barré Syndrome (GBS), by time course and steroid responsiveness. Unlike AIDP, CIDP typically has a more indolent course and all of the published criteria for CIDP recognize time to greatest weakness of longer than 8 weeks to differentiate CIDP from AIDP (which reaches nadir in 4 weeks or less). Some CIDP patients may have a more acute onset and may present as multiple occurrences of AIDP with ventilator failure (24). Typically most patients present with weakness, both proximal and distal, paresthesias, and sensory loss that may be slowly progressive or have a more relapsing/remitting course. Unlike AIDP, back pain and autonomic symptoms are less common although autonomic abnormalities are frequently present on testing (25). While some patients may identify a preceding infection or injury, most patients with CIDP do not. In addition, CIDP may also present in patients with hereditary or diabetic neuropathy (although the extent to which these underyling disorders increase CIDP risk remains controversial), making diagnosis more difficult. Another differentiation between AIDP and CIDP is the less common occurrence of bulbar involvement or respiratory compromise in CIDP. CIDP typically responds to corticosteroid therapy, whereas AIDP does not.
CIDP occurs slightly more often in men in all ages, and has its highest prevalence in middle age (ages 30-60). CIDP has been recognized world-wide, with varying prevalence (partly due to diagnostic criteria) from 1.0 to 8.9 per 100,000 persons (24;26-29). Prevalence is lowest in the United Kingdom (30), with the highest rate in Olmstead County, MN (26). CIDP has also been recognized to have a higher prevalence in patients with HIV infection (31;32). Whether the prevalence of CIDP is increased in diabetes mellitus has been under debate (26).
The prognosis of CIDP is variable and is reminiscent of multiple sclerosis in its heterogeneity. Some patients (20-65%) follow a relapsing remitting course, others a more progressive course . Over time, most patients with CIDP without associated conditions respond to treatment, especially if CSF protein is elevated . Presence of monoclonal proteins portend poor prognosis and lack of response to immuno-modulatory treatment, causing some to suggest this should not be categorized with idiopathic CIDP (33-35). Predominantly distal weakness also has poorer prognosis. It is not clear if this is related to presence of associated monoclonal protein-patients with DADS more likely to have an IgM monoclonal protein than CIDP patients (36;37). Respiratory failure requiring ventilator support is rare, but can occur (24). CNS lesions can also occur, varying from T2 hyperintense white matter lesions, atrophic cervical cord, and abnormal brainstem evoked potentials, although prominent upper motor neuron features would be atypical and suggest an alternative diagnosis (38-41).
Other CIDP Variants
Pure Sensory CIDP
Pure sensory CIDP has been reported by several authors as a CIDP variant with purely or predominantly sensory involvement, which is unlike typical CIDP where weakness is a required symptom. Patients with sensory CIDP often have findings in motor nerves despite their lack of motor symptoms and may progress to develop motor symptoms over time (6;42). Patients who have sensory CIDP typically respond as well to immune-modulating treatment (43). Sensory CIDP is to be differentiated from demyelinating neuropathies with monoclonal gammopathy, as these neuropathies, while sensory predominant in symptoms, have a much different prognosis and respond poorly to standard treatments for CIDP.
Multifocal Motor and Sensory Demyelinating Neuropathy/Lewis-Sumner Syndrome
Typical CIDP has asymmetric conduction velocity slowing, but clinically patients have symmetric weakness and sensory loss on exam. Five patients who had a presentation resembling mononeuritis multiplex were reported by Lewis and colleagues with primarily upper limb symptoms in the distribution of single nerves (5). Patients had elevated CSF protein and demyelination on nerve biopsy, further confirming a demyelinating process rather than a mononeuritis multiplex(5). Patients had a favorable response to corticosteroids as well as IVIG.
Distal Acquired Demyelinating Neuropathy (DADS) was first coined by Katz and colleagues to describe patients with distal weakness and demyelinating features on electrophysiological studies (44). However, subsequent articles have suggested that DADS with monoclonal paraproteinemia, which is often a typical presentation of neuropathy associated with IgM paraproteinemias, not be included as a CIDP variant because of the poor response to treatment (45-47).
Diagnostic Criteria
Electrodiagnostic Criteria
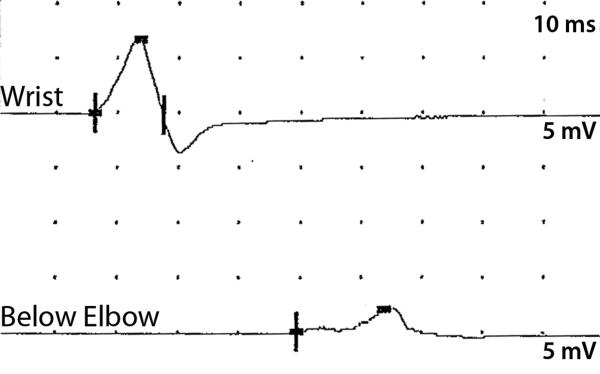
There are currently seventeen published sets of electrodiagnostic criteria for acquired demyelinating disorders (GBS/AIDP and CIDP). Many criteria were established for research study patient inclusion such as the INCAT criteria and AAN criteria (48;49). Features observed in both CIDP and AIDP/GBS which have been carried forward by Dyck's publication and in most criteria include asymmetric conduction velocity slowing to distinguish CIDP from uniform conduction velocity slowing observed in dysmyelinating inherited neuropathies (50). Prolonged distal latency and F-wave latencies have also been included in most criteria. However, the electrodiagnostic hallmark of acquired demyelination has been conduction block (decrease in amplitude of the compound muscle action potential - CMAP - at more proximal stimulation sites) and temporal dispersion (prolonged CMAP duration after proximal stimulation compared to distal stimulation). (figure 1)
Figure 1.
Temporal dispersion and conduction block observed recording from the ulnar nerve at the abductor digiti minimi in a patient with CIDP. The proximal amplitude is lower with longer duration typical of temporal dispersion.
The first criteria were put forth were by Kelly in 1983 to distinguish neuropathies associated with monoclonal protein as axonal or demyelinating (8). These criteria were further refined by Albers et al. in 1985 for AIDP(51) . Albers and Kelly in 1989(52) revised the initial Kelly criteria and specified conduction velocity slowing, prolonged distal latency, prolonged F-wave latency or temporal dispersion in 2 or more nerves with specificity CMAP amplitude. A third set was proposed by an AAN Ad Hoc Committee Task Force for research criteria (49) but this criterion has been criticized in multiple publications for low sensitivity (53-55); spurring further sets of criteria including other metrics such as distal CMAP duration (56), CSF protein elevation (see further next section)(57), or conduction block (58).
Wilson et al. compared electrodiagnostic features of patients with CIDP (biopsy proven), diabetes and monoclonal gammopathy and found that F-wave latency was best able to distinguish CIDP from other neuropathies with demyelinating features (59;60). Variability in severity of conduction block (20-50%) and number of motor nerves displaying abnormalities also significantly affects sensitivity and specificity. Other authors have suggested using a treatment responsive approach (61) which may be helpful in practice but is not practical for research purposes and may generate significantly increased health care costs.
Cytoalbuminologic Dissociation and cerebrospinal fluid analysis
Approximately 90 % of patients with CIDP demonstrate elevated CSF protein (greater than 45 mg/dL) (62-64). CSF pleocytosis is not typically seen, and often suggests a co-infection, such as HIV (65). The AAN Ad Hoc criteria specifically exclude patients with CSF cell count >10/mm3. This is one of the distinguishing factors that separate CIDP from MMN, as MMN patients typically have normal CSF protein (66). Patients with CIDP and diabetes have higher CSF protein than patients with CIDP alone(67), although diabetics tend to have baseline higher CSF protein without the presence of CIDP.
Nerve Pathology
As previously mentioned, peripheral nerve pathology was included in the initial Dyck review in 1975. Paranodal and intermodal segmental demyelination on teased fibers, edema, onion bulb formation, epineurial and endoneurial inflammation and axonal degeneration were observed (1). Biopsies performed for research purposes have also identified activated Cd4 or CD8 T cells and elevated soluble adhesion molecules, chemokines and matrix metalloproteinases (68). Macrophages are the most common inflammatory cell in biopsies although T cells are also highly abundant in subgroups of CIDP patients (69;70).
However, there is not a specific pathologic finding for CIDP. Sural nerve biopsy is not commonly performed for diagnostic confirmation of CIDP as characteristic features are often absent and there is considerable overlap between demyelinating findings observed by Dyck et al (1) and findings in biopsies from patients without clinical CIDP (1). This may be in line with the asymmetric process and “sural sparing” which is observed electrophysiologically (71). Skin biopsy, especially of glabrous skin may also demonstrate segmental demyelination and could be used as an alternative in future studies of CIDP(72). Fascicular nerve biopsy of motor nerves may prove to be more sensitive than traditional sensory nerve biopsies (73) especially in multifocal motor neuropathy.
The diagnosis of CIDP is based primarily on recognition of a characteristic history and typical clinical features. Electrophsyiological studies, CSF examination, and rarely nerve biopsy are useful confirmatory tests and are often used to exclude other disorders. Because diagnostic criteria have largely been devised for research settings, and are thus meant to have high specificity, the absence of typical electrophysiological or pathologic features in a patient with otherwise typical clinical features of CIDP should not necessarily exclude the diagnosis. Similarly, the observation of demyelinating features on nerve conduction studies or nerve biopsy in a patient lacking clinical features of CIDP should be interpreted with caution.
Treatment of CIDP
Treatments for CIDP are similar to those for GBS, except they are broader and more varied. In controlled studies, corticosteroids, intravenous immune globulin and plasma exchange (plasmapheresis) have been shown to have similar efficacy, improving strength and function in approximately 65 to 70% of patients (74) Other therapies directed against the presumed autoimmune basis of CIDP have been tried with success in case reports and small series, but high quality randomized controlled trials have yet to be performed(74)
Corticosteroids
Corticosteroids have been used to treat CIDP for more than 60 years. One of the first reports of CIDP discussed its recurrence and response to corticosteroids treatments, implying that this previously unreported type of neuropathy could be treated, a rare expectation in 1958 (Austin)(3) Since this first report, corticosteroids became the benchmark treatment for CIDP until other forms of immune therapy were tried. In a small study of 28 patients who completed a controlled 3 month trial of prednisone in CIDP, prednisone led to a small, but significant improvement over no treatment in several measures of strength, sensation, and some attributes of nerve conduction(75) No difference was noted in the response rate in those patients with progressive compared to recurrent CIDP (75).
Corticosteroids are usually given in high daily doses initially to produce a rapid response, followed by tapering over months to years to low dose daily or alternate day treatment. A popular treatment regimen was proposed by Dalakas and Engel in 1981(76). Patients are started on 80-100 mg of prednisone per day for 1-2 months. This dose is tapered over time with many patients remaining on low dose prednisone (10-20 mg every other day) to sustain remission (76). Improvement may occur over several weeks, but is often lower with gradual recovery over months to years. Many neurologists prefer to use a somewhat lower dose of corticosteroids and taper more rapidly, hoping to avoid side effects.
An alternative to daily oral prednisone or prednisolone is administration of high dose corticosteroids over several, in the hopes of avoiding long term side effects. Van Schaik and colleagues compared the response rate of patients with definite or probable CIDP to pulsed high dose dexamethasone every 4 weeks to daily oral prednisolone (77). After one year, there was no difference between the two groups in the percentage of patients who achieved remission. A substantial proportion of patients in both groups were in remission at one year. Adverse events did not differ statistically between the two treatment groups. In a follow up study of those patients, the authors reported that a cure or long term remission could be achieved in about one quarter of patients with CIDP after 1-2 courses of pulse dexamethasone or 8 month treatment with daily oral prednisolone(78).
Intravenous Immunoglobulin
Intravenous immunoglobulin (IVIG) has many immune modulatory effects which may underlie its reported benefit for CIDP. Immunoglobulin blocks antibody production via negative feedback on the bone marrow, inhibition of complement activation, and downregulation of cytokines, adhesion molecules and Fc receptors on macrophages(74). IVIG has been shown to be beneficial in CIDP in numerous studies. A randomized, double-blind placebo controlled multicenter study compared IVIG at a dose of 1 gm/kg on days 1, 2 and 21 placebo. Patients were followed for 42 days.(79) The average muscle score improved in the IVIG group compared to the placebo group at day 42 (p=0.019) and 11 subjects in the IVIG group showed improvement in the functional disability scale (79). Parallel improvement was also observed in 3 nerve conduction parameters. The longest evidenced-based clinical trial of IVIG in CIDP was reported by Hughes et al in 2008(80). The ICE trial randmozied117 CIDP patients in a double-blind, placebo-controlled, response-conditional crossover trial of proprietary IVIG (Gamunex). Patients in the treatment arm received 1g/kg of IVIG every 3 weeks for up to 24 weeks in an initial blinded treatment period. Patients who did not improve were moved to the alternate treatment regimen. Patients who completed the 24 weeks period and improved were re-randomized to a blinded 24 week extension phase. Fifty-four percent of patients receiving IVIG improved by at least one point on the adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) compared to 21% of patients receiving placebo (p>0.0002)(80). Statistically significant improvement was also observed in grip strength in the dominant and non-dominant hand. In the second phase of the study, subjects receiving IVIG experienced a longer time to relapse. Side effects did not limit the completion of the trial. In a follow up publication, the most common drug-related adverse events were headache and fever(81)
A recent Cochrane Review analyzed 7 randomized controlled trials of IVIG versus placebo, plasma exchange, or corticosteroids in cases of definite or probable CIDP(82). The authors found a significantly higher proportion of participants improved in disability within one month after receiving IVIG compared to placebo. There was no significant difference between IVIG and plasma exchange at 6 weeks and between IVIG and prednisolone at 2 to 6 weeks(82). One study suggested that IVIG responsiveness may relate to a specific haplotype of a single nucleotide polymorphisms (SNPs) of TAG-1(83).
Given the constraints of infusing IVIG in the hospital, an infusion center, or at home, interest has developed in the infusion of immunglobulin subcutaneously. This preparation of immunoglobulin can be administered using 2-4 small subcutaneous needles connected to a line attached to a small battery powered infusion pump. Patients or care takers can be taught to perform the infusion without nursing assistance. A small case series suggests that subcutaneous IVIG is well tolerated at a similar monthly dose to intravenous delivery with similar efficacy. Four of 5 patients preferred subcutaneous delivery(84). Over time, SQ infusion of immune globulin may become the preferred method of treating CIDP long term with immunoglobulin.
Plasma Exchange
Plasma exchange (PEx) has been used to treat GBS for decades and in a large controlled study was shown to be more beneficial than best medical management in GBS in 1985(85). Dyck and colleagues performed a randomized sham controlled trial of PEx in 15 patients with CIDP (86). After 3 weeks of treatment, there was a statistically significant improvement of nerve conduction parameters favoring patients who had received PEx, and in the neurologic disability score in 5 patients and in subset scores for weakness and reflexes in 4 patients. The authors concluded that PEx helps some, but not all patients with CIDP(86). Similar benefit was reported in another double-blind, sham-controlled , cross-over study(87). In this study, 80% of patients improved after undergoing plasma exchange. The improvement in motor functions correlated with the electrophysiologic data. Of interest, all but 2 patients required long term immunosuppressive drug therapy to achieve stabilization of disease(88). A Cochrane Review noted short term benefit in about two-thirds of patients with CIDP, but cautioned that rapid deterioration may occur afterwards. They reinforced the potential for adverse events such as difficulty with venous access, use of citrate and hemodynamic changes during or after exchange(89).
Immunoadsorption (IA) is a process that uses an immunosorbent to purify a substance. One study of tryptophan-immune adsorbers in14 patients with CIDPdemonstrated significant improvement in INCAT scores in 10 after one treatment series(90). IA was considered safe and well-tolerated.
Several studies have compared IVIG to PEx. One carefully-designed crossover study of IVIG versus PEx, found no statistical differences between the two treatments(91). For most patients, the benefits were short lived and required continued intermittent treatment with the same agent. Another study examined the benefit of various treatments in 67 consecutive patients with CIDP over 4 years(92). Although the response rate was similar to IVIG, steroids and PEx, the functional improvement as measured by the Rankin Score was greatest after PEx. Of patients who did not respond to initial therapy with one of the 3 agents, 35% responded to the second tand of those who failed to improve after 2 modalities, 27% responded to the third (92). The overall response rate to one of the 3 therapies was 66%. In a larger study from 11 centers in Italy, the percentage of responders to first-line therapy (steroids, IVIG or PEx) was 69%(93). This percentage increased to 81% after a change to another therapy. There was a better response to steroids or IVIG than to PEx. Adverse effects were the highest after receiving PEx. In a randomized controlled trial of IVIG versus oral prednisolone in CIDP, both treatments produced a significant improvement in the primary outcome(48). Non-statistically significant changes in secondary outcomes favored the IVIG group.
Immunosuppessants
Many oral and intravenous immunosuppressants have been used to treat CIDP including azathioprine, cyclophosphamide, cyclosporine, methotrexate, mycophenylate mofetil, rituximab, interferon, and alemtuzumab(74). Many have reported in case reports or small series, and there have been few randomized trials.
Azathioprine
A single small randomized 9 month trial of alternate day dosing of prednisone therapy alone or with azathioprine given as 2 mg/kg found no treatment benefit(94). The lack of response may have been due to the short duration of treatment (9 months) or the dosing of azathioprine. Many neuromuscular experts routinely use azathioprine as a steroid sparing agent based on clinical experience despite the lack of strong evidence from the literature.
Cyclophosphamide
Pulse cyclophosphamide, infused once per month for 6 months, was used in 15 patients with CIDP(95). The dosing was 1 g/M2 given over 1.5 -2 hours. Eleven patients achieved complete remission and one patient improved in his functional scale. Three patients did not improve and only one person worsened. Adverse effects included nausea, vomiting, anemia and hair loss. High dose cyclophosphamide (200 mg/kg) has also been used to good effect in refractory patients (96)
Cyclosporin
Four studies (including one of two children) suggest cyclosporine may be beneficial for CIDP(97-100). In the largest study, patients with both progressive and relapsing CIDP improved after receiving cyclosporin(97). The initial dosing was 8-11 mg/kg in 8 patients and 3-7 mg/kg in 10 patients. The dosing was reduced stepwise over 6 months. All of the patients with progressive CIDP improved and the incidence of relapses declined in the group with recurrent worsening CIDP. In a second study of 6 patients with CIDP and two with IgG monoclonal gammopathies who were treated with cyclosporine in a dose of 3-5 mg/kg, improvement was recorded in 3 patients and no change in 5 patients(98).
Mycophenylate mofetil
Mycophenylate mofetil induces immune suppression by selectively blocking purine synthesis in lymphocytes and inhibiting the proliferation of B and T cells(101). Studies of mycophenylate in CIDP have shown benefit in several patients, but the results are not as impressive as in other illnesses or when compared to other immune suppressants. In one report, almost 75% of patients with myasthenia gravis improved after the initiation of mycophenylate 33% for CIDP and inflammatory myopathy. Favorable results were reported in 2 patients with CIDP treated with mycophenylate(102). In a study of 21 patients there was a modest improvement in 30%, permitting a reduction of steroids or IVIG therapy(103). Another study found no clinically significant benefit or reduction in the dosing of corticosteroids or other immunosuppressants in 5 patients with treatment-resistent CIDP who were taking mycophenylate(104).
Methotrexate
Methotrexate has been used for decades to treat inflammatory myopathies such as polymyositis and dermatomyosities. A 40 week study of methotrexate compared to placebo did not demonstrate a reduction in the dosing of corticosteroids or IVIG of 20% (the primary outcome) in patients taking methotrexate (15 mg weekly) compared to placebo(105). In addition, there was no clinically or statistically significant difference in secondary outcomes.
Rituximab
Rituximab is a chimeric anti-CD20 monoclonal protein that is approved for the treatment of B-cell lymphoproliferative diseases and which is now used to treat B-cell mediated disorders. A retrospective, observational study of 13 patients with treatment refractory CIDP demonstrated a response in 9 patients, 6 of whom improved clinically(106). Three patients maintained the improvement they had achieved from treatment with IVIG or plasma exchange. Seven of the patients who responded had associated hematologic diseases. The response occurred as early as 2 months in some patients. In an early study of the use of rituximab in patients with IVIG-dependent immune polyneuropathy, two patients with CIDP who were given rituximab ( 375mg/M2 IV weekly for 4 weeks) did not experience a reduction in IVIG requirements(107). In single case reports, rituximab was effective in a patient with CIDP and idiopathic thrombocytopenic purpura and another who had CIDP and SLE(108;109). Rituximab has been described as useful in childhood onset CIDP (110).
Alemtuzumab
Alemtuzumab is a recombinant, humanized monoclonal protein that is directed against the CD52 antigen(111). CD 52 is expressed on most B and T lymphocytes, macrophages and monocytes. Because of its mechanism of action, alemtuzumab became an attractive choice as a possible therapeutic agent for the treatment of CIDP. Seven patients with CIDP, who were refractory to conventional immunosuppression, were treated with 9 courses of alemtruzumab, the dose ranging from 60-150 mg(112). Two patients experienced a prolonged remission, two patients a partial remission, and three no clear benefit from alemtuzumab. Three patients developed an autoimmune disease after treatment with alemtruzumab. Thus, alemtruzumab may have a future in the treatment of refractory CIDP, but a concern over the development autoimmune disease may limit its utility.
Interferon
The interferons were developed decades ago to treat demyelinating diseases of the central nervous system. Because of the role of various interferons in the inflammatory process, they have also been considered as potential treatments in CIDP. Sabatelli and colleagues reported two patients with treatment refractory CIDP who achieved complete and sustained recovery after treatment with interferon alpha-2a(113). Nine of 16 patients in another study experienced improved strength and sensation after receiving interferon-alpha 2a for 6 weeks(114). All patients who were studied had failed to respond to at least one conventional treatment for CIDP(114).
Individual case reports have suggested efficacy of interferon beta in CIDP (115) (116;117). In some cases, improvement was observed in electrophysiologic parameters(116). However, several studies have failed to demonstrate treatment efficacy of interferon –beta 1a as primary therapy of CIDP (118;119) or in patients who were IVIG dependent(120).
Cocito et al reviewed the clinical and electrophysiologic data of 110 patients with CIDP followed at 10 Italian centers to assess the response rate to immunosuppressive and immunomodulatory therapies prescribed in patients who were non-responders to conventional treatments (corticosteroids, IVIG, and plasma exchange)(121). Approximately one fourth of patients experienced benefit when given one of the immunosuppressive or immunomodulatory therapies. None of the 3 patients who received interferon-beta 1a improved. The response rate to each drug is as follows: azathioprine 27%, rituximab 33%, cyclosporine 25%, cyclophosphamide 38%, methotrexate 17%, mycophenylate mofetil 25%, and alpha interferon 36%(121). A Cochrane Review examined randomized and quasi-randomized trials of immunosuppressive agents for the treatment of CIDP and concluded the evidence is inadequate to decide whether azathioprine, interferon beta, or any other immunosuppressive drug or interferon is beneficial in CIDP(122).
Stem Cell Transplantation
Autologous peripheral blood stem cell transplantation (PBSCT) is an attractive treatment for autoimmune inflammatory conditions as it offers the potential for restructuring the immune system and reaction to antigens. Mahdi-Rogers and colleagues treated 3 patients with CIDP, two patients with POEMS Syndrome, and one with a neuropathy from an IgM paraprotein with (PBSCT). Two of the three patients with CIDP improved, but one relapsed after 18 months. Four of the 6 patients developed neutrophenic septicemia and pneumonia(123). Thus, PBSCT may offer improvement in a highly select cadre of patients with CIDP, but its serious adverse effects will preclude its use for all but the most refractory patients.
Conclusions
Despite significant advancements in treatment options for CIDP, further work on elucidating the pathogenesis is needed. While there is substantial evidence for an immune dysregulation etiology, better understanding may improve selection of therapeutic agents for interventional studies rather than the current “shotgun” approach in which all immunosuppressive therapies are tried. In addition, more specific biomarkers are needed to guide therapeutic decisions and improve interventional trials, however no biomarkers have met sufficient sensitivity and specificity for clinical use (124-126).
Table 1.
Characteristics of CIDP
| Equal prevalence in men and women |
| Time course of 2 months or more |
| Disease Evolution: Chronic progressive, stepwise progressive, relapsing and remitting |
| Symmetric proximal and distal weakness |
| Large fiber more than small fiber involvement |
| Hyporeflexia or areflexia |
| Cranial nerve involvement rare |
| Respiratory failure rare |
| Nerve conduction abnormalities: slowing of conduction velocities, prolonged distal latencies, conduction block, temporal dispersion |
| CSF protein: greater than 45 mg/dl, fewer than 10 WBCs |
| Nerve biopsy: may show inflammation, demyelination, and axon loss |
| Responsive to immunotherapy. |
Table 2.
Treatment Options for CIDP
| Treatments with Class B or higher efficacy | Other Treatments |
|---|---|
| Corticosteroids | Azathioprine |
| Intravenous Immunoglobulin (IVIg) | Methotrexate |
| Plasma Exchange | Cyclosporin A |
| Cyclophosphomide (pulse IV) | |
| Mycophenolate | |
| Interferons | |
| Rituximab | |
| Alemtuzumab | |
| Stem Cell Transplantation | |
| Immunoadsorption |
Acknowledgments
This work is, in part, supported by grants from NINDS K23 NS056009 (A.C.P.),
Reference List
- 1.Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc. 1975 Nov;50(11):621–637. [PubMed] [Google Scholar]
- 2.Burns TM. Chronic inflammatory demyelinating polyradiculoneuropathy. Arch Neurol. 2004 Jun;61(6):973–975. doi: 10.1001/archneur.61.6.973. [DOI] [PubMed] [Google Scholar]
- 3.AUSTIN JH. Recurrent polyneuropathies and their corticosteroid treatment; with five-year observations of a placebo-controlled case treated with corticotrophin, cortisone, and prednisone. Brain. 1958 Jun;81(2):157–192. doi: 10.1093/brain/81.2.157. [DOI] [PubMed] [Google Scholar]
- 4.Katz JS, Saperstein DS, Gronseth G, Amato AA, Barohn RJ. Distal acquired demyelinating symmetric neuropathy. Neurology. 2000 Feb 8;54(3):615–620. doi: 10.1212/wnl.54.3.615. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RA, Sumner AJ, Brown MJ, Asbury AK. Multifocal demyelinating neuropathy with persistent conduction block. Neurology. 1982 Sep;32(9):958–964. doi: 10.1212/wnl.32.9.958. [DOI] [PubMed] [Google Scholar]
- 6.Oh SJ, Joy JL, Kuruoglu R. “Chronic sensory demyelinating neuropathy”: chronic inflammatory demyelinating polyneuropathy presenting as a pure sensory neuropathy. J Neurol Neurosurg Psychiatry. 1992 Aug;55(8):677–680. doi: 10.1136/jnnp.55.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pestronk A, Cornblath DR, Ilyas AA, et al. A treatable multifocal motor neuropathy with antibodies to GM1 ganglioside. Ann Neurol. 1988 Jul;24(1):73–78. doi: 10.1002/ana.410240113. [DOI] [PubMed] [Google Scholar]
- 8.Kelly JJ., Jr The electrodiagnostic findings in peripheral neuropathy associated with monoclonal gammopathy. Muscle Nerve. 1983 Sep;6(7):504–509. doi: 10.1002/mus.880060706. [DOI] [PubMed] [Google Scholar]
- 9.Cocito D, Durelli L, Isoardo G. Different clinical, electrophysiological and immunological features of CIDP associated with paraproteinaemia. Acta Neurol Scand. 2003 Oct;108(4):274–280. doi: 10.1034/j.1600-0404.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 10.McCombe PA, Pollard JD, McLeod JG. Chronic inflammatory demyelinating polyradiculoneuropathy. A clinical and electrophysiological study of 92 cases. Brain. 1987 Dec;110(Pt 6):1617–1630. doi: 10.1093/brain/110.6.1617. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel CM, Hughes RA, Moore SE, Smith KJ, Walsh FS. Induction of experimental autoimmune neuritis with peripheral myelin protein-22. Brain. 1998 Oct;121(Pt 10):1895–1902. doi: 10.1093/brain/121.10.1895. [DOI] [PubMed] [Google Scholar]
- 12.Hughes RA, Allen D, Makowska A, Gregson NA. Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2006 Mar;11(1):30–46. doi: 10.1111/j.1085-9489.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara M, Suzuki S, Takada K, Kusunoki S. Antibodies to LM1 and LM1-containing ganglioside complexes in Guillain-Barre syndrome and chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 2011 Oct 28;239(1-2):87–90. doi: 10.1016/j.jneuroim.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Chi LJ, Xu WH, Zhang ZW, Huang HT, Zhang LM, Zhou J. Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010 Dec;15(4):345–356. doi: 10.1111/j.1529-8027.2010.00294.x. [DOI] [PubMed] [Google Scholar]
- 15.Archelos JJ, Maurer M, Jung S, et al. Inhibition of experimental autoimmune neuritis by an antibody to the lymphocyte function-associated antigen-1. Lab Invest. 1994 May;70(5):667–675. [PubMed] [Google Scholar]
- 16.Kieseier BC, Tani M, Mahad D, et al. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain. 2002 Apr;125(Pt 4):823–834. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- 17.Mahad DJ, Howell SJ, Woodroofe MN. Expression of chemokines in cerebrospinal fluid and serum of patients with chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2002 Sep;73(3):320–323. doi: 10.1136/jnnp.73.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei FJ, Ishizu T, Murai H, et al. Th1 shift in CIDP versus Th2 shift in vasculitic neuropathy in CSF. J Neurol Sci. 2005 Jan 15;228(1):75–85. doi: 10.1016/j.jns.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Melendez-Vasquez C, Redford J, Choudhary PP, et al. Immunological investigation of chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol. 1997 Mar;73(1-2):124–134. doi: 10.1016/s0165-5728(96)00189-0. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt B, Toyka KV, Kiefer R, Full J, Hartung HP, Pollard J. Inflammatory infiltrates in sural nerve biopsies in Guillain-Barre syndrome and chronic inflammatory demyelinating neuropathy. Muscle Nerve. 1996 Apr;19(4):474–487. doi: 10.1002/(SICI)1097-4598(199604)19:4<474::AID-MUS8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Kiefer R, Dangond F, Mueller M, Toyka KV, Hafler DA, Hartung HP. Enhanced B7 costimulatory molecule expression in inflammatory human sural nerve biopsies. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):362–368. doi: 10.1136/jnnp.69.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van R, I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain. 2000 Oct;123(Pt 10):2020–2029. doi: 10.1093/brain/123.10.2020. [DOI] [PubMed] [Google Scholar]
- 23.Murata K, Dalakas MC. Expression of the co-stimulatory molecule BB-1, the ligands CTLA-4 and CD28 and their mRNAs in chronic inflammatory demyelinating polyneuropathy. Brain. 2000 Aug;123(Pt 8):1660–1666. doi: 10.1093/brain/123.8.1660. [DOI] [PubMed] [Google Scholar]
- 24.Zivkovic SA, Peltier AC, Iacob T, Lacomis D. Chronic inflammatory demyelinating polyneuropathy and ventilatory failure: report of seven new cases and review of the literature. Acta Neurol Scand. 2011 Jul;124(1):59–63. doi: 10.1111/j.1600-0404.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa JJ, Dyck PJ, Laughlin RS, et al. Autonomic dysfunction in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2012 Mar 6;78(10):702–708. doi: 10.1212/WNL.0b013e3182494d66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughlin RS, Dyck PJ, Melton LJ, III, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009 Jul 7;73(1):39–45. doi: 10.1212/WNL.0b013e3181aaea47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajabally YA, Simpson BS, Beri S, Bankart J, Gosalakkal JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: study of a UK population. Muscle Nerve. 2009 Apr;39(4):432–438. doi: 10.1002/mus.21206. [DOI] [PubMed] [Google Scholar]
- 28.McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol. 1999 Dec;46(6):910–913. [PubMed] [Google Scholar]
- 29.Iijima M, Koike H, Hattori N, et al. Prevalence and incidence rates of chronic inflammatory demyelinating polyneuropathy in the Japanese population. J Neurol Neurosurg Psychiatry. 2008 Sep;79(9):1040–1043. doi: 10.1136/jnnp.2007.128132. [DOI] [PubMed] [Google Scholar]
- 30.Lunn MP, Manji H, Choudhary PP, Hughes RA, Thomas PK. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south east England. J Neurol Neurosurg Psychiatry. 1999 May;66(5):677–680. doi: 10.1136/jnnp.66.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson DM, Olney RK. Peripheral neuropathies associated with human immunodeficiency virus infection. Neurol Clin. 1992 Aug;10(3):685–711. [PubMed] [Google Scholar]
- 32.Brannagan TH, III, Zhou Y. HIV-associated Guillain-Barre syndrome. J Neurol Sci. 2003 Apr 15;208(1-2):39–42. doi: 10.1016/s0022-510x(02)00418-5. [DOI] [PubMed] [Google Scholar]
- 33.Simmons Z, Albers JW, Bromberg MB, Feldman EL. Long-term follow-up of patients with chronic inflammatory demyelinating polyradiculoneuropathy, without and with monoclonal gammopathy. Brain. 1995 Apr;118(Pt 2):359–368. doi: 10.1093/brain/118.2.359. [DOI] [PubMed] [Google Scholar]
- 34.Tackenberg B, Lunemann JD, Steinbrecher A, et al. Classifications and treatment responses in chronic immune-mediated demyelinating polyneuropathy. Neurology. 2007 May 8;68(19):1622–1629. doi: 10.1212/01.wnl.0000260972.07422.ea. [DOI] [PubMed] [Google Scholar]
- 35.Wadwekar V, Kalita J, Misra UK. Does the chronic inflammatory demyelinating polyradiculoneuropathy due to secondary cause differ from primary? Neurol India. 2011 Sep;59(5):664–668. doi: 10.4103/0028-3886.86537. [DOI] [PubMed] [Google Scholar]
- 36.Larue S, Bombelli F, Viala K, et al. Non-anti-MAG DADS neuropathy as a variant of CIDP: clinical, electrophysiological, laboratory features and response to treatment in 10 cases. Eur J Neurol. 2011 Jun;18(6):899–905. doi: 10.1111/j.1468-1331.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 37.Mygland A, Monstad P. Chronic acquired demyelinating symmetric polyneuropathy classified by pattern of weakness. Arch Neurol. 2003 Feb;60(2):260–264. doi: 10.1001/archneur.60.2.260. [DOI] [PubMed] [Google Scholar]
- 38.Mendell JR, Kolkin S, Kissel JT, Weiss KL, Chakeres DW, Rammohan KW. Evidence for central nervous system demyelination in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1987 Aug;37(8):1291–1294. doi: 10.1212/wnl.37.8.1291. [DOI] [PubMed] [Google Scholar]
- 39.Gigli GL, Carlesimo A, Valente M, et al. Evoked potentials suggest cranial nerves and CNS involvement in chronic relapsing polyradiculoneuropathy. Eur Neurol. 1989;29(3):145–149. doi: 10.1159/000116398. [DOI] [PubMed] [Google Scholar]
- 40.Ohtake T, Komori T, Hirose K, Tanabe H. CNS involvement in Japanese patients with chronic inflammatory demyelinating polyradiculoneuropathy. Acta Neurol Scand. 1990 Feb;81(2):108–112. doi: 10.1111/j.1600-0404.1990.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 41.Uncini A, Gallucci M, Lugaresi A, Porrini AM, Onofrj M, Gambi D. CNS involvement in chronic inflammatory demyelinating polyneuropathy: an electrophysiological and MRI study. Electromyogr Clin Neurophysiol. 1991 Sep;31(6):365–371. [PubMed] [Google Scholar]
- 42.Berger AR, Herskovitz S, Kaplan J. Late motor involvement in cases presenting as “chronic sensory demyelinating polyneuropathy”. Muscle Nerve. 1995 Apr;18(4):440–444. doi: 10.1002/mus.880180411. [DOI] [PubMed] [Google Scholar]
- 43.Sinnreich M, Klein CJ, Daube JR, Engelstad J, Spinner RJ, Dyck PJ. Chronic immune sensory polyradiculopathy: a possibly treatable sensory ataxia. Neurology. 2004 Nov 9;63(9):1662–1669. doi: 10.1212/01.wnl.0000142507.12763.58. [DOI] [PubMed] [Google Scholar]
- 44.Katz JS, Saperstein DS, Gronseth G, Amato AA, Barohn RJ. Distal acquired demyelinating symmetric neuropathy. Neurology. 2000 Feb 8;54(3):615–620. doi: 10.1212/wnl.54.3.615. [DOI] [PubMed] [Google Scholar]
- 45.Larue S, Bombelli F, Viala K, et al. Non-anti-MAG DADS neuropathy as a variant of CIDP: clinical, electrophysiological, laboratory features and response to treatment in 10 cases. Eur J Neurol. 2011 Jun;18(6):899–905. doi: 10.1111/j.1468-1331.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 46.Remiche G, Kentos A, Mavroudakis N. Distal acquired demyelinating symmetric neuropathy associated with anti-GM1 antibodies: is this a CIDP variant? Acta Neurol Belg. 2010 Mar;110(1):103–106. [PubMed] [Google Scholar]
- 47.Sander HW, Latov N. Research criteria for defining patients with CIDP. Neurology. 2003 Apr 1;60(8 Suppl 3):S8–15. doi: 10.1212/wnl.60.8_suppl_3.s8. [DOI] [PubMed] [Google Scholar]
- 48.Hughes R, Bensa S, Willison H, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001 Aug;50(2):195–201. doi: 10.1002/ana.1088. [DOI] [PubMed] [Google Scholar]
- 49.Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology. 1991 May;41(5):617–618. [PubMed] [Google Scholar]
- 50.Bromberg MB. Review of the evolution of electrodiagnostic criteria for chronic inflammatory demyelinating polyradicoloneuropathy. Muscle Nerve. 2011 Jun;43(6):780–794. doi: 10.1002/mus.22038. [DOI] [PubMed] [Google Scholar]
- 51.Albers JW, Donofrio PD, McGonagle TK. Sequential electrodiagnostic abnormalities in acute inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 1985 Jul;8(6):528–539. doi: 10.1002/mus.880080609. [DOI] [PubMed] [Google Scholar]
- 52.Albers JW, Kelly JJ., Jr Acquired inflammatory demyelinating polyneuropathies: clinical and electrodiagnostic features. Muscle Nerve. 1989 Jun;12(6):435–451. doi: 10.1002/mus.880120602. [DOI] [PubMed] [Google Scholar]
- 53.Sander HW, Latov N. Research criteria for defining patients with CIDP. Neurology. 2003 Apr 1;60(8 Suppl 3):S8–15. doi: 10.1212/wnl.60.8_suppl_3.s8. [DOI] [PubMed] [Google Scholar]
- 54.Nicolas G, Maisonobe T, Le FN, Leger JM, Bouche P. Proposed revised electrophysiological criteria for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2002 Jan;25(1):26–30. doi: 10.1002/mus.1214. [DOI] [PubMed] [Google Scholar]
- 55.Haq RU, Fries TJ, Pendlebury WW, Kenny MJ, Badger GJ, Tandan R. Chronic inflammatory demyelinating polyradiculoneuropathy: a study of proposed electrodiagnostic and histologic criteria. Arch Neurol. 2000 Dec;57(12):1745–1750. doi: 10.1001/archneur.57.12.1745. [DOI] [PubMed] [Google Scholar]
- 56.Thaisetthawatkul P, Logigian EL, Herrmann DN. Dispersion of the distal compound muscle action potential as a diagnostic criterion for chronic inflammatory demyelinating polyneuropathy. Neurology. 2002 Nov 26;59(10):1526–1532. doi: 10.1212/01.wnl.0000034172.47882.20. [DOI] [PubMed] [Google Scholar]
- 57.Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve. 2001 Mar;24(3):311–324. doi: 10.1002/1097-4598(200103)24:3<311::aid-mus1001>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 58.Nicolas G, Maisonobe T, Le FN, Leger JM, Bouche P. Proposed revised electrophysiological criteria for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2002 Jan;25(1):26–30. doi: 10.1002/mus.1214. [DOI] [PubMed] [Google Scholar]
- 59.Wilson J, Chawla J, Fisher M. Sensitivity and specificity of electrodiagnostic criteria for CIDP using ROC curves: comparison to patients with diabetic and MGUS associated neuropathies. J Neurol Sci. 2005 Apr 15;231(1-2):19–28. doi: 10.1016/j.jns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Wilson JR, Park Y, Fisher MA. Electrodiagnostic criteria in CIDP: comparison with diabetic neuropathy. Electromyogr Clin Neurophysiol. 2000 Apr;40(3):181–185. [PubMed] [Google Scholar]
- 61.Latov N. Diagnosis of CIDP. Neurology. 2002 Dec 24;59(12 Suppl 6):S2–S6. doi: 10.1212/wnl.59.12_suppl_6.s2. [DOI] [PubMed] [Google Scholar]
- 62.Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Arch Neurol. 1989 Aug;46(8):878–884. doi: 10.1001/archneur.1989.00520440064022. [DOI] [PubMed] [Google Scholar]
- 63.Hattori N, Misu K, Koike H, et al. Age of onset influences clinical features of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2001 Feb 15;184(1):57–63. doi: 10.1016/s0022-510x(00)00493-7. [DOI] [PubMed] [Google Scholar]
- 64.Hahn AF, Hartung H, Dyck PJ. Chronic Inflammatory Demyelinating Polyradiculoneuropathy. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th ed. Elsevier Saunders; Philadelphia: 2005. pp. 2221–2253. [Google Scholar]
- 65.Chimowitz MI, Audet AM, Hallet A, Kelly JJ., Jr HIV-associated CIDP. Muscle Nerve. 1989 Aug;12(8):695–696. doi: 10.1002/mus.880120811. [DOI] [PubMed] [Google Scholar]
- 66.Nobile-Orazio E. Multifocal motor neuropathy. J Neuroimmunol. 2001 Apr 2;115(1-2):4–18. doi: 10.1016/s0165-5728(01)00266-1. [DOI] [PubMed] [Google Scholar]
- 67.Kalita J, Misra UK, Yadav RK. A comparative study of chronic inflammatory demyelinating polyradiculoneuropathy with and without diabetes mellitus. Eur J Neurol. 2007 Jun;14(6):638–643. doi: 10.1111/j.1468-1331.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 68.Sommer C, Toyka K. Nerve biopsy in chronic inflammatory neuropathies: in situ biomarkers. J Peripher Nerv Syst. 2011 Jun;16(Suppl 1):24–29. doi: 10.1111/j.1529-8027.2011.00301.x. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt B, Toyka KV, Kiefer R, Full J, Hartung HP, Pollard J. Inflammatory infiltrates in sural nerve biopsies in Guillain-Barre syndrome and chronic inflammatory demyelinating neuropathy. Muscle Nerve. 1996 Apr;19(4):474–487. doi: 10.1002/(SICI)1097-4598(199604)19:4<474::AID-MUS8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 70.Sommer C, Koch S, Lammens M, Gabreels-Festen A, Stoll G, Toyka KV. Macrophage clustering as a diagnostic marker in sural nerve biopsies of patients with CIDP. Neurology. 2005 Dec 27;65(12):1924–1929. doi: 10.1212/01.wnl.0000188879.19900.b7. [DOI] [PubMed] [Google Scholar]
- 71.Bromberg MB, Albers JW. Patterns of sensory nerve conduction abnormalities in demyelinating and axonal peripheral nerve disorders. Muscle Nerve. 1993 Mar;16(3):262–266. doi: 10.1002/mus.880160304. [DOI] [PubMed] [Google Scholar]
- 72.Saporta MA, Katona I, Lewis RA, Masse S, Shy ME, Li J. Shortened internodal length of dermal myelinated nerve fibres in Charcot-Marie-Tooth disease type 1A. Brain. 2009 Dec;132(Pt 12):3263–3273. doi: 10.1093/brain/awp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor BV, Dyck PJ, Engelstad J, Gruener G, Grant I, Dyck PJ. Multifocal motor neuropathy: pathologic alterations at the site of conduction block. J Neuropathol Exp Neurol. 2004 Feb;63(2):129–137. doi: 10.1093/jnen/63.2.129. [DOI] [PubMed] [Google Scholar]
- 74.Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011 Sep;7(9):507–517. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- 75.Dyck PJ, O'Brien PC, Oviatt KF, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol. 1982 Feb;11(2):136–141. doi: 10.1002/ana.410110205. [DOI] [PubMed] [Google Scholar]
- 76.Dalakas MC, Engel WK. Chronic relapsing (dysimmune) polyneuropathy: pathogenesis and treatment. Ann Neurol. 1981;9(Suppl):134–145. doi: 10.1002/ana.410090719. [DOI] [PubMed] [Google Scholar]
- 77.van S, I, Eftimov F, van Doorn PA, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol. 2010 Mar;9(3):245–253. doi: 10.1016/S1474-4422(10)70021-1. [DOI] [PubMed] [Google Scholar]
- 78.Eftimov F, Vermeulen M, van Doorn PA, Brusse E, van S., I Long-term remission of CIDP after pulsed dexamethasone or short-term prednisolone treatment. Neurology. 2012 Apr 3;78(14):1079–1084. doi: 10.1212/WNL.0b013e31824e8f84. [DOI] [PubMed] [Google Scholar]
- 79.Mendell JR, Barohn RJ, Freimer ML, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001 Feb 27;56(4):445–449. doi: 10.1212/wnl.56.4.445. [DOI] [PubMed] [Google Scholar]
- 80.Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008 Feb;7(2):136–144. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- 81.Donofrio PD, Bril V, Dalakas MC, et al. Safety and tolerability of immune globulin intravenous in chronic inflammatory demyelinating polyradiculoneuropathy. Arch Neurol. 2010 Sep;67(9):1082–1088. doi: 10.1001/archneurol.2010.223. [DOI] [PubMed] [Google Scholar]
- 82.Eftimov F, Winer JB, Vermeulen M, de HR, van S., I Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2009;(1):CD001797. doi: 10.1002/14651858.CD001797.pub2. [DOI] [PubMed] [Google Scholar]
- 83.Iijima M, Koike H, Sobue G. [Efficacy and availability of intravenous immunoglobulin in chronic inflammatory demyelinating polyneuropathy]. Nihon Rinsho. 2012 Apr;70(4):715–721. [PubMed] [Google Scholar]
- 84.Cocito D, Serra G, Falcone Y, Paolasso I. The efficacy of subcutaneous immunoglobulin administration in chronic inflammatory demyelinating polyneuropathy responders to intravenous immunoglobulin. J Peripher Nerv Syst. 2011 Jun;16(2):150–152. doi: 10.1111/j.1529-8027.2011.00340.x. [DOI] [PubMed] [Google Scholar]
- 85.The Guillain-Barre syndrome Study Group Plasmapheresis and acute Guillain-Barre syndrome. Neurology. 1985 Aug;35(8):1096–1104. [PubMed] [Google Scholar]
- 86.Dyck PJ, Daube J, O'Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med. 1986 Feb 20;314(8):461–465. doi: 10.1056/NEJM198602203140801. [DOI] [PubMed] [Google Scholar]
- 87.Hahn AF, Bolton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham-controlled, cross-over study. Brain. 1996 Aug;119(Pt 4):1055–1066. doi: 10.1093/brain/119.4.1055. [DOI] [PubMed] [Google Scholar]
- 88.Hahn AF, Bolton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham-controlled, cross-over study. Brain. 1996 Aug;119(Pt 4):1055–1066. doi: 10.1093/brain/119.4.1055. [DOI] [PubMed] [Google Scholar]
- 89.Mehndiratta MM, Hughes RA, Agarwal P. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2004;(3):CD003906. doi: 10.1002/14651858.CD003906.pub2. [DOI] [PubMed] [Google Scholar]
- 90.Galldiks N, Burghaus L, Dohmen C, et al. Immunoadsorption in patients with chronic inflammatory demyelinating polyradiculoneuropathy with unsatisfactory response to first-line treatment. Eur Neurol. 2011;66(4):183–189. doi: 10.1159/000331011. [DOI] [PubMed] [Google Scholar]
- 91.Dyck PJ, Litchy WJ, Kratz KM, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 1994 Dec;36(6):838–845. doi: 10.1002/ana.410360607. [DOI] [PubMed] [Google Scholar]
- 92.Gorson KC, Allam G, Ropper AH. Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without a monoclonal gammopathy. Neurology. 1997 Feb;48(2):321–328. doi: 10.1212/wnl.48.2.321. [DOI] [PubMed] [Google Scholar]
- 93.Cocito D, Paolasso I, Antonini G, et al. A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2010 Feb;17(2):289–294. doi: 10.1111/j.1468-1331.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 94.Dyck PJ, O'Brien P, Swanson C, Low P, Daube J. Combined azathioprine and prednisone in chronic inflammatory-demyelinating polyneuropathy. Neurology. 1985 Aug;35(8):1173–1176. doi: 10.1212/wnl.35.8.1173. [DOI] [PubMed] [Google Scholar]
- 95.Good JL, Chehrenama M, Mayer RF, Koski CL. Pulse cyclophosphamide therapy in chronic inflammatory demyelinating polyneuropathy. Neurology. 1998 Dec;51(6):1735–1738. doi: 10.1212/wnl.51.6.1735. [DOI] [PubMed] [Google Scholar]
- 96.Brannagan TH, III, Pradhan A, Heiman-Patterson T, et al. High-dose cyclophosphamide without stem-cell rescue for refractory CIDP. Neurology. 2002 Jun 25;58(12):1856–1858. doi: 10.1212/wnl.58.12.1856. [DOI] [PubMed] [Google Scholar]
- 97.Barnett MH, Pollard JD, Davies L, McLeod JG. Cyclosporin A in resistant chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 1998 Apr;21(4):454–460. doi: 10.1002/(sici)1097-4598(199804)21:4<454::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 98.Mahattanakul W, Crawford TO, Griffin JW, Goldstein JM, Cornblath DR. Treatment of chronic inflammatory demyelinating polyneuropathy with cyclosporin-A. J Neurol Neurosurg Psychiatry. 1996 Feb;60(2):185–187. doi: 10.1136/jnnp.60.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takeuchi A, Shirai S, Horiuchi K, et al. Successful cyclosporine treatment in 2 patients with refractory CIDP, involving monitoring of both AUC(0-4h) and trough levels. Rinsho Shinkeigaku. 2012;52(3):172–177. doi: 10.5692/clinicalneurol.52.172. [DOI] [PubMed] [Google Scholar]
- 100.Visudtibhan A, Chiemchanya S, Visudhiphan P. Cyclosporine in chronic inflammatory demyelinating polyradiculoneuropathy. Pediatr Neurol. 2005 Nov;33(5):368–372. doi: 10.1016/j.pediatrneurol.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Chaudhry V, Cornblath DR, Griffin JW, O'Brien R, Drachman DB. Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology. 2001 Jan 9;56(1):94–96. doi: 10.1212/wnl.56.1.94. [DOI] [PubMed] [Google Scholar]
- 102.Mowzoon N, Sussman A, Bradley WG. Mycophenolate (CellCept) treatment of myasthenia gravis, chronic inflammatory polyneuropathy and inclusion body myositis. J Neurol Sci. 2001 Apr 1;185(2):119–122. doi: 10.1016/s0022-510x(01)00478-6. [DOI] [PubMed] [Google Scholar]
- 103.Gorson KC, Amato AA, Ropper AH. Efficacy of mycophenolate mofetil in patients with chronic immune demyelinating polyneuropathy. Neurology. 2004 Aug 24;63(4):715–717. doi: 10.1212/01.wnl.0000134676.05850.c0. [DOI] [PubMed] [Google Scholar]
- 104.Umapathi T, Hughes R. Mycophenolate in treatment-resistant inflammatory neuropathies. Eur J Neurol. 2002 Nov;9(6):683–685. doi: 10.1046/j.1468-1331.2002.00478.x. [DOI] [PubMed] [Google Scholar]
- 105.Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurol. 2009 Feb;8(2):158–164. doi: 10.1016/S1474-4422(08)70299-0. [DOI] [PubMed] [Google Scholar]
- 106.Benedetti L, Briani C, Franciotta D, et al. Rituximab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a report of 13 cases and review of the literature. J Neurol Neurosurg Psychiatry. 2011 Mar;82(3):306–308. doi: 10.1136/jnnp.2009.188912. [DOI] [PubMed] [Google Scholar]
- 107.Gorson KC, Natarajan N, Ropper AH, Weinstein R. Rituximab treatment in patients with IVIg-dependent immune polyneuropathy: a prospective pilot trial. Muscle Nerve. 2007 Jan;35(1):66–69. doi: 10.1002/mus.20664. [DOI] [PubMed] [Google Scholar]
- 108.Benedetti L, Franciotta D, Beronio A, et al. Rituximab efficacy in CIDP associated with idiopathic thrombocytopenic purpura. Muscle Nerve. 2008 Aug;38(2):1076–1077. doi: 10.1002/mus.21073. [DOI] [PubMed] [Google Scholar]
- 109.Sanz PG, Garcia Mendez CV, Cueto AL, et al. Chronic inflammatory demyelinating polyradiculoneuropathy in a patient with systemic lupus erythematosus and good outcome with rituximab treatment. Rheumatol Int. 2011 Sep 16; doi: 10.1007/s00296-011-2130-5. [DOI] [PubMed] [Google Scholar]
- 110.D'Amico A, Catteruccia M, De BF, et al. Rituximab in a childhood-onset idiopathic refractory chronic inflammatory demyelinating polyneuropathy. Eur J Paediatr Neurol. 2012 May;16(3):301–303. doi: 10.1016/j.ejpn.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Brannagan TH, III, Patterson SK. Alemtuzumab: the future of chronic inflammatory demyelinating polyradiculoneuropathy treatment? Expert Rev Clin Immunol. 2010 May;6(3):319–321. doi: 10.1586/eci.10.23. [DOI] [PubMed] [Google Scholar]
- 112.Marsh EA, Hirst CL, Llewelyn JG, et al. Alemtuzumab in the treatment of IVIG-dependent chronic inflammatory demyelinating polyneuropathy. J Neurol. 2010 Jun;257(6):913–919. doi: 10.1007/s00415-009-5437-3. [DOI] [PubMed] [Google Scholar]
- 113.Sabatelli M, Mignogna T, Lippi G, et al. Interferon-alpha may benefit steroid unresponsive chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 1995 May;58(5):638–639. doi: 10.1136/jnnp.58.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorson KC, Ropper AH, Clark BD, Dew RB, III, Simovic D, Allam G. Treatment of chronic inflammatory demyelinating polyneuropathy with interferon-alpha 2a. Neurology. 1998 Jan;50(1):84–87. doi: 10.1212/wnl.50.1.84. [DOI] [PubMed] [Google Scholar]
- 115.Choudhary PP, Thompson N, Hughes RA. Improvement following interferon beta in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. 1995 Mar;242(4):252–253. doi: 10.1007/BF00919601. [DOI] [PubMed] [Google Scholar]
- 116.Vallat JM, Hahn AF, Leger JM, et al. Interferon beta-1a as an investigational treatment for CIDP. Neurology. 2003 Apr 1;60(8 Suppl 3):S23–S28. doi: 10.1212/wnl.60.8_suppl_3.s23. [DOI] [PubMed] [Google Scholar]
- 117.Villa AM, Garcea O, Di EM, Saizar R, Sica RE. Interferon beta-1a in chronic inflammatory demyelinating polyneuropathy: case report. Arq Neuropsiquiatr. 2004 Sep;62(3B):892–894. doi: 10.1590/s0004-282x2004000500031. [DOI] [PubMed] [Google Scholar]
- 118.Hadden RD, Sharrack B, Bensa S, Soudain SE, Hughes RA. Randomized trial of interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1999 Jul 13;53(1):57–61. doi: 10.1212/wnl.53.1.57. [DOI] [PubMed] [Google Scholar]
- 119.Kuntzer T, Radziwill AJ, Lettry-Trouillat R, et al. Interferon-beta1a in chronic inflammatory demyelinating polyneuropathy. Neurology. 1999 Oct 12;53(6):1364–1365. doi: 10.1212/wnl.53.6.1364. [DOI] [PubMed] [Google Scholar]
- 120.Hughes RA, Gorson KC, Cros D, et al. Intramuscular interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2010 Feb 23;74(8):651–657. doi: 10.1212/WNL.0b013e3181d1a862. [DOI] [PubMed] [Google Scholar]
- 121.Cocito D, Grimaldi S, Paolasso I, et al. Immunosuppressive treatment in refractory chronic inflammatory demyelinating polyradiculoneuropathy. A nationwide retrospective analysis. Eur J Neurol. 2011 Dec;18(12):1417–1421. doi: 10.1111/j.1468-1331.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 122.Hughes RA, Swan AV, van Doorn PA. Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2004;(4):CD003280. doi: 10.1002/14651858.CD003280.pub2. [DOI] [PubMed] [Google Scholar]
- 123.Mahdi-Rogers M, Kazmi M, Ferner R, et al. Autologous peripheral blood stem cell transplantation for chronic acquired demyelinating neuropathy. J Peripher Nerv Syst. 2009 Jun;14(2):118–124. doi: 10.1111/j.1529-8027.2009.00221.x. [DOI] [PubMed] [Google Scholar]
- 124.Brannagan TH., III Current diagnosis of CIDP: the need for biomarkers. J Peripher Nerv Syst. 2011 Jun;16(Suppl 1):3–13. doi: 10.1111/j.1529-8027.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- 125.Latov N. Biomarkers of CIDP in patients with diabetes or CMT1. J Peripher Nerv Syst. 2011 Jun;16(Suppl 1):14–17. doi: 10.1111/j.1529-8027.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- 126.Willison HJ. Biomarkers in experimental models of antibody-mediated neuropathies. J Peripher Nerv Syst. 2011 Jun;16(Suppl 1):60–62. doi: 10.1111/j.1529-8027.2011.00310.x. [DOI] [PubMed] [Google Scholar]