Abstract
Identifying cross-sectional and longitudinal correspondence in the abdomen on computed tomography (CT) scans is necessary for quantitatively tracking change and understanding population characteristics, yet abdominal image registration is a challenging problem. The key difficulty in solving this problem is huge variations in organ dimensions and shapes across subjects. The current standard registration method uses the global or body-wise registration technique, which is based on the global topology for alignment. This method (although producing decent results) has substantial influence of outliers, thus leaving room for significant improvement. Here, we study a new image registration approach using local (organ-wise registration) by first creating organ-specific bounding boxes and then using these regions of interest (ROIs) for aligning references to target. Based on Dice Similarity Coefficient (DSC), Mean Surface Distance (MSD) and Hausdorff Distance (HD), the organ-wise approach is demonstrated to have significantly better results by minimizing the distorting effects of organ variations. This paper compares exclusively the two registration methods by providing novel quantitative and qualitative comparison data and is a subset of the more comprehensive problem of improving the multi-atlas segmentation by using organ normalization.
Keywords: Organ localization, abdomen, image registration, random forest
1. INTRODUCTION
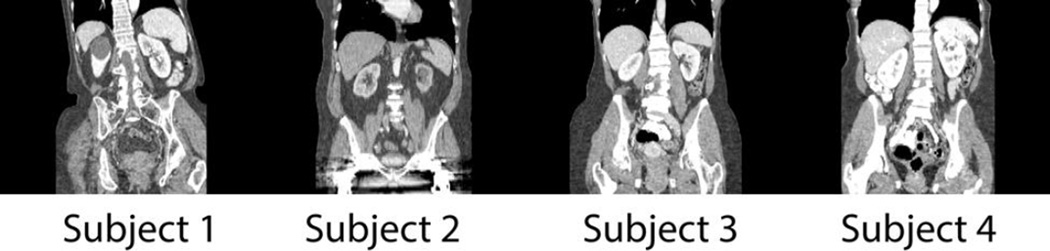
Image registration is a major area of research in medical image processing with widespread applications ranging from atlas-based segmentation, image guided surgery, biomarker screening, longitudinal analysis to diagnosing diseases. While volumetric brain registrations have been reasonably handled, registration on clinically acquired abdominal computed tomography (CT) is exceptionally difficult for not only the huge variations in organ shapes and sizes from across subjects, but also the large deformation between the organs of interest (Figure 1). Thus the quality of the image registrations in abdomen is important and requires evaluation.
Figure 1.
Illustration of coronal slices of four CT scans to demonstrate the variations in image quality, body sizes, and organ appearances.
Klein et al. assessed 14 non-linear registration tools on 80 MRIs of the human brain [1]. Murphy et al. compared 20 registration algorithms on 30 thoracic CT pairs in the EMPIRE10 challenge [2]. These two evaluations targeted the registration approaches designed for the brain and thorax, respectively; however, there has not been a registration tool tailored for image registration in human abdomen so far. Currently, general-purpose registration tools initially designed for brain structures are applied to abdomen [3, 4]. In a previous study [5], we followed the framework of Klein et al. [6] to evaluate four non-rigid registration tools including FNIRT [7], IRTK [8], ANTs [9], and NiftyReg [10] (all with default parameters) on their performances of abdominal CT registration regarding volumetric overlap and surface distance error. The results indicated that (1) all evaluated registration tools had a large portion of catastrophic registration failures that could undermine the further processing like the atlas-based segmentation, and (2) although better parameter selection may enhance the registration performances, new perspectives of addressing the challenges in abdomen were critical to improve the robustness of abdominal registrations.
Recently, random forest (RF) techniques have been employed to localize the abdominal organs in the form of bounding boxes [2]. This provides an opportunity to approach the abdominal registration problem in a discontinuous manner, which might be beneficial to overcome the distorting effects between the abdominal organs. In this study, we chose the registration tool with the best performances in [5], and compare its traditional registrations between the whole abdominal CT scans (body-wise with the registrations between organ-specific regions of interest (ROIs) localized by RF (organ-wise). Note this work was an extension of [5] on the same 20 datasets and studied in multi-atlas labeling and statistical fusion in [6]. Here here we focus on the evaluating the efficacy of using organ-wise registration.
2. METHODS
Data
For uniformity, the dataset used to compare the two registration methods is the same as the dataset used to previously evaluate five different registration tools using the body-wise approach [5]. It comprises of 20 anonymous and randomly selected CT scans in NIFTI format from an ongoing colorectal cancer chemotherapy trial under Institutional Review Board (IRB) supervision. Various field of views (approx. 300 × 300 × 400 mm ~ 500 × 500 × 700 mm) and resolutions (approx. 0.6 × 0.6 × 3.0 mm ~ 1.0 × 1.0 × 5.0 mm) are found in the 20 datasets. Note this differs from [5] in that (1) the images were not cropped before registrations, and (2) the adrenal glands were divided into two separate labels. Image orientations were normalized in the NIFTI header and thirteen abdominal organs were considered as ROIs, namely spleen, right kidney, left kidney, gall bladder, esophagus, liver, stomach, aorta, inferior vena cava, portal and splenic vein, pancreas, right adrenal gland and left adrenal gland. The ROIs were labelled by two experienced undergraduate students using the Medical Image Processing And Visualization (MIPAV [11]) software and verified by a radiologist to enable assessment of correspondence.
Experimental Design
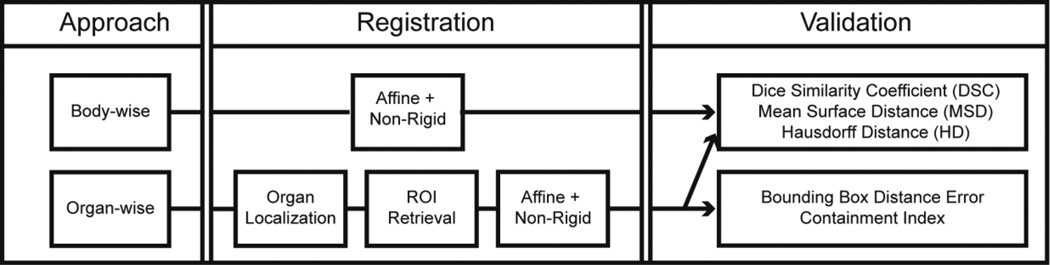
Briefly, this experimental design followed Figure 2. Body-wise and organ-wise registrations were both evaluated on the 20 CT scans. A leave-one-out cross validation (LOOCV) scheme was used across the 20 datasets, i.e., when one scan was used as target, the remaining 19 scans were considered as references to register towards the target. Thus a total of 380 outcomes (20 × 19) were generated for the body-wise registration, while 4940 outcomes (20 × 19 × 13) were obtained for the organ-wise approach.
Figure 2.
Pipelines for the body-wise and organ-wise registrations and validation.
For both approaches, NiftyReg was used as the registration tool. Briefly, affine followed by non-rigid registrations were performed to align the references to the target; this involved five coarse-to-fine levels, and limited the upper intensity threshold to 500 HU. Note the registration parameters were optimized and provided by the developers of NiftyReg, which differed from the previous default settings [5]. The registrations were applied between the whole abdominal CT scans for the body-wise approach, while between the organ-specific ROIs for the organ-wise approach.
The registered atlases were validated against the manual labels using DSC, MSD, and HD. For organ-wise registrations, registered atlases for each specific organ were first transferred back to the body space, and validated in a body-wise manner. The results in [5] were also included for comparison as the body-wise registrations with default parameters.
Body-wise Approach
The NiftyReg registrations were applied directly to the whole range of abdominal CT scans under LOOCV.
Organ-wise Approach
Organ-specific ROIs were generated using random forest following [12]. Specifically, four trees were trained with fourteen levels to learn the six boundary positions of each organ by using ten external training datasets. During training, the features to be learnt were collected by the differences of the mean intensities with two feature boxes randomized in offset (0 ~ 100 mm for × and y direction, 0 ~10 mm for z direction) and size (0 ~ 70 mm for each direction). During testing, 10% of the voxels falling into the leaf nodes with least uncertainty were used for boundary estimation. The organ-specific ROIs were then defined by padding 5 cm to each side of bounding boxes to include adequate background tissues.
When performing organ-wise registrations using NifyReg, the organ-specific ROIs were estimated on the target scan, while derived from the manual segmentations on the atlases under LOOCV. The organ localization was also validated as the prerequisite for the tested organ-wise registrations. Performance was assessed by the mean distance error of the estimated bounding boxes to those from the manual labeling. Containment index was collected for each localized organ before and after padding the ROI as the percentage of organ volumes contained within the bounding boxes.
3. RESULTS
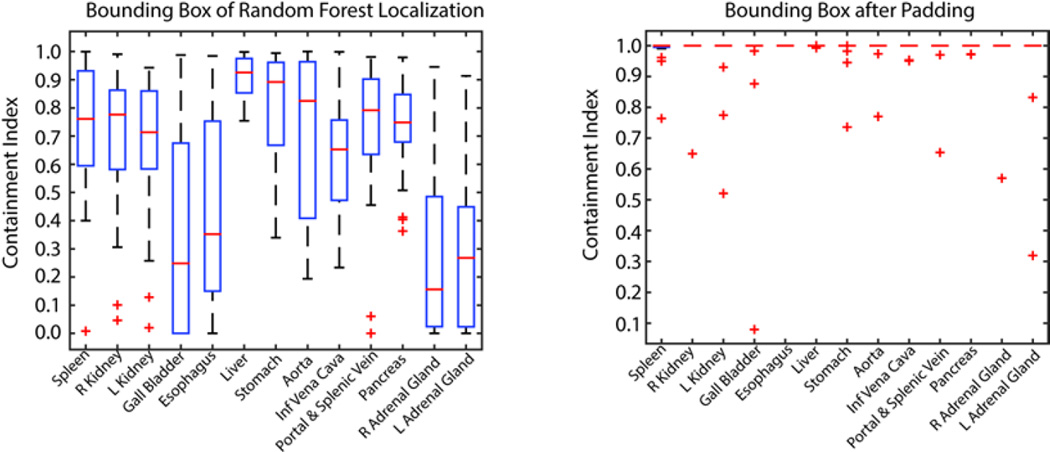
The mean distance error of the estimated bounding boxes using random forest localization was 14.71 mm. The mean containment indexes for the bounding boxes before and after the margin padding were 0.61 and 0.98, respectively. 17 out of 20 datasets had full containment on all organs after the padding (Figure 3).
Figure 3.
Containment indexes for organ-wise registration showing the ratio of organ volume captured inside the bounding boxes before and after padding of 5 cm to each side of the estimated bounding box by random forest.
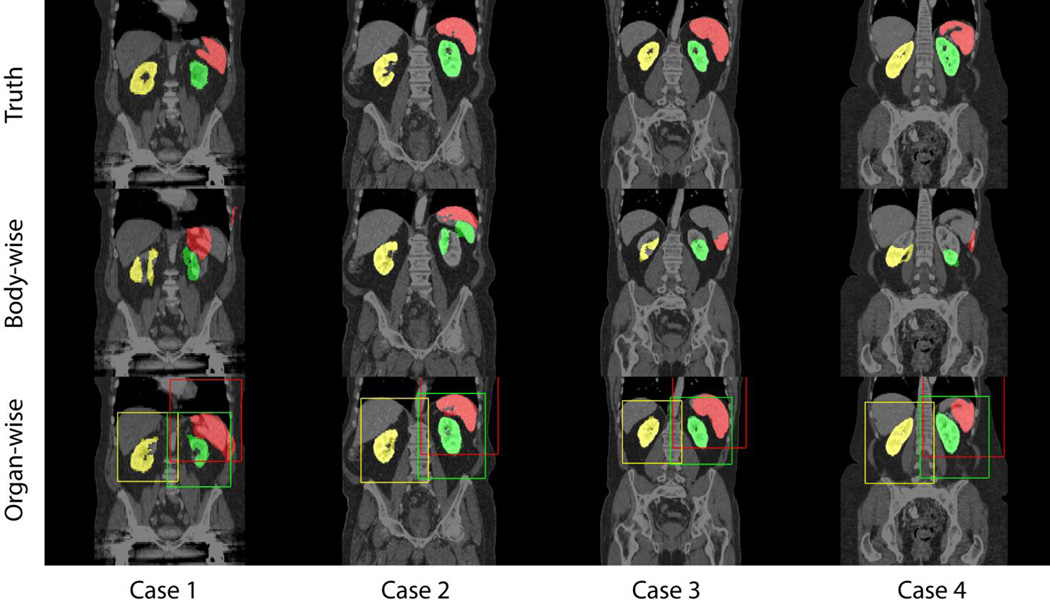
On four randomly selected registration cases, large improvements were observed for the organ-wise registrations compared to the body-wise counterparts on three selected organs, i.e., spleen, right kidney and left kidney (Figure 4).
Figure 4.
Qualitative comparisons between body-wise and organ-wise approaches on spleen (red), right kidney (yellow), and left kidney (blue). Padded bounding boxes in correspondent colors indicate the ROIs for organ-wise registrations.
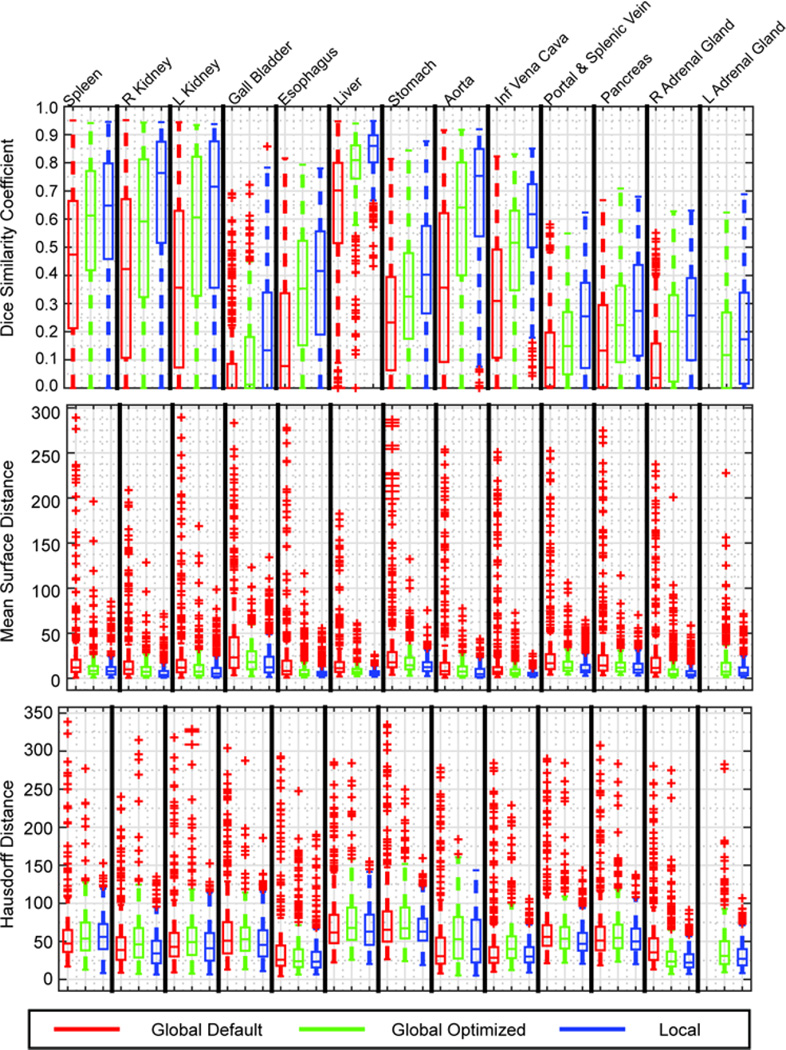
Quantitatively, the organ-wise registrations performed consistently better than the global-wise registrations using default or optimized parameters in DSC, MSD, and HD. Using the Wilcoxon signed-rank test, organ-wise approach represents significantly larger DSC values for all organs (Δ=0.10 by median, p-value < 0.005), smaller MSD (Δ=−2.16mm by median, p-value < 0.001) and HD (Δ=−6.82mm by median, p-value <0.01) for all organs except for spleen as compared to the body-wise approaches (Figure 5).
Figure 5.
Quantitative comparisons between body-wise and organ-wise approaches using Dice Similarity Coefficient (DSC), Mean Surface Distance (MSD) and Hausdorff Distance (HD). Global default (red) represnts results in previous study using default parameters of NiftyReg, where its performances on both adrenal glands are demonstrated as the “R Adrenal Gland” in the boxplot. Global optimized (green) represents body-wise registrations using optimized parameters of NiftyReg. Local (blue) indicates the proposed organ-wise registrations.
4. DISCUSSION
Image registration of human anatomy in medical data has been a challenging problem, especially beyond the cranial vault; the uniform smoothness constraint on deformation field of traditional non-rigid registration breaks when different anatomical structures of interest slide with respect to each other without a natural constraint like the skull. This sliding problem is not uncommon in medical imaging applications involving registrations, including atlas-based segmentation, image-guided surgery, and longitudinal analysis. Efforts on treating the sliding anatomies have been fallen into (1) piece-wise registration and (2) deformation regularization, where the former scheme codes transformations for sub-regions of the images [13, 14], and the latter extends the plausibility of image correspondences during deformations [15, 16].
In this study, we take a shortcut to handle the implicit discontinouity in abdominal anatomy by performing organ-wise registration after localizing the ROIs for each organ. Our approach gets around the global alignment, while focusing on the local context around the organs of interest, and turns out to be effective comparing to the traditional body-wise registration. On the other hand, the organ localization by random forest, as the prerequisite for this approach, leaves substantial room for improvement with possible directions on optimizing the feature box selection and using hiearchical identification [17]. Replacing the form of organ-specific ROIs with more anatomically reasonable shape with super-voxel methods might also improve the registrations [18].
Acknowledgments
This research was supported by NIH 1R03EB012461, NIH 2R01EB006136, NIH R01EB006193, ViSE/VICTR VR3029, NIH UL1 RR024975-01, NIH UL1 TR000445-06, NIH P30 CA068485, and AUR GE Radiology Research Academic Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy K, Van Ginneken B, Reinhardt J, et al. Evaluation of methods for pulmonary image registration: The EMPIRE10 study. Grand Challenges in Medical Image Analysis. 2010;2010 [Google Scholar]
- 3.Wolz R, Chu C, Misawa K, et al. Automated abdominal multi-organ segmentation with subject-specific atlas generation. Medical Imaging, IEEE Transactions on. 2013;32(9):1723–1730. doi: 10.1109/TMI.2013.2265805. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z, Burke RP, Lee CP, et al. Efficient multi-atlas abdominal segmentation on clinically acquired CT with SIMPLE context learning. Medical image analysis. 2015 doi: 10.1016/j.media.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CP, Xu Z, Burke RP, et al. Evaluation of five image registration tools for abdominal CT. 94131N-94131N-7. doi: 10.1117/12.2081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z, Gertz AL, Baucom RB, et al. Improving Multi-Atlas Abdominal Organ Segmentation via Organ-Wise Normalization. MICCAI 2015 Workshop and Challenge: Multi-Atlas Labeling Beyond Cranial Vault (in press) 2015 [Google Scholar]
- 7.Jenkinson M, Beckmann CF, Behrens TE, et al. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Rueckert D, Sonoda LI, Hayes C, et al. Nonrigid registration using free-form deformations: application to breast MR images. Medical Imaging, IEEE Transactions on. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 9.Avants BB, Epstein CL, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modat M, Ridgway GR, Taylor ZA, et al. Fast free-form deformation using graphics processing units. Computer methods and programs in biomedicine. 2010;98(3):278–284. doi: 10.1016/j.cmpb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 11.McAuliffe MJ, Lalonde FM, McGarry D, et al. Medical image processing, analysis and visualization in clinical research. :381–386. [Google Scholar]
- 12.Criminisi A, Robertson D, Konukoglu E, et al. Regression forests for efficient anatomy detection and localization in computed tomography scans. Medical image analysis. 2013;17(8):1293–1303. doi: 10.1016/j.media.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Arsigny V, Pennec X, Ayache N. Polyrigid and polyaffine transformations: a novel geometrical tool to deal with non-rigid deformations–application to the registration of histological slices. Medical image analysis. 2005;9(6):507–523. doi: 10.1016/j.media.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Risser L, Vialard F-X, Baluwala HY, et al. Piecewise-diffeomorphic image registration: Application to the motion estimation between 3D CT lung images with sliding conditions. Medical image analysis. 2013;17(2):182–193. doi: 10.1016/j.media.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Pace DF, Niethammer M, Aylward SR. [Sliding geometries in deformable image registration] Springer; 2012. [Google Scholar]
- 16.Schmidt-Richberg A, Ehrhardt J, Werner R, et al. [Slipping objects in image registration: improved motion field estimation with direction-dependent regularization] Springer; 2009. [DOI] [PubMed] [Google Scholar]
- 17.Cuingnet R, Prevost R, Lesage D, et al. [Automatic detection and segmentation of kidneys in 3D CT images using random forests] Springer; 2012. [DOI] [PubMed] [Google Scholar]
- 18.Marai GE, Laidlaw DH, Crisco JJ. Super-resolution registration using tissue-classified distance fields. Medical Imaging, IEEE Transactions on. 2006;25(2):177–187. doi: 10.1109/TMI.2005.862151. [DOI] [PubMed] [Google Scholar]