Abstract
Since mitochondria play roles in amino acid metabolism, carbohydrate metabolism and fatty acid oxidation, defects in mitochondrial function often compromise the lives of those who suffer from these complex diseases. Detecting mitochondrial metabolic changes is vital to the understanding of mitochondrial disorders and mitochondrial responses to pharmacological agents. Although mitochondrial metabolism is at the core of metabolic regulation, the detection of subtle changes in mitochondrial metabolism may be hindered by the overrepresentation of other cytosolic metabolites obtained using whole organism or whole tissue extractions.
Here we describe an isolation method that detected pronounced mitochondrial metabolic changes in Drosophila that were distinct between whole-fly and mitochondrial enriched preparations. To illustrate the sensitivity of this method, we used a set of Drosophila harboring genetically diverse mitochondrial DNAs (mtDNA) and exposed them to the drug rapamycin. Using this method we showed that rapamycin modifies mitochondrial metabolism in a mitochondrial-genotype-dependent manner. However, these changes are much more distinct in metabolomics studies when metabolites were extracted from mitochondrial enriched fractions. In contrast, whole tissue extracts only detected metabolic changes mediated by the drug rapamycin independently of mtDNAs.
Keywords: Developmental Biology, Issue 103, Mitochondrial isolates, metabolomics, Drosophila, mitochondrial DNAs, mitochondrial physiology, pharmacology
Introduction
The goal of this procedure is to develop enriched mitochondrial fractions that yield enough mitochondrial metabolites for metabolomics studies using Drosophila melanogaster. In our experience, metabolomics analysis using whole cellular extraction methods are unable to detect subtle mitochondrial metabolite changes in Drosophila. However, mitochondrial fractioning prior to metabolomics analysis increases the sensitivity to identify mitochondrial metabolite shifts.
Mitochondria are cellular organelles responsible for providing 90% of the energy that cells need for normal function1. In recent years it has been recognized that mitochondria play a much more dynamic role in cellular and organismal function than merely producing adenosine triphosphate(ATP), and are now recognized as hubs for the regulation of metabolic homeostasis2,3. Mitochondria are the result of an endosymbiotic process in which distinct microbial lineages merged ~1.5 billion years ago4. As mitochondria evolved into true organelles, genes from the endosymbiont were incorporated in the emerging nuclear genome. In animals today, approximately 1,500 mitochondrial proteins are nuclear-encoded while 37 genes remain in the mtDNA, 13 of which encode mitochondrial proteins that are subunits of the enzyme complexes of oxidative phosphorylation5. Coordination between mitochondria and nuclear compartments is needed to maintain proper mitochondrial function.
Using the methods described here we were able to detect mitochondrial metabolic changes in Drosophila that result from manipulation of the coordination between mitochondrial and nuclear genomes. We used a strain of Drosophila in which mtDNA from its sister species D. simulans was placed on a single D. melanogaster nuclear background6. This ‘disrupted’ mitonuclear genotype was compared to the ‘native’, or co-evolved mitonuclear genotype of D. melanogaster carrying the same nuclear genome with its native D. melanogaster mtDNA. The D. melanogaster and D. simulans mtDNAs differ by ~100 amino acids and >500 synonymous substitutions that affect mitonuclear communication7,8. We generated whole fly extracts and mitochondrial enriched extracts to study metabolite shifts in response to pharmacological stress. Here we show that when using mitochondrial enriched fractions we detect pronounced shifts in mitochondrial metabolites between the native, co-evolved genotype carrying the D. melanogaster mtDNAs and the disrupted genotype carrying D. simulans mtDNA. In contrast, the metabolite changes between these two genotypes are subtle using normal methods that utilize whole fly extract. Therefore, this method provided a path to understand how mtDNAs mediate mitochondrial changes in response to different drugs.
Protocol
1. Reagents and Solutions
- Preparation of fly food and media
- Heat fly ingredients 11% sugar, 2% autolyzed yeast, 5.2% cornmeal, agar 0.79% w/v in water in a hotplate set at 90 °C. Stir regularly until a homogenous slightly dense mixture is obtained. Prepare a final volume of 550 ml of fly food for a total of 100 vials with 5 ml fly food in each.
- Remove from the heating source, stir occasionally until the food cools down to 80 °C and add 0.2% tegosept-methyl 4-hydroxybenzoate dissolved in 95% ethanol.
- Split the food in two equal volumes. Add 1.1 ml of 50 mM rapamycin dissolved in ethanol to one volume and 1.1 ml of ethanol to the other volume. Stir the rapamycin and the ethanol solutions into the food. Be sure that the temperature decreases to 50 °C before adding drugs.
- Isolation Buffer
- Prepare 500 ml of Isolation Buffer [225 mM Mannitol, 75 mM Sucrose, 10 mM 3-(N-morpholino) propanesulfonic acid (MOPS) and 1 mM ethylenediaminetetraacetic acid (EDTA) , 2.5 mg/ml bovine serum albumin (BSA)] in water.
- Adjust the pH to 7.2 using sodium hydroxide. The initial pH will be acidic.
- Use sterile filter storage bottles with a 0.22 µm pore size to sterilize solution and store it at 4 °C.
- Wash Buffer
- Prepare 500 ml of wash buffer [225 mM Mannitol, 75 mM Sucrose, 10 mM KCl, 10 mM Tris HCl and 5 mM KH2PO4] in water.
- Adjust the pH to 7.2 using sodium hydroxide. The initial pH will be acidic.
- Use sterile filter storage bottles with a 0.22 µm pore size to sterilize solution and store it at 4 °C.
2. Rearing of the Drosophila Strains
To control larval density during development, place 25 pairs of parents in to a culture bottle and allow them to lay eggs for 48 hr.
Remove parents after 48 hr to regulate egg density.
Repeat steps 2.1 and 2.2 using the emerging F1 offspring, so two generations of this density control is achieved.
- To collect adults, anesthetize the flies using carbon dioxide (CO2) and separate by sex.
- Use a single stage regulator to delivered pure CO2 from a high pressured tank at a continuous flow of 5 psi. To avoid static electricity, use plastic tubing to catalyze the CO2 into a 500 ml filtering flask with water.
- Use a rubber stopper with one hole to seal the flask. Use plastic tubing to connect the lateral aperture of the flask to a CO2 pad.
- Place the flies in the pad for no more than 10-15 min. Allow to recover from anesthesia for 24 hr before placing them in experimental conditions.
To generate enough metabolites for mitochondrial enriched fractions, use 300 flies per sample. Here, use females, but gender may differ in other experiments. Transfer 150 flies to a homemade 1 L demography cage. Allow access to 5 ml of fly food in a vial.
Transfer the remaining 150 flies to another 1 L cage. Do not overpopulate cages with more than 150 flies.
Use six replicate samples per experimental condition. Since whole animal extracts yield more metabolites, to generate 1 sample of whole animal isolates, transfer 50 female flies to one cage. As for the mitochondrial isolates, use six replicate samples per experimental condition.
Place cages at 25 °C with cycle of 12 hr light and 12 hr dark.
Provide fresh food every 2 or 3 days in a vial with 5 ml of food to maintain food quality.
Maintain flies on food for 10 days. This step is needed for the rapamycin treatment7. Other treatments or conditions will differ.
3. Isolation of Mitochondrial Fractions
Dump flies from one cage (150 flies) into a glass-teflon dounce homogenizer filled with 1 ml of chilled isolation buffer.
Homogenize by moving the pestle up and down for 15 strokes. Keep the mortar on ice to keep mitochondria intact.
Transfer homogenate to a 1.5 ml tube and centrifuge at 300 x g for 5 min at 4 °C.
Take the supernatant and centrifuge at 6,000 x g for 10 min at 4 °C to enrich for mitochondria.
As the supernatant contains the cytosolic fraction, discard the supernatant or use it for alternative experiments. Resuspend the pellet in 300 µl of wash buffer.
Repeat steps 3.1 - 3.5 for the second cage. Combine the resuspended pellets of both cages in a cryogenic microcentrifuge tube.
Centrifuge at 6,000 x g for 10 min at 4 °C.
Discard the supernatant and flash-freeze the pellet in liquid nitrogen.
- Store the mitochondrial enriched fractions at -80 °C or lower temperature.
- Alternatively, for whole animal preparation, place the adults in a cryogenic microcentrifuge tube. Flash-freeze the vial in liquid nitrogen and store adults at -80 °C or lower temperature.
Representative Results
Using the protocol explained above, we performed metabolomic analysis on mitochondrial enriched fractions and whole animal extracts to test the effect of the drug rapamycin on divergent mtDNAs 7. We delivered 200 µM of rapamycin by dissolving the drug in the fly food. Flies were exposed to rapamycin for 10 days. Metabolites from whole fly extracts and from mitochondrial extracts were obtained by using gas chromatography mass spectrometry (GC/MS) and liquid chromatography-tandem mass spectrometry (LC/MS/MS) using standard solvent extraction methods.
Figure 1 shows an enrichment of the membrane transport protein porin as indicator of mitochondrial enrichment in the mitochondrial fractions. Porin protein was undetectable in the cytosolic fractions. Conversely, tubulin protein was not detected in the mitochondrial fraction, suggesting little contamination of cytosolic proteins. Figure 2 and Figure 3 show principle components analysis (PCA) of the complete metabolite profiles from the ‘native’ D. melanogaster strain (OreR) and the ‘disrupted’ strain of flies harboring mtDNA from D. simulans and nuclear DNA from D. melanogaster strain OreR (sm21). Both strains were exposed to the drug rapamycin. The data are presented as a bi-plot of principle component axes 1 and 2 to visualize the effect of treatment and mtDNAs7,9. Metabolites obtained using a standard whole fly extract are shown in Figure 2A. Figure 2B shows a similar PCA plot for metabolites in the mitochondrial enriched extracts. For whole fly extracts (Figure 2A), rapamycin treatment sifts the samples to significantly lower values of PC2. Yet, changes in metabolites due to mtDNA genotype are subtle, since OreR and sm21 mtDNAs exposed to rapamycin or control vehicle are closely adjacent or overlap in PCA space. However, when using enriched mitochondrial extracts, mtDNAs show distinct rapamycin effects on mitochondrial metabolite profiles. In particular, rapamycin has a strong effect on the metabolite profiles of the OreR strain, shown by the displacement along the PC1 axis. But, on the ‘disrupted’ sm21 mtDNA genotype, the metabolite profile for the control-food condition is displaced from that of the native OreR strain (red and black polygons, respectively, in Figure 2B). As a result, the rapamycin treatment has noticeably less of an effect on the shift of the metabolite landscapes in the disrupted mtDNA genotype, sm21 (compare red and blue polygons in Figure 2B).
Figure 3 shows PCA plots of metabolites involved in energy homeostasis (a subset of all metabolites, limited to cofactors, vitamins and Krebs cycle intermediates), often located inside of the mitochondria. Here again, metabolite shifts from enriched mitochondrial fractions show a different behavior than those from whole animal extracts.
These results illustrate how metabolic information obtained by mitochondrial enriched extract and whole animal extracts differ but complement each other, with the mitochondrial enriched extract being more sensitive to metabolic shifts in the mitochondria.
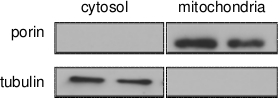 Figure 1. Western blot analysis showing effective separation of
mitochondria in mitochondrial fractions. Antibodies used were against porin (a
mitochondrial outer membrane protein) and tubulin (a cytosolic protein). Protein abundance
of mitochondrial and cytosolic fractions were quantified using bicinchoninic acid (BCA)
assay. 30 µg of total protein were loaded in each lane. Two independent extractions were
performed.
Figure 1. Western blot analysis showing effective separation of
mitochondria in mitochondrial fractions. Antibodies used were against porin (a
mitochondrial outer membrane protein) and tubulin (a cytosolic protein). Protein abundance
of mitochondrial and cytosolic fractions were quantified using bicinchoninic acid (BCA)
assay. 30 µg of total protein were loaded in each lane. Two independent extractions were
performed.
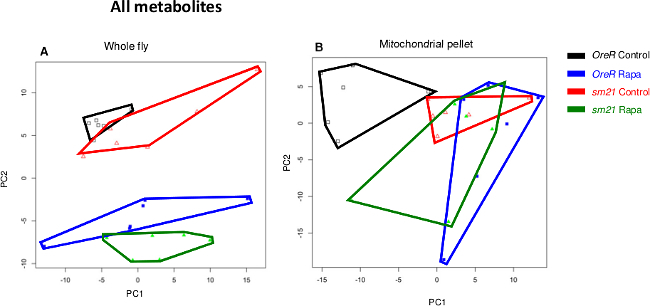 Figure 2. Principle component analysis (PCA) of metabolites obtained using whole
animal samples vs. mitochondrial enriched extracts on flies carrying
OreR and
sm21 mitochondrial haplotypes.
(A) PCA of 210 metabolites identified in whole animal samples.
(B) PCA of 230 metabolites detected on the mitochondrial extracts. Polygons
surrounding points are intended to aid the visualization of the six replicate samples for
each treatment. Please click
here to view a larger version of this figure.
Figure 2. Principle component analysis (PCA) of metabolites obtained using whole
animal samples vs. mitochondrial enriched extracts on flies carrying
OreR and
sm21 mitochondrial haplotypes.
(A) PCA of 210 metabolites identified in whole animal samples.
(B) PCA of 230 metabolites detected on the mitochondrial extracts. Polygons
surrounding points are intended to aid the visualization of the six replicate samples for
each treatment. Please click
here to view a larger version of this figure.
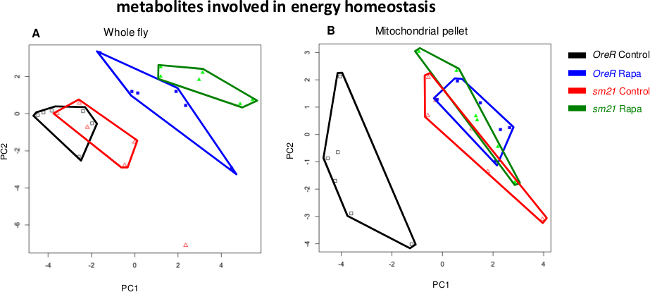 Figure 3. Principal component analysis of metabolites involved in energy
homeostasis on flies carrying OreR and
sm21 mitochondrial haplotypes.
(A) PCA of 12 energy metabolites from whole fly extracts and 13 energy
metabolites from mitochondrial extracts. (B) Polygons surrounding points are
intended to aid the visualization of the six replicate samples for each treatment. Please click
here to view a larger version of this figure.
Figure 3. Principal component analysis of metabolites involved in energy
homeostasis on flies carrying OreR and
sm21 mitochondrial haplotypes.
(A) PCA of 12 energy metabolites from whole fly extracts and 13 energy
metabolites from mitochondrial extracts. (B) Polygons surrounding points are
intended to aid the visualization of the six replicate samples for each treatment. Please click
here to view a larger version of this figure.
Discussion
The most critical steps in this protocol are: 1) rearing enough flies in abundant space. It is very important to not overpopulate the demography cages with more than 150 flies each; 2) changing the food of the cages frequently to avoid food competition and nutritional stress; and 3) maintaining all samples at 4 °C to ensure integrity during the isolation of the mitochondrial fraction. It is also recommended to chill the isolation buffer, the wash buffer, and the glass-teflon dounce homogenizer before use. To reduce cytosolic contamination of the enriched mitochondrial factions, the mitochondrial pellet from step 3.5 could be washed with wash buffer.
The extraction of metabolites from enriched mitochondrial fractions requires a high number of replicate samples (6 replicates/experimental condition). In total, 24 cages are used for Figures 1 and 2. We recommend performing the extraction of all the samples the same day to decrease experimental variability. Although feasible, this protocol entails careful planning and preparation of materials needed ahead of time. Labeling of the tubes ahead of time, and designing a plan to ensured consistency of waiting times between samples is needed to preserve sample quality and to decrease variation between replicates.
The buffers normally used to extract mitochondria contain sugars10,11; hence, the analysis of particular sugar metabolites may be affected by the extraction method. Although we have not used them in our analysis, KCl extraction buffers may be an alternative to mitochondrial isolation buffers that contain sugars12.
Due to the homeostatic nature of metabolic networks and a complex system of cellular signals that mediated this response, changes in mitochondrial metabolism could be detected by shifts in whole cell metabolite pools. In the case of mitochondrial regulation of such systems, subtle metabolite shifts may be hindered unless enough metabolites are extracted from mitochondrial enriched fractions. In model systems where plenty of mitochondria can be isolated from tissue, high resolution metabolomics has successfully detected changes in mitochondrial phenotypes13. However, in Drosophila melanogaster, harboring enough quality metabolites for a good composition of a sample may present a challenge. Using this protocol we obtained high quantity, quality and variety of metabolites to demonstrate that different extraction protocols reveal distinct metabolite shifts for whole-fly vs. mitochondrial fractions. Although the Western blot of mitochondrial and cytosolic proteins suggests little contamination of proteins in the two fractions, to confirm detailed quantitative measurements of mitochondrial metabolites, additional steps to limit contamination of cytosolic metabolites in the mitochondrial fraction could be applied. Measurement of metabolites before freezing the sample and comparison of these metabolites to those obtained after the freeze/thaw step may provide a more accurate quantification of mitochondrial specific metabolites. However, in this model where we manipulate the mitochondrial genome, the procedures we have used to prepare samples are particularly informative and may point to novel pathways of metabolic reprogramming mediated through mitochondria.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by Adelphi University faculty development grant and grant R15GM113156 from NIGMS awarded to EVC, grant R01GM067862 from NIGMS and grant R01AG027849 from NIA awarded to DMR.
References
- Scheffler IE. Mitochondria (Scheffler, Mitochondria) Wiley-Liss; 2007. p. 484. [Google Scholar]
- Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins. Cell. 2008;132(2):171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo N. Mitochondrial pathology: stress signals from the energy factory. Trends in molecular medicine. 2014;20(5):282–292. doi: 10.1016/j.molmed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440(7084):623–630. doi: 10.1038/nature04546. [DOI] [PubMed] [Google Scholar]
- Lane N. Power, Sex, Suicide: Mitochondria and the Meaning of Life. Vol. 368. USA: Oxford University Press; 2006. [Google Scholar]
- Montooth KL, Meiklejohn CD, Abt DN, Rand DM. Mitochondrial-nuclear epistasis affects fitness within species but does not contribute to fixed incompatibilities between species of Drosophila. Evolution; international journal of organic evolution. 2010;64(12):3364–3379. doi: 10.1111/j.1558-5646.2010.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Cuesta E, Holmbeck MA, Rand DM. Rapamycin increases mitochondrial efficiency by mtDNA-dependent reprogramming of mitochondrial metabolism in Drosophila. Journal of cell science. 2014;127(Pt 10):2282–2290. doi: 10.1242/jcs.142026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C-T, Ingelmo P, Rand DM. G×G×E for Lifespan in Drosophila: Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity. PLoS genetics. 2014;10(5):e1004354. doi: 10.1371/journal.pgen.1004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madala NE, Piater LA, Steenkamp PA, Dubery IA. Multivariate statistical models of metabolomic data reveals different metabolite distribution patterns in isonitrosoacetophenone-elicited Nicotiana tabacum and Sorghum bicolor cells. SpringerPlus. 2014;3:254. doi: 10.1186/2193-1801-3-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeboom GH, Schneider WC, Pallade GE. Cytochemical Studies Of Mammalian Tissues I . Isolation Of Intact Mitochondria From Rat Liver Some Biochemical Properties Of Mitochondria And Submicroscopic Particulate Material. Journal of Biological Chemistry. 1948;172:619–635. [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nature protocols. 2007;2(2):287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Corcelli A, Saponetti MS, et al. Mitochondria isolated in nearly isotonic KCl buffer: focus on cardiolipin and organelle morphology. Biochimica et biophysica acta. 2010;1798(3):681–687. doi: 10.1016/j.bbamem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Roede JR, Park Y, Li S, Strobel FH, Jones DP. Detailed mitochondrial phenotyping by high resolution metabolomics. PloS one. 2012;7(3):e33020. doi: 10.1371/journal.pone.0033020. [DOI] [PMC free article] [PubMed] [Google Scholar]


