Abstract
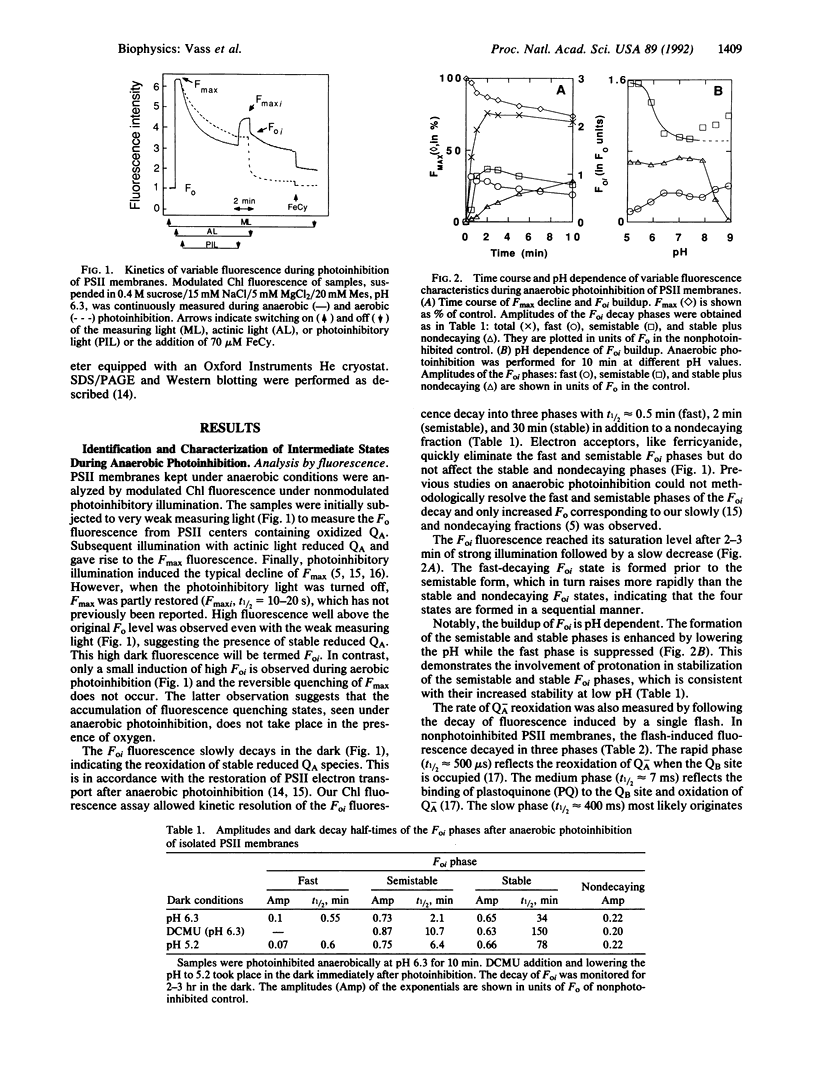
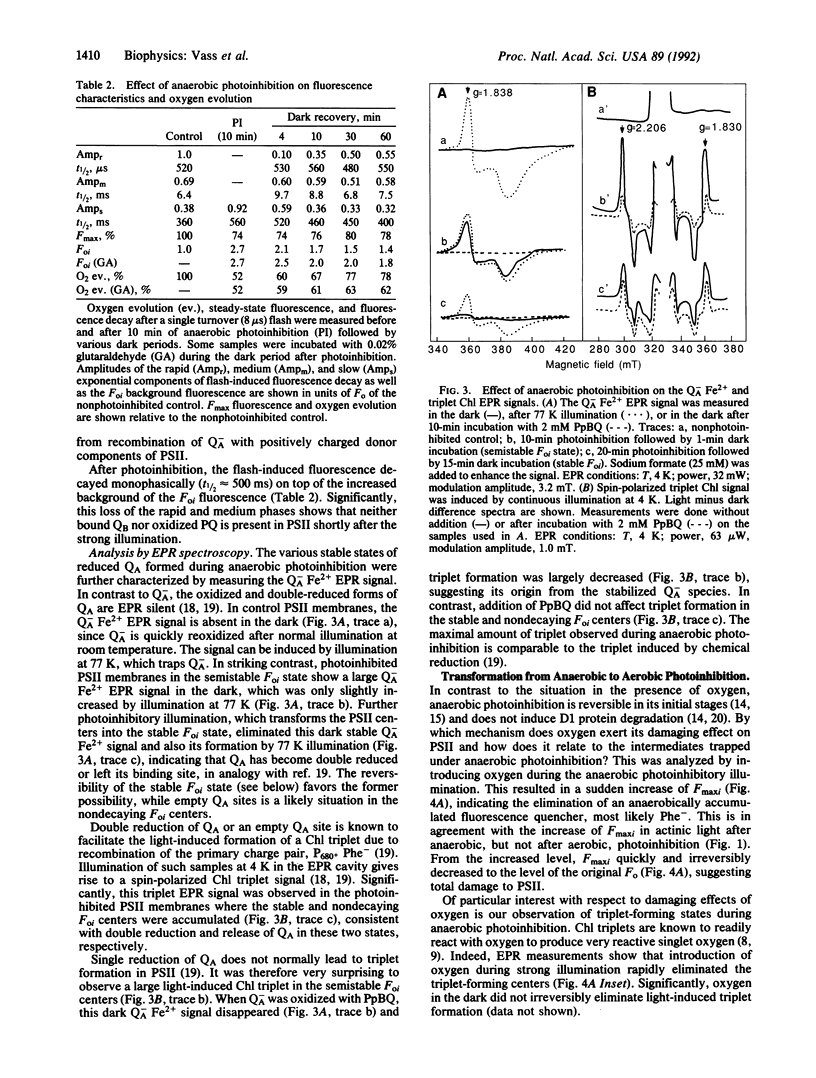
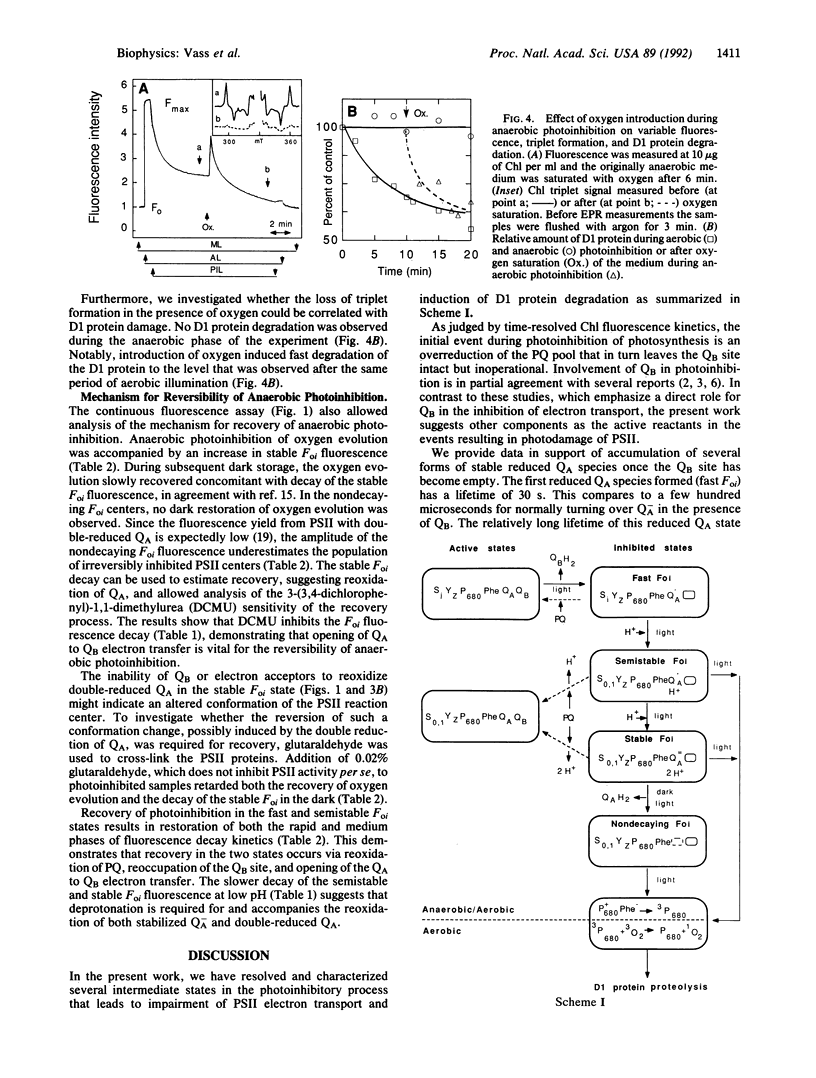
Photoinhibition of photosynthesis was studied in isolated photosystem II membranes by using chlorophyll fluorescence and electron paramagnetic resonance (EPR) spectroscopy combined with protein analysis. Under anaerobic conditions four sequentially intermediate steps in the photoinhibitory process were identified and characterized. These intermediates show high dark chlorophyll fluorescence (Foi) with typical decay kinetics (fast, semistable, stable, and nondecaying). The fast-decaying state has no bound QB but possesses a single reduced QA species with a 30-s decay half-time in the dark (QB, second quinone acceptor; QA, first quinone acceptor). In the semistable state, Q-A is stabilized for 2-3 min, most likely by protonation, and gives rise to the Q-A Fe2+ EPR signal in the dark. In the stable state, QA has become double reduced and is stabilized for 0.5-2 hr by protonation and a protein conformational change. The final, nondecaying state is likely to represent centers where QA H2 has left its binding site. The first three photoinhibitory states are reversible in the dark through reestablishment of QA to QB electron transfer. Significantly, illumination at 4 K of anaerobically photoinhibited centers trapped in all but the fast state gives rise to a spinpolarized triplet EPR signal from chlorophyll P680 (primary electron donor). When oxygen is introduced during anaerobic illumination, the light-inducible chlorophyll triplet is lost concomitant with induction of D1 protein degradation. The results are integrated into a model for the photoinhibitory process involving initial loss of bound QB followed by stable reduction and subsequent loss of QA facilitating chlorophyll P680 triplet formation. This in turn mediates light-induced formation of highly reactive and damaging singlet oxygen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan F. E., Becker D. W., Cheniae G. M. Studies on the Photoactivation of the Water-Oxidizing Enzyme: II. Characterization of Weak Light Photoinhibition of PSII and Its Light-Induced Recovery. Plant Physiol. 1986 Sep;82(1):261–269. doi: 10.1104/pp.82.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal T., Aro E. M., Carlberg I., Andersson B. Restoration of light induced photosystem II inhibition without de novo protein synthesis. FEBS Lett. 1990 Jul 16;267(2):203–206. doi: 10.1016/0014-5793(90)80925-9. [DOI] [PubMed] [Google Scholar]
- Jegerschöld C., Styring S. Fast oxygen-independent degradation of the D1 reaction center protein in photosystem II. FEBS Lett. 1991 Mar 11;280(1):87–90. doi: 10.1016/0014-5793(91)80210-t. [DOI] [PubMed] [Google Scholar]
- Jegerschöld C., Virgin I., Styring S. Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction. Biochemistry. 1990 Jul 3;29(26):6179–6186. doi: 10.1021/bi00478a010. [DOI] [PubMed] [Google Scholar]
- Kirilovsky D., Etienne A. L. Protection of reaction center II from photodamage by low temperature and anaerobiosis in spinach chloroplasts. FEBS Lett. 1991 Feb 25;279(2):201–204. doi: 10.1016/0014-5793(91)80149-w. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Marder J. B., Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989 Jan 27;56(2):241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Miller A. F., Brudvig G. W. A guide to electron paramagnetic resonance spectroscopy of Photosystem II membranes. Biochim Biophys Acta. 1991 Jan 3;1056(1):1–18. doi: 10.1016/s0005-2728(05)80067-2. [DOI] [PubMed] [Google Scholar]
- Shipton C. A., Barber J. Photoinduced degradation of the D1 polypeptide in isolated reaction centers of photosystem II: evidence for an autoproteolytic process triggered by the oxidizing side of the photosystem. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6691–6695. doi: 10.1073/pnas.88.15.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]



