Abstract
Objective
The aim of this study was to describe the clinical features and outcomes of Chinese patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer.
Method
The clinical data and survival statuses of 732 patients with operable HER2-positive breast cancer who were treated at the Department of Breast Surgery of the Shanghai Cancer Center from January 1, 2007, to December 31, 2011, were collected. The patients were divided into two groups according to treatment with and without trastuzumab. Disease-free survival (DFS) and overall survival were calculated using the Kaplan–Meier method and log-rank test. The associations of the patient characteristics with prognosis were analyzed via Cox regression.
Results
A total of 732 women with HER2-positive breast cancer were included in this study, among whom 258 (35.2%) received trastuzumab. The median follow-up duration was 41 months. By the end of the follow-up period, 86 (12%) women experienced local recurrence or metastasis. Patients who received both anti-HER2 therapy and chemotherapy exhibited a longer DFS than those who received chemotherapy alone (P=0.001). Tumor size, lymph node status, and family history of breast cancer were associated with median DFS, and tumor size, lymph node status, clinical stage, age, and body mass index were associated with median overall survival. Patients who received both neoadjuvant chemotherapy and trastuzumab exhibited a higher rate of pathological complete remission. In the neoadjuvant group, the patients who received both anti-HER2 therapy and chemotherapy exhibited a longer DFS than those who received chemotherapy alone (P=0.049).
Conclusion
Significant clinical features were observed in the Chinese patients with HER2-positive breast cancer. Furthermore, targeted anti-HER2 therapy may improve the prognosis of these patients.
Keywords: breast cancer, trastuzumab, HER2, disease-free survival, chemotherapy
Introduction
Amplification of the human epidermal growth factor receptor 2 (HER2) is observed in 15%–20% of all the patients with breast cancer.1,2 In the era prior to the availability of HER2-directed therapy, patients with HER2-positive (HER2+) breast cancer were observed to have more aggressive disease, poorer prognosis, and shorter overall survival (OS) than patients with other subtypes of breast cancer.3,4 In 1998, trastuzumab was approved for use, either alone or in combination with chemotherapy, for the treatment of metastatic HER2+ disease based on the results of a pivotal study conducted by Slamon et al.5,6 Trastuzumab, the first biological agent developed and approved for use in breast cancer, is a humanized monoclonal antibody against HER2 that has been shown to improve disease-free survival (DFS) after chemotherapy in women with early-stage HER2+ breast cancer. Additionally, it displays clinical activity and prolongs survival in patients with early-stage or metastatic breast cancer exhibiting HER2 overexpression (defined as a score of III based on immunohistochemistry [IHC] or evidence of gene amplification based on fluorescence in situ hybridization [FISH]).7
The final outcomes of the NSABP B-31 (The National Surgical Adjuvant Breast and Bowel Project B-31)/NCCTG-N9831 (North central cancer treatment group N9831) trials were presented at the 2012 ESMO meeting, and these results clarified the optimal duration of treatment with adjuvant trastuzumab in patients with HER2+ disease.8,9 Recent clinical trial results have indicated that a standard duration of 1 year of adjuvant trastuzumab treatment is optimal rather than a longer or shorter treatment duration.10 Although some clinical trials have found that new anti-HER2 combination therapies, such as lapatinib with trastuzumab, may improve the survival of patients with HER2+ breast cancer, this evidence should be further validated in additional trials.11 Other targeted anti-HER2 therapies, such as pertuzumab and T-DM1 (kadcyla, ado-trastuzumab emtansine), are currently under investigation. Further clinical trials need to be conducted before these compounds can be used in the clinical setting. Overall, an increasing number of new therapeutic agents are being developed for HER2+ patients.
Methods
Patients
Clinical data were collected for 732 consecutive female patients with HER2+ breast cancer treated at the Department of Breast Surgery of the Shanghai Cancer Center from January 1, 2007, to December 31, 2011. These data included medical histories and the results of physical examinations and imaging and pathological assessments. Women were eligible for enrollment in this study if the following criteria were met: 1) an age of >18 years; 2) primary histologically confirmed unilateral invasive carcinoma; 3) receipt of mastectomy or breast-conserving surgery with sentinel lymph node (LN) biopsy or axillary LN dissection within 2 weeks of diagnosis or cessation of neoadjuvant chemotherapy (NCT; axillary LN dissection was performed for sentinel LN biopsy-positive patients); 4) cancer classification as American joint committee on cancer stages I–III; 5) HER2 overexpression based on an IHC score of III and HER2-positivity based on FISH; 6) a left ventricular ejection fraction of ≥50%, as measured by echocardiography (ECHO) within 2 weeks preceding treatment with trastuzumab; and 7) receipt of a bone scan, chest X-ray/computed tomography imaging, or an abdominal ultrasound examination to exclude the presence of metastatic disease preceding the initial treatment. The exclusion criteria were as follows: 1) cancer classification as American joint committee on cancer stage IV; 2) a left ventricular ejection fraction of <50%, as measured by ECHO; 3) a history of myocardial infarction within 6 months prior to recruitment; 4) a history of congestive heart failure; or 5) no follow-up data.
Treatment and follow-up
Trastuzumab (Herceptin; Roche Pharma Ltd, Basel, Switzerland) was administered as follows: an 8 mg/kg loading dose on the first week, followed by 6 mg/kg every 3 weeks, or a 4 mg/kg loading dose on the first week, followed by 2 mg/kg weekly for 1 year. This drug was administered during or after chemotherapy. Some patients had participated in a trastuzumab-based clinical trial, and others had received chemotherapy, radiation, endocrine therapy, and/or anti-HER2+ therapy according to the standard treatment guidelines based on the tumor size, cancer stage, prior surgical approach used, and intrinsic subtypes of breast cancer. In the trastuzumab + NCT group, trastuzumab was administered for 1 year, beginning at initiation of chemotherapy, and the ECHO was checked every 3 months during the treatment. The clinical response and pathological complete remission (pCR) were then evaluated. pCR was defined as the absence of invasive carcinoma in both the breast tissue and the LNs. The patients were also examined for residual ductal carcinoma in situ. The clinical response to NCT was evaluated by breast magnetic resonance imaging and was determined according to the response evaluation criteria in solid tumors 1.1.12 The cutoff value for estrogen or progesterone positivity was positive nuclear staining in 1% of tumor cells.
All patients who were treated at the Fudan University Shanghai Cancer Center were instructed to attend regular follow-up visits by phone. All information regarding the adjuvant treatment of these patients was recorded; in addition, the site and time of the first detected relapse or metastasis were recorded, as well as the cause and time of death. We analyzed the data for these patients with HER2+ breast cancer to determine their clinical characteristics. We compared DFS and other end points in the women who were treated with and without trastuzumab to determine whether trastuzumab improves the prognosis of patients with HER2+ breast cancer.
Our study was approved by the independent ethical committee/institutional review board of the Fudan University Shanghai Cancer Center (Shanghai Cancer Center Ethical Committee). All patients provided written informed consent before inclusion in this study.
Statistical analysis
The primary end point of this study was DFS, which was defined as the interval from the date of definitive surgery to the first occurrence of any of the following events: breast cancer recurrence at any site; metastasis; the development of ipsilateral or contralateral breast cancer, including ductal carcinoma in situ but not lobular carcinoma in situ; or death from any cause without documentation of a cancer-related event. New primary breast cancer was counted as a DFS event, while other primary cancers were not. OS was assessed as a secondary end point. The chi-square test was used to evaluate the difference of patient characteristics between groups treated with and without trastuzumab. The patient characteristics include the age at diagnosis, tumor size, cancer stage, LN status, type of chemotherapy received, menopausal status, sex hormone expression, and body mass index (BMI). DFS and OS were calculated using the Kaplan–Meier method. Univariate survival differences were tested for significance with a log-rank test. All the potentially important factors identified in univariate analysis with a P-value <0.3 were included in the multivariate analysis. The Cox regression model was used to analyze the abilities of these characteristics to predict patient prognosis. All statistical tests were two-sided, and a P-value of <0.05 was considered significant. All analyses were performed using IBM SPSS Version 20 (IBM Corporation, Armonk, NY, USA).
Results
Survey of patients with HER2+ breast cancer at our center
Data were collected from 732 female patients with HER2+ breast cancer who were treated at the Department of Breast Surgery of the Shanghai Cancer Center from January 1, 2007, to December 31, 2011. HER2 overexpression was defined as a score of III based on IHC and positivity for HER2 gene amplification based on FISH. At our center, patients with HER2+ breast cancer accounted for ~20% of all patients with breast cancer, and this percentage is identical to that in a previous report.1 We calculated the numbers of patients with HER2+ breast cancer and of those who were treated with trastuzumab every year from 2007 to 2011 in the study cohort. These numbers increased gradually each year (Figure 1).
Figure 1.
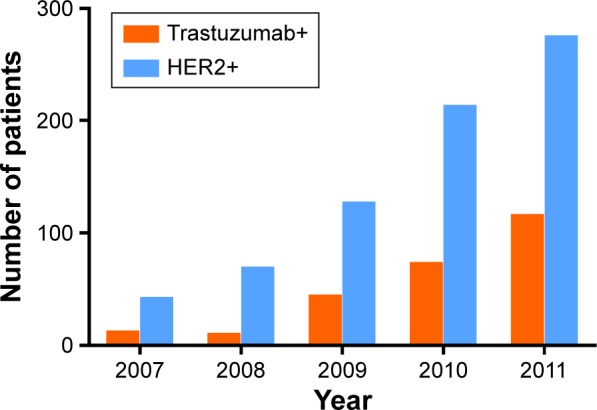
The proportion of trastuzumab+ (combination of trastuzumab and chemotherapy) patients out of all HER2-positive (HER2+) patients.
Note: The number of HER2+ patients and patients treated with trastuzumab (trastuzumab+) increased gradually each year from 2007 to 2011.
Abbreviation: HER2, human epidermal growth factor receptor 2.
Patient characteristics
A total of 732 women with HER2+ disease were included in this study, of whom 258 (35.2%) were treated with trastuzumab (trastuzumab+ group); in addition, a total of 459 (62.7%) patients were not treated with trastuzumab (trastuzumab− group), and the treatment received could not be identified for 15 patients (2%). The median follow-up duration was 41 months. Eighty-six (12%) women were diagnosed with local recurrence or metastasis. The women who received trastuzumab experienced fewer events than those who did not. The median age of the patients was 51 years (range, 20–86 years). The majority of the patients were older than 50 years (52.2%), 9.1% of patients were no older than 35 years, and 38.8% of patients were between 36 years and 50 years. A total of 439 (61.2%) patients had a large-size tumor (>2 cm), and 520 (72.5%) were in clinical stage II or III. In addition, 258 (35.98%) women harbored a Ki-67-positive tumor (>14% Ki-67-positive cells in the biopsy specimen) and 123 harbored a Ki-67-negative tumor (≤14%). A total of 459 (64.0%) women exhibited no axillary LN involvement. Moreover, 625 patients received chemotherapy, among whom 418 received taxane-based chemotherapy. The majority of these patients (54.3%) were of normal weight, but 290 (40.4%) were overweight or obese. The majority of the patients were postmenopausal (51.2%) and exhibited no LN involvement (64%). Forty-six patients (6.3%) had a confirmed family history of breast cancer. Additionally, 55.5% of all patients harbored a hormone receptor-positive (HR+) tumor, and the remaining patients (44.5%) harbored a hormone receptor-negative (HR−) tumor. Table 1 shows the patient characteristics according to age at diagnosis, tumor size, cancer stage, LN status, histologic grade, embolus status, receipt of NCT, chemotherapy, endocrine therapy, and/or radiotherapy, menopausal status, sex hormone expression, Ki-67 expression, and BMI.
Table 1.
Characteristics of HER2-positive patients
| No (%) of patients
| |||
|---|---|---|---|
| Characteristics | Total
|
Trastuzumab+
|
Trastuzumab−
|
| 717 | 258 | 459 | |
| Patient age (years) | |||
| ≤35 | 65 (9.1) | 33 (12.8) | 32 (7.0) |
| 36–50 | 278 (38.8) | 116 (45.0) | 162 (35.3) |
| >50 | 374 (52.2) | 109 (42.2) | 265 (57.7) |
| Tumor size (mm) | |||
| ≤20 | 246 (34.3) | 87 (33.7) | 159 (34.6) |
| 21–50 | 383 (53.4) | 135 (52.3) | 248 (54) |
| >50 | 56 (7.8) | 31 (12.0) | 25 (5.4) |
| Unknown | 32 (4.5) | 5 (1.9) | 27 (5.9) |
| Cancer stage | |||
| DCIS | 21 (2.9) | 0 (0.0) | 21 (4.6) |
| I | 176 (24.5) | 58 (22.5) | 118 (25.7) |
| II | 418 (58.3) | 151 (58.5) | 267 (58.2) |
| III | 102 (14.2) | 49 (19.0) | 53 (11.5) |
| LN status | |||
| 0 | 459 (64.0) | 153 (59.3) | 306 (66.7) |
| 1–3 | 122 (17.0) | 55 (21.3) | 67 (14.6) |
| ≥4 | 127 (17.7) | 49 (19.0) | 78 (17.0) |
| Unknown | 9 (1.3) | 1 (0.4) | 8 (1.7) |
| Histologic grade | |||
| I–II | 311 (43.4) | 122 (47.3) | 189 (41.2) |
| II–III | 75 (10.5) | 34 (13.2) | 41 (8.9) |
| III | 132 (18.4) | 49 (19.0) | 83 (18.1) |
| Unknown and DCIS | 199 (27.8) | 53 (20.5) | 146 (27.8) |
| Embolus | |||
| Yes | 160 (22.3) | 78 (30.2) | 82 (17.9) |
| No | 351 (49.0) | 114 (44.2) | 237 (51.6) |
| Unknown | 206 (28.7) | 66 (25.6) | 140 (30.5) |
| NCT | |||
| Yes | 139 (19.4) | 64 (24.8) | 75 (16.3) |
| No | 578 (80.6) | 194 (75.2) | 384 (83.7) |
| Chemotherapy | |||
| No | 92 (12.8) | 5 (1.9) | 87 (19.0) |
| Anthracycline-based | 194 (27.1) | 36 (14.0) | 158 (34.4) |
| Taxane | 418 (58.3) | 217 (84.1) | 201 (43.8) |
| Others | 7 (1.0) | 0 (0.0) | 7 (1.5) |
| Unknown | 6 (0.8) | 0 (0.0) | 6 (1.3) |
| History of mother suffered BC | |||
| Yes | 46 (6.4) | 18 (7.0) | 28 (6.1) |
| No | 671 (93.6) | 240 (93.0) | 431 (93.9) |
| Menopause | |||
| Yes | 367 (51.2) | 117 (45.3) | 250 (54.5) |
| No | 350 (48.8) | 141 (54.7) | 209 (45.5) |
| Sex hormone receptor | |||
| Positive | 398 (55.5) | 134 (51.9) | 264 (57.5) |
| Negative | 319 (44.5) | 124 (48.1) | 195 (42.5) |
| Endocrine therapy | |||
| Yes | 370 (51.6) | 130 (50.4) | 240 (52.3) |
| No | 324 (45.2) | 119 (46.1) | 205 (44.7) |
| Unknown | 23 (3.2) | 9 (3.5) | 14 (3.1) |
| Radiotherapy | |||
| Yes | 299 (41.7) | 151 (58.5) | 148 (32.3) |
| No | 411 (57.3) | 106 (41.1) | 305 (66.4) |
| Unknown | 7 (1.0) | 1 (0.4) | 6 (1.3) |
| Ki-67 (%) | |||
| ≤14 | 123 (17.2) | 49 (19.0) | 74 (16.1) |
| >14 | 258 (36.0) | 113 (43.8) | 145 (31.6) |
| Unknown | 336 (46.9) | 96 (37.2) | 240 (52.3) |
| BMI (kg/m2) | |||
| <18.5 | 26 (3.6) | 8 (3.1) | 18 (3.9) |
| 18.5–23.9 | 389 (54.3) | 142 (55.0) | 247 (53.8) |
| ≥24 | 290 (40.4) | 104 (40.3) | 186 (40.5) |
| Unknown | 12 (1.7) | 4 (1.6) | 8 (1.7) |
Abbreviations: BC, breast cancer; BMI, body mass index; DCIS, ductal carcinoma in situ; HER2, human epidermal growth factor receptor 2; LN, lymph node; NCT, neoadjuvant chemotherapy.
Trastuzumab prolongs DFS in HER2+ patients
Figure 2 shows that the patients who received anti-HER2 therapy combined with chemotherapy exhibited a longer DFS than those who received chemotherapy alone (median DFS, 41 months vs 40 months; P=0.001, odds ratio [OR]: 0.422; 95% confidence interval [CI]: 0.248–0.718); however, the median OS duration did not significantly differ between the two groups (Figure S1). The median OS of the trastuzumab+ and the trastuzumab− groups was 41 months and 43 months, respectively (P=0.352; OR: 0.642; 95% CI: 0.252–1.633). Patients in the trastuzumab+ group had a relatively lower median OS because some died of noncancer reasons. The earliest death event occurred in the 24th month after surgery in the trastuzumab+ group, while the time of the trastuzumab−group was much shorter, namely eleventh month. In the trastuzumab+ group, DFS events occurred in 17 (6.6%) patients; however, the DFS event rate was much higher in the 69 (15%) patients in the trastuzumab group (P=0.001). The earliest occurrence time of any event of the trastuzumab+ group was 24 months, while that of the trastuzumab− group was much shorter, only 11 months.
Figure 2.
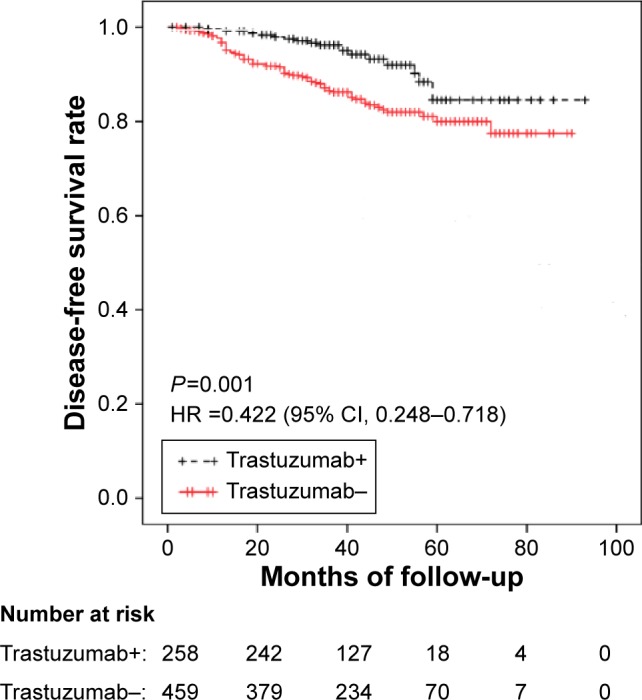
DFS of the patients according to treatment with trastuzumab.
Note: Patients who received both trastuzumab and chemotherapy (trastuzumab+ group) exhibited a longer DFS than those who received chemotherapy alone (trastuzumab− group) (P=0.001) (95% CI: 0.248–0.718; HR: 0.422).
Abbreviations: DFS, disease-free survival; HR, hazard ratio; CI, confidence interval.
Survival does not differ between the HR+ HER2+ and HR− HER2+ groups
After dividing all the patients into two groups according to their HR statuses, neither the median DFS nor OS duration was found to significantly differ between the HR+ HER2+ and HR− HER2+ patients. Similar results were obtained for the trastuzumab+ and trastuzumab− groups.
Impacts of other factors on prognosis
As shown in Tables 2 and 3, the patients harboring a small tumor (≤2 cm) exhibited a longer DFS than those harboring a large tumor (>5 cm) (median DFS: 42 months vs 37 months; hazard ratio: 0.164; 95% CI: 0.076–0.355; P<0.001). The patients ranging in age from 36 years to 50 years exhibited a longer OS than those >50 years of age (median OS, 42 months vs 41 months; hazard ratio: 0.606; 95% CI: 0.378–0.973; P=0.038). Additionally, the patients with stage I or II cancer exhibited a better prognosis than those with stage III cancer (P=0.000). Similarly, those with 0–3 involved LNs, those lacking an embolus, those who were premenopausal, and those who were not treated with radiation (radiotherapy−) exhibited a better prognosis than those with more than four involved LNs (P<0.001, P=0.031), those harboring an embolus (P=0.011), those who were menopausal (P=0.026), and those who were treated with radiation (P<0.001), respectively. Other factors such as histological grade, receipt of chemotherapy, Ki-67 expression, BMI, and family history were not significantly associated with DFS based on Cox regression analysis. The patients who were 36–50 years of age (P=0.007), those harboring a small tumor (≤2 cm) (P=0.004), those with stage I or II cancer (P=0.004, P=0.002), and those lacking LN involvement exhibited a longer OS than those who were >50 years of age, those harboring a large tumor (>5 cm), those with stage III cancer, and those with more than four involved LNs (P=0.001), respectively. The histological grade, receipt of chemotherapy, Ki-67 expression, embolus status, BMI, menopausal status, receipt of radiotherapy, and family history did not significantly impact the median OS duration. However, BMI was significantly associated with the median OS duration when it was considered as a categorical variable after combination of the overweight and obese categories (P=0.035). The patients with a BMI of >25 kg/m2 (obese and overweight) exhibited a shorter median OS duration than those with a BMI of ≤25 kg/m2 (normal and underweight).
Table 2.
Cox regression hazard model to predict the effect on DFS
| Category | HR | 95% CI | P-value |
|---|---|---|---|
| Patient age (years) | 0.115 | ||
| ≤35 | 0.904 | 0.430–1.898 | 0.789 |
| 36–50 | 0.606 | 0.378–0.973 | 0.038 |
| >50 | |||
| Tumor size (mm) | 0.000 | ||
| ≤20 | 0.164 | 0.076–0.355 | 0.000 |
| 21–50 | 0.579 | 0.324–1.037 | 0.066 |
| >50 | |||
| Cancer stage | 0.000 | ||
| DCIS | 0.000 | 0.000–1.194E+205 | 0.957 |
| I | 0.118 | 0.052–0.269 | 0.000 |
| II | 0.385 | 0.245–0.605 | 0.000 |
| III | |||
| LN status | 0.000 | ||
| 0 | 0.166 | 0.100–0.277 | 0.000 |
| 1–3 | 0.556 | 0.326–0.948 | 0.031 |
| ≥4 | |||
| Histologic grade | 0.977 | ||
| I–II | 1.070 | 0.142–8.057 | 0.948 |
| II–III | 0.914 | 0.512–1.631 | 0.760 |
| III | 1.050 | 0.480–2.296 | 0.902 |
| Unknown and DCIS | |||
| Embolus | 0.538 | 0.333–0.870 | 0.011 |
| Chemotherapy | 0.207 | ||
| No | 0.621 | 0.075–5.165 | 0.659 |
| Anthracycline-based | 0.976 | 0.131–7.281 | 0.981 |
| Taxane | 1.377 | 0.190–9.955 | 0.751 |
| Others | |||
| History of mother suffered BC | 7.277 | 1.013–52.257 | 0.048 |
| Menopause | 0.611 | 0.396–0.944 | 0.026 |
| Sex hormone receptor | 1.063 | 0.697–1.621 | 0.778 |
| Endocrine therapy | 1.216 | 0.782–1.891 | 0.385 |
| Radiotherapy | 0.378 | 0.242–0.588 | 0.000 |
| Ki-67 (%) | |||
| ≤14 | 0.689 | 0.362–1.312 | 0.689 |
| >14 | |||
| BMI (kg/m2) | 0.657 | ||
| <18.5 | 1.180 | 0.422–3.298 | 0.753 |
| 18.5–23.9 | 0.844 | 0.549–1.299 | 0.441 |
| ≥24 |
Abbreviations: BC, breast cancer; BMI, body mass index; CI, confidence interval; DCIS, ductal carcinoma in situ; DFS, disease-free survival; HR, hazard ratio; LN, lymph node.
Table 3.
Cox regression hazard model to predict the effect on OS
| Category | HR | 95% CI | P-value |
|---|---|---|---|
| Patient age (years) | 0.026 | ||
| ≤35 | 0.961 | 0.285–3.238 | 0.949 |
| 36–50 | 0.136 | 0.032–0.583 | 0.007 |
| >50 | |||
| Tumor size (mm) | 0.016 | ||
| ≤20 | 0.123 | 0.029–0.516 | 0.004 |
| 21–50 | 0.478 | 0.176–1.296 | 0.147 |
| >50 | |||
| Cancer stage | 0.002 | ||
| DCIS | 0.000 | 0.000–6.264E+296 | 0.971 |
| I | 0.050 | 0.006–0.387 | 0.004 |
| II | 0.277 | 0.124–0.619 | 0.002 |
| III | |||
| LN status | 0.003 | ||
| 0 | 0.188 | 0.070–0.507 | 0.001 |
| 1–3 | 0.727 | 0.271–1.955 | 0.528 |
| ≥4 | |||
| Histologic grade | 0.296 | ||
| I–II | 0.000 | 0.000–6.264E+296 | 0.981 |
| II–III | 0.439 | 0.512–1.631 | 0.124 |
| III | 1.212 | 0.480–2.296 | 0.744 |
| Unknown and DCIS | |||
| Embolus | 1.238 | 0.445–3.443 | 0.682 |
| Chemotherapy | 0.851 | ||
| No | 2001.174 | 0.075–2.718E+87 | 0.936 |
| Anthracycline-based | 2727.280 | 0.131–3.9E+87 | 0.933 |
| Taxane | 3573.678 | 0.190–5.056E+87 | 0.931 |
| Others | |||
| History of mother suffered BC | 1.943 | 0.263–14.378 | 0.515 |
| Menopause | 0.431 | 0.180–1.033 | 0.059 |
| Sex hormone receptor | 1.050 | 0.476–2.316 | 0.905 |
| Endocrine therapy | 0.677 | 0.280–1.636 | 0.386 |
| Radiotherapy | 0.720 | 0.323–1.604 | 0.422 |
| Ki-67 (%) | |||
| ≤14 | 0.581 | 0.162–2.082 | 0.404 |
| >14 | |||
| BMI (kg/m2) | 0.417 | ||
| <18.5 | 0.851 | 0.112–6.473 | 0.876 |
| 18.5–23.9 | 0.578 | 0.256–1.304 | 0.187 |
| ≥24 |
Abbreviations: BC, breast cancer; BMI, body mass index; DCIS, ductal carcinoma in situ; HR, hazard ratio; LN, lymph node; OS, overall survival.
Trastuzumab improves the clinical outcomes of patients with HER2+ who have received NCT
A total of 139 patients received NCT, including 61 who received both anti-HER2 therapy and chemotherapy (trastuzumab+ group) and 78 who received chemotherapy alone. Among these 139 patients, 40 (28.8%) experienced pCR. Among these 40 patients, 27 were in the trastuzumab+ group and the remaining 13 were in the trastuzumab− group (Figure 3). The patients in the trastuzumab+ group experienced a higher rate of pCR than those in the trastuzumab− group (P=0.001).
Figure 3.
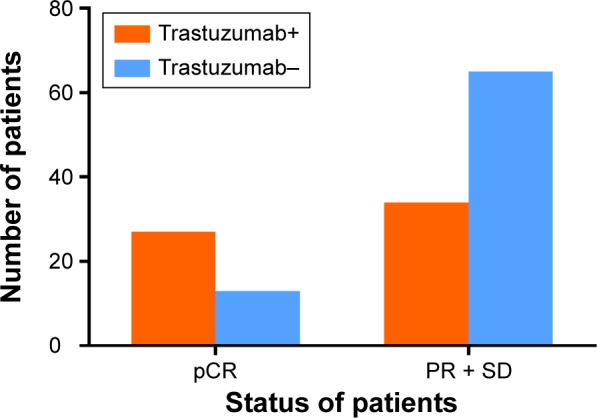
Chemotherapeutic response in the NCT group (n=139) according to treatment with trastuzumab.
Note: Patients who received both trastuzumab and chemotherapy (trastuzumab+ group) exhibited a higher pCR rate than those who received chemotherapy alone (trastuzumab− group) (P=0.001).
Abbreviations: NCT, neoadjuvant chemotherapy; pCR, pathological complete remission; PR, partial response; SD, stable disease.
Similar to the HER2+ patients, among the patients who received NCT, those who received both anti-HER2 therapy and chemotherapy exhibited a longer DFS than those who received chemotherapy alone (P=0.049) (95% CI: 0.213–0.998; hazard ratio: 0.461) (Figure 4). However, the median OS duration did not significantly differ between these patient subgroups.
Figure 4.
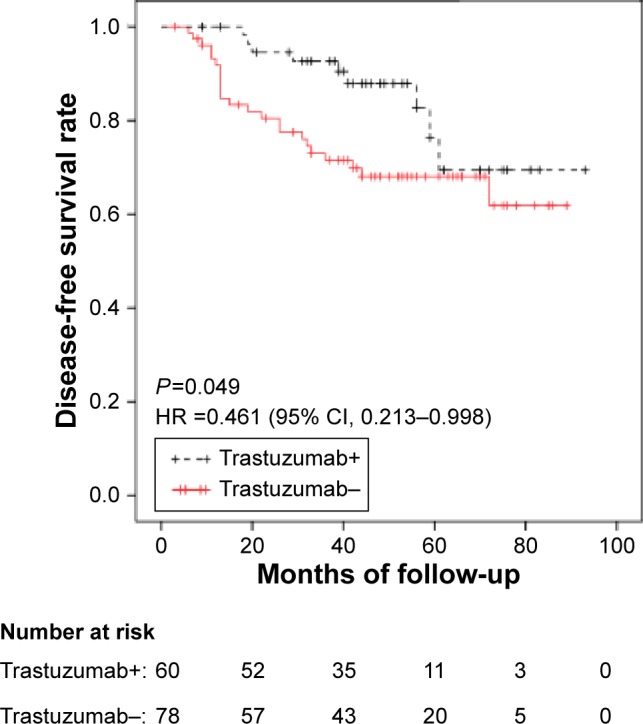
In the NCT group, patients who received both trastuzumab and chemotherapy (trastuzumab+ group) exhibited a longer DFS than those who received chemotherapy alone (trastuzumab− group) (P=0.049) (95% CI: 0.213–0.998; HR: 0.461).
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NCT, neoadjuvant chemotherapy.
Discussion
Trastuzumab, a humanized monoclonal antibody against HER2, has been shown to improve the prognosis of women with HER2+ breast cancer. In our study, a total of 723 HER2+ patients were analyzed who were treated at the Department of Breast Surgery of the Shanghai Cancer Center from January 1, 2007, to December 31, 2011. The median patient age was 51 years, and the majority of the patients were >50 years of age. The percentage of postmenopausal women was 51.2%. Most of the patients were of normal weight, but 290 (40.4%) were overweight or obese. The patients who received both anti-HER2 therapy and chemotherapy exhibited a longer DFS than those who received chemotherapy alone. Trastuzumab also prolonged DFS among the HER2+ patients who received NCT.
The HER2+ patients were observed to have more aggressive disease and a poorer prognosis. Many studies have reported that trastuzumab improves the prognosis of patients with HER2+ breast cancer, which is in accordance with our results. The majority of the patients in our study were postmenopausal women; thus, we concluded that elderly Chinese women are more likely to develop HER2+ breast cancer than other breast cancer subtypes. Most of the HER2+ patients harbored large tumors, had later stage cancer, and carried a Ki-67-positive tumor (>14%). These results are consistent with the aggressive characteristics of HER2+ breast cancer. When a cutoff BMI of >25 was used to stratify the patients according to body weight, we found that the obese and overweight women exhibited a shorter median OS than the normal weight and underweight women. The patients who received both anti-HER2 therapy and chemotherapy exhibited a longer DFS than those who received chemotherapy alone (P=0.001) (95% CI: 0.248–0.718; hazard ratio: 0.422). Therefore, trastuzumab decreased the risk of recurrence or metastasis by 57.8%; this percentage is similar to that observed in the Herceptin Adjuvant trial.13
Beginning in 2007, some patients at the Fudan University Shanghai Cancer Center participated in several international multicenter clinical trials, such as ALTTO and BETH.11 In 2009, trastuzumab was accepted for the treatment of patients with HER2+ breast cancer in the People’s Republic of China; thus, after that time, increasing numbers of patients with HER2+ breast cancer were treated with both trastuzumab and chemotherapy. Neither the median DFS nor OS significantly differed between the HR+ HER2+ and HR− HER2+ patients. We propose that HR− HER2+ patients might benefit from additional cycles of chemotherapy or from more powerful chemotherapeutic agents. Cox regression analysis revealed that DFS was associated with tumor size, clinical stage, LN status, age, embolus status, menopausal status, and radiotherapy and that OS was associated with tumor size, cancer stage, and LN status. These results are consistent with those of other studies. Although most designers of clinical trials have recommended the combination of trastuzumab with taxane-based chemotherapy, no significant differences were found among the patients according to the chemotherapy regimen received in our study.14 Thus, this combination should be validated in additional trials. Among the 139 patients who received NCT, 40 (28.8%) experienced pCR. The patients in the trastuzumab+ group exhibited a higher pCR rate than those in the trastuzumab− group (P=0.001). Thus, the patients who received both NCT and trastuzumab exhibited a higher pCR rate than those who received chemotherapy alone. This conclusion is consistent with those of several neoadjuvant trials (eg, MDACC, NOAH, and TECHNO).15–17 The patients who received both anti-HER2 therapy and chemotherapy exhibited a longer DFS than those who received chemotherapy alone.
In addition to trastuzumab, other targeted anti-HER2 therapies, such as lapatinib, pertuzumab, and T-DM1, are currently under investigation. US Food and Drug Administration (FDA) approved pertuzumab, which can be used in combination with trastuzumab for metastatic HER2+ breast cancer treatment in 2012.18,19 Whether trastuzumab combined with pertuzumab will be used as first-line treatment for early HER2+ breast cancer depends on outcomes of more large-scale multicenter clinical research studies. Further clinical trials must be conducted before these compounds can be used in the clinical setting. In the future, additional novel therapies for HER2+ patients will be developed.
Our findings may influence clinical practice, as they contribute to current knowledge regarding the characteristics of Chinese patients with HER2+ breast cancer. Trastuzumab has been demonstrated to improve the survival of HER2+ patients in many studies, in accordance with our results. Our results may enhance the confidence of Chinese physicians with regard to the treatment of HER2+ patients with trastuzumab. These patients may easily achieve pCR following combined treatment with chemotherapy and trastuzumab. Anti-HER2 therapy consisting of trastuzumab and chemotherapy is the standard treatment of HER2+ breast cancer. The HER2+ patients in this study were recommended to receive 1 year of treatment. However, many patients could not afford trastuzumab because of its high cost and because it is on the list of medications whose costs are not covered in most provinces and cities in the People’s Republic of China. A total of 258 patients received trastuzumab, and none experienced congestive heart failure or other serious adverse events during treatment.
Our study contained several limitations. First, all the studied patients were HER2+ women, and patients with HER2-negative breast cancer were not included as a control group because this patient population is large. Second, we performed retrospective database analysis rather than prospective cohort analysis; this approach may have led to unaccounted biases. Third, several cancer centers have published results of studies of HER2+ patients with similar conclusion. Fourth, the follow-up duration was not adequate (the median follow-up duration was 41 months), and the results that may be influenced by a longer follow-up period require further validation. Fifth, other anti-HER2 therapies, such as lapatinib (used in the ALLTO trial11), were not analyzed.
Conclusion
Significant clinical features were observed in the HER2+ patients, and significant differences were detected between the patients with HER2+ breast cancer who received both trastuzumab and chemotherapy and those who received chemotherapy alone. The patients who received NCT combined with trastuzumab exhibited a higher pCR rate than those who received other regimens. Furthermore, the prognosis of patients with HER2+ breast cancer has improved with the advent of targeted anti-HER2 therapy.
Supplementary material
OS of the patients according to treatment with trastuzumab.
Note: No statistical significance was identified between patients who received both trastuzumab and chemotherapy (trastuzumab+ group) and patients who received chemotherapy alone (trastuzumab− group) (P=0.325) (95% CI: 0.252–1.633; hazard ratio: 0.642).
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
Acknowledgments
This work was supported by grants from the Research Project of the Fudan University Shanghai Cancer Center (YJ201401), the National Natural Science Foundation of China (81372848, 81370075, and 81260389), the Municipal Project for Developing Emerging and Frontier Technology in Shanghai Hospitals (SHDC12010116), the Cooperation Project of Conquering Major Diseases of the Shanghai Municipality Health System (2013ZYJB0302), the Innovation Team of the Ministry of Education (IRT1223), the Shanghai Key Laboratory of Breast Cancer (12DZ2260100), and the Guangdong Province Science and Technology Key Project (2012A030400066).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology. College of American Pathologists American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Srirajaskanthan R, Shah T, Watkins J, Marelli L, Khan K, Caplin ME. Expression of the HER-1-4 family of receptor tyrosine kinases in neuroendocrine tumours. Oncol Rep. 2010;23(4):909–915. doi: 10.3892/or_00000714. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. Herceptin Adjuvant (HERA) Trial Study Team 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 9.Pivot X, Romieu G, Debled M, et al. PHARE trial investigators 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 10.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson L. Breast cancer: ALTTO: wake-up call for setting up clinical trials. Nat Rev Clin Oncol. 2013;10(3):121. doi: 10.1038/nrclinonc.2013.20. [DOI] [PubMed] [Google Scholar]
- 12.Sohaib A. RECIST rules. Cancer Imaging. 2012;12:345–346. doi: 10.1102/1470-7330.2012.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 14.Goldhirsch A, Winer EP, Coates AS, et al. Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 17.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 18.Swain SM, Baselga J, Kim SB, et al. CLEOPATRA Study Group Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(5):442–447. doi: 10.1200/JCO.2014.57.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OS of the patients according to treatment with trastuzumab.
Note: No statistical significance was identified between patients who received both trastuzumab and chemotherapy (trastuzumab+ group) and patients who received chemotherapy alone (trastuzumab− group) (P=0.325) (95% CI: 0.252–1.633; hazard ratio: 0.642).
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.


