Abstract
Background
Clinical outcomes are worse in patients with COPD and chronic bronchitis. N-acetylcysteine (NAC) is commonly prescribed for such patients but with uncertain clinical benefits. We postulated that oral NAC, at much larger doses than those ordinarily prescribed, would improve clinical outcomes in a subset of patients with COPD and chronic bronchitis.
Objective
The aim of this study was to determine whether very high-dose NAC would improve respiratory health status in patients with COPD and chronic bronchitis.
Methods
Patients with COPD and chronic bronchitis were enrolled in a randomized, controlled, double-blinded trial. Patients received oral NAC (1,800 mg) or matching placebo twice daily for 8 weeks in addition to their usual respiratory medications. The primary outcome, respiratory health status, was assessed by changes in the St George’s Respiratory Questionnaire. The effects of NAC on lung function and circulating markers of oxidative stress and inflammation were also evaluated.
Results
We terminated the study prematurely because new external information suggested the possibility of a safety issue. Of the planned 130 patients, 51 were randomized and 45 (22 in the placebo arm and 23 in the NAC arm) completed the study. There was no statistically significant difference between changes in the St George’s Respiratory Questionnaire total score, comparing NAC to placebo (adjusted mean difference, 0.1 U; 95% CI, −7.8 to 8.18 U; P=0.97). There were also no significant NAC-related improvements in any of the secondary outcomes.
Conclusion
In this 8-week trial, we were unable to show any clinical benefit from a very high dose of NAC in patients with COPD and chronic bronchitis.
Keywords: COPD, chronic bronchitis, N-acetylcysteine
Introduction
Chronic obstructive pulmonary disease (COPD) remains a major public health problem. Advanced COPD severely restricts work opportunities and impairs the ability to perform household chores, social activities, and family activities.1 Inhaled long-acting bronchodilators and inhaled corticosteroids are widely prescribed for patients with COPD. These drugs reduce exacerbation risk but have only a modest effect on symptoms and overall respiratory health status. In the majority of patients, these classes of drugs fail to confer improvements in the St George’s Respiratory Questionnaire (SGRQ) that are considered clinically significant.2,3
Chronic bronchitis, traditionally defined as chronic cough and phlegm for at least 3 months a year for at least two consecutive years, is common among patients with COPD, especially in current smokers.4,5 When adjustments are made for age, sex, and severity of airflow obstruction, patients with chronic bronchitis experience more exacerbations, have worse respiratory health status, and have higher mortality than do patients without chronic bronchitis, highlighting the need for therapies that might improve symptoms and other clinical outcomes in this subset of patients with COPD.5
N-acetylcysteine (NAC) possesses well-described antioxidant, anti-inflammatory, and mucolytic properties, making it attractive as a potential COPD therapy.6 Oral NAC is thought to enhance the synthesis of glutathione (GSH), a tripeptide with critical systemic antioxidant and anti-inflammatory effects. Increased levels of GSH in the lung might improve clinical outcomes by suppressing oxidant-induced inflammation and mucus production and by reducing the viscosity of mucus via the cleavage of mucin disulfide bonds.7
Oral NAC is widely prescribed in many countries and has been evaluated in numerous placebo-controlled, randomized clinical trials. Oral NAC, usually given in split doses of 300–1,200 mg daily, may have a modest effect in preventing COPD exacerbations and may also improve symptoms of cough and sputum, though results have not been entirely consistent.8–11 NAC has a good safety profile and has been administered in oral doses of 6,000–8,000 mg daily for several months to HIV-infected patients without evident ill effects.12 We hypothesized that NAC doses substantially higher than those usually used in patients with COPD might improve the clinical outcomes while remaining both tolerable and safe. We also reasoned that patients with both COPD and chronic bronchitis might be most likely to benefit.
Methods
Study population and trial sites
We conducted a randomized, double-blinded, placebo- controlled trial comparing high-dose NAC, 1,800 mg twice daily, versus placebo for 8 weeks in COPD patients with chronic cough and sputum production. Principal eligibility criteria were: 1) ratio of postbronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity of <0.70 along with an FEV1 <65% of predicted, 2) age >40 years and ≤85 years, 3) current or past history of cigarette smoking of at least ten pack-years, 4) no COPD exacerbation in the last 4 weeks, and 5) presence of chronic bronchitis. We defined chronic bronchitis as a positive response to questions 1 and 2 of the SGRQ, meaning that cough was present “several days per week” or “almost every day” and sputum production was present “several days per week” or “almost every day.”13 This definition correlates well with the more traditional definition of chronic bronchitis but identifies more patients with this phenotype.14 Principal exclusion criteria were: 1) primary clinical diagnosis of asthma, 2) uncompensated heart failure, 3) cirrhosis with ascites and edema, 4) estimated glomerular filtration rate of <30 mL/min/1.73 m2, 5) use of long-acting nitrates, and 6) inability to provide informed consent. Patients with a propensity to develop edema from heart failure or liver disease were excluded because each tablet of the study drug contained 200 mg of sodium for a daily total of 800 mg. Patients taking long-acting nitrates were excluded because NAC may potentiate drugs of this class.15 The trial was conducted with a common protocol at three sites in the Minneapolis–St Paul metropolitan area: the Minneapolis VA Health Care System, the HealthPartners Research Foundation, and the University of Minnesota Medical Center. The Institutional Review Boards at each participating site approved the study (Minneapolis VA Health Care System, the HealthPartners Research Foundation, and the University of Minnesota Medical Center), and all patients gave written informed consent. An independent Data Monitoring Committee was established to review the study progress and safety. The trial was registered with ClinicalTrials.gov (NCT01599884).
Randomization
At each site, patients were randomized 1:1 to active drug or placebo in permuted blocks of size two. Research pharmacists at each site were the only study personnel with access to the randomization list, and they assigned the treatment accordingly. All other study personnel and study patients were fully blinded to the allocation arm.
Intervention
The NAC was synthesized and formulated according to Good Manufacturing Practice standards by BioAdvantex Pharma, Inc. (Mississauga, ON, Canada), which donated the study drug and matching placebo. Effervescent tablets containing 900 mg of NAC were packaged in airtight packets made of aluminum foil to prevent oxidation. Placebo tablets were indistinguishable from active drug in terms of appearance, effervescence, taste, and odor. Patients were instructed to take two tablets dissolved in water or fruit juice twice daily. The duration of study drug administration was 8 weeks. Patients were allowed to continue all of their usual medications, except for guaifenesin and other over-the-counter antitussives.
Study outcomes
The primary outcome was change in the total score of the SGRQ, a well-validated measure of health status in patients with chronic lung disease.13 The SGRQ is scored on a scale of 0–100 with 100 representing the worst respiratory health status. This instrument was self-administered at baseline and again at the end of the 8-week study. As secondary clinical outcomes, we evaluated changes in the three domains of the SGRQ, the Chronic Bronchitis Symptoms Assessment Scale (CBSAS), and the Short Form-36 Health Survey (SF-36).16,17 We assessed lung function with postbronchodilator spirometry, systemic inflammation with serum C-reactive protein levels, and systemic oxidative stress with plasma 8-isoprostane levels.18 We further assessed oxidative stress and biochemical efficacy of NAC, by measuring changes in thiol redox couples, GSH/GSH disulfide (GSSG) and cysteine/cystine (Cys/CySS) in plasma. Trained laboratory technicians separated and processed plasma from collected blood, closely following the methodology described by Jones and Liang.19 Samples were immediately frozen and then shipped to the Clinical Pediatric Center Biomarkers Core Laboratory at Emory University for the measurement of the thiol redox couples. Plasma samples were also processed for the measurement of 8-isoprostane, as described previously.20 Measurements of plasma thiol redox couples and 8-isoprostane were performed only in the subset of patients who were randomized at the Minneapolis VA Health Care System.
Monitoring adverse events
Patients were questioned during a mid-study telephone call at week 4 and at the final study visit at week 8 regarding all adverse events, including possible drug reactions and health care utilization. Reports of serious adverse events were promptly filed with the Institutional Review Board per institutional policies. As an added safety measure, common laboratory tests, including a complete blood count, electrolytes, creatinine, alanine aminotransferase, and prothrombin time, were obtained at baseline and at the 8-week visit.
Sample size and data analysis
The trial was designed to detect a between-group difference of 4 U in the mean change of the total score for the SGRQ from baseline to 8 weeks. The 4-U change is widely accepted as the minimal clinically important difference for the SGRQ.21 For our sample size calculation, we assumed a SD of 7.5 U for the change in SGRQ measurements, based on a prior trial of similar patients with COPD and similar duration conducted at the Minneapolis VA Health Care System.22 Based on these assumptions, 55 patients in each arm would provide 80% power for detecting a four-point difference between groups with a two-tailed alpha error of 0.05. To account for the potential dropout of patients, we had planned for a total sample size of 130 patients, 65 patients in each arm.
Baseline comparisons of treatment groups applied two sided Student’s t-test to continuous measures and chi-square tests to categorical measures, while Wilcoxon signed-rank sum tests were used for highly skewed continuous measures and Fisher’s exact test was used for categorical measures with low expected cell counts. The primary analysis of the changes in the total SGRQ score from baseline to the 8-week visit compared the two treatment arms, including only those patients who had completed the study, using an initial t-test and an additional planned analysis regressing the change in SGRQ total score on the study arm, baseline total score, and age, sex, and % predicted FEV1. The secondary outcomes were analyzed in a similar manner. Changes in outcomes were compared using a simple t-test and an analysis regressing the change in outcome on the intervention arm, respective baseline measure, age, sex, and baseline percentage predicted FEV1. Residual diagnostics for the SGRQ, CBSAS, SF-36, and lung spirometric outcomes indicated no problems with the analyses mentioned earlier, and diagnostic results for the measures of systemic oxidative stress and inflammation indicated some lack of normality and skewness. Additional analyses of the logarithmic transformation of these measures yielded better diagnostic measures of fit for some of the outcomes, but the results were comparable to those from the initial analyses.
Study termination
Due to a potential safety issue, we terminated the trial after 51 patients had been randomized. This decision was largely based on the study of Sayin et al23 that was published after we had started the study. They showed that vitamin E and NAC given orally stimulated tumor growth and proliferation in mouse models of B-RAF- and K-RAS-induced lung cancer by reducing oxidative stress and DNA cell damage and by suppressing activation of p53, a key tumor suppressor protein. They were able to replicate these findings in cell lines from both human and mouse lung tumors, suggesting that these findings may have relevance to human lung cancer. We terminated the trial because of concerns about administering very large doses of NAC to patients with severe COPD who were at a very high risk for developing lung cancer. The study team was unblinded to the efficacy outcomes only after the decision had been made to terminate the trial.
Results
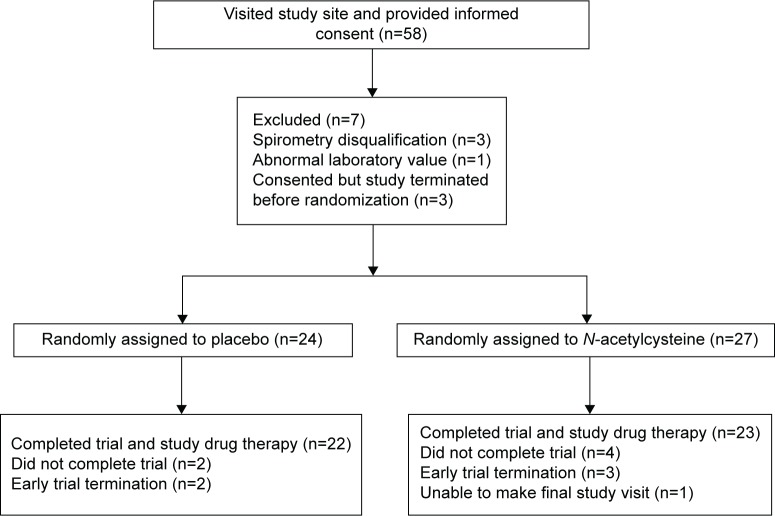
The first patient was randomized in September 2012, and the last randomization occurred in February 2014. Of the 58 patients who provided informed consent, 51 were randomized, and 45 completed the trial: 22 being assigned to placebo and 23 to NAC (Figure 1). Failure to complete the study was due to the termination of the trial prior to the scheduled final visit in five patients, while one patient was physically unable to make the final visit. All patients were Caucasian with both treatment arms having an average age of ~70 years, an average FEV1 of ~40% of predicted, and a high frequency of prior exacerbations and cardiovascular events (Table 1). As might be anticipated in a trial with a relatively small sample size, there were imbalances. Patients in the placebo arm were more likely to be current smokers but less likely to be using home oxygen. Baseline scores for the symptom domain of the SGRQ and of the CBSAS suggest that the symptoms of chronic bronchitis were somewhat more severe among patients in the placebo arm. Based upon the counts of returned study drug, the overall compliance was estimated at 94% in the placebo arm and 93% in the NAC arm.
Figure 1.
Flow chart of screening, randomization, and follow-up.
Table 1.
Baseline characteristics
| Placebo (N=22) | N-acetylcysteine (N=23) | P-value | |
|---|---|---|---|
| Age, years (mean ± SD) | 68.9±8.2 | 70.1±7.7 | 0.62 |
| Sex, M/F | 19/3 | 18/5 | 0.38a |
| Caucasian race, n (%) | 22 (100) | 23 (100) | 1.00 |
| Smoking history | |||
| Current smoker, n (%) | 11 (50) | 3 (13) | 0.006 |
| Pack-years (mean ± SD) | 60±40 | 74±38 | 0.11a |
| Respiratory medications at baseline | |||
| LAMA, n (%) | 15 (68) | 18 (78) | 0.44 |
| LABA, n (%) | 17 (77) | 21 (91) | 0.19 |
| ICS, n (%) | 16 (73) | 19 (83) | 0.42 |
| Home oxygen, n (%) | 6 (27) | 14 (61) | 0.02 |
| FEV1 (L), mean ± SD | 1.15±0.50 | 1.11±0.44 | 0.78 |
| % predicted FEV1, mean ± SD | 41±12 | 40±13 | 0.70 |
| FVC (L), mean ± SD | 2.73±0.88 | 2.65±0.70 | 0.73 |
| FEV1/FVC, mean ± SD | 0.42±0.11 | 0.43±0.14 | 0.73 |
| ≥1 exacerbation past year, n (%) | 16 (73) | 18 (78) | 0.67 |
| ≥1 COPD hospital admission past year, n (%) | 7 (32) | 5 (22) | 0.44 |
| History of cardiovascular disease, n (%) | 14 (64) | 18 (78) | 0.28 |
| SGRQ | |||
| Symptoms, mean ± SD | 69±18 | 54±17 | 0.004a |
| Activity, mean ± SD | 70±22 | 71±21 | 0.95a |
| Impacts, mean ± SD | 42±22 | 37±20 | 0.39 |
| Total, mean ± SD | 56±20 | 49±17 | 0.18a |
| CBSAS, mean ± SD | 22.5±8.3 | 17.6±7.5 | 0.04 |
| SF-36 | |||
| Physical, mean ± SD | 36±7 | 38±7 | 0.05a |
| Mental, mean ± SD | 50±11 | 50±11 | 0.93a |
Notes:
Fisher’s exact test was used for categorical measure or Wilcoxon signed-rank sum test was used for continuous measure; if skewness measure for distribution in either group was >0.70, Wilcoxon test was used, and if 50% of expected cell counts were <5, then Fisher’s exact test was used.
Abbreviations: CBSAS, Chronic Bronchitis Symptoms Assessment Scale; F, female; FEV1, forced expiratory volume in one 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting beta adrenergic; LAMA, long-acting antimuscarinic; M, male; SGRQ, St George’s Respiratory Questionnaire; SF-36, Short Form-36 Health Survey; SD, standard deviation.
In the primary outcome, the total SGRQ score improved from baseline to the 8-week visit in both treatment arms with differences that were not statistically significant (adjusted mean difference, 0.1 U; 95% CI, −7.8 to 8.18 U; P=0.97; Table 2). Similarly, there were no statistically significant treatment-related differences in any of the individual SGRQ domains, in the CBSAS, or in either the physical or the mental component of the SF-36. Compared to placebo, NAC had no statistically significant effect on any of the spirometric parameters, except for a marginal improvement in the unadjusted FEV1 in patients who received NAC (Table 3). There was no statistically significant effect on plasma concentrations of any component of the redox pairs, GSH/GSSG and Cys/CySS (Table 4). NAC also had no statistically significant effect on reducing the levels of serum C-reactive protein or plasma 8-isoprostane, compared to placebo (Table 4).
Table 2.
Changes in survey scores over 8 weeks, according to treatment assignment
| Baseline (mean ± SD) | Study end(mean ± SD) | Change (mean ± SD) |
N-acetylcysteine–placebo
|
||||
|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) | P-value | Adjusted mean difference (95% CI) | P-value | ||||
| SGRQ | |||||||
| Symptoms | |||||||
| Placebo (n=22) | 69.3±17.7 | 59.5±21.2 | −9.8±17.8 | 6.3 (−4.6, 17.3) | 0.25 | 1.6 (−10.4, 13.5) | 0.79 |
| N-acetylcysteine (n=23) | 54.2±17.4 | 50.7±20.3 | −3.5±18.6 | ||||
| Activity | |||||||
| Placebo (n=22) | 69.8±21.9 | 66.1±21.5 | −3.7±14.5 | −0.3 (−8.7, 8.2) | 0.95 | −1.1 (−8.5, 6.3) | 0.76 |
| N-acetylcysteine (n=23) | 70.4±20.9 | 66.4±16.9 | −4.0±13.6 | ||||
| Impact | |||||||
| Placebo (n=22) | 42.3±22.5 | 34.8±22.8 | −7.5±20.1 | 3.0 (−7.6, 13.6) | 0.57 | −0.4 (−10.0, 9.2) | 0.93 |
| N-acetylcysteine (n=23) | 36.9±19.9 | 32.4±17.9 | −4.5±14.9 | ||||
| Total | |||||||
| Placebo (n=22) | 55.5±20.0 | 48.4±20.7 | −7.1±16.1 | 3.2 (−5.3, 11.8) | 0.45 | 0.1 (−7.8, 8.1) | 0.97 |
| N-acetylcysteine (n=23) | 49.6±16.9 | 45.8±14.9 | −3.9±12.1 | ||||
| CBSAS | |||||||
| Placebo (n=22) | 22.5±8.3 | 19.6±8.0 | −2.8±5.2 | 0.6 (−2.4, 3.6) | 0.69 | −0.8 (−3.8, 2.2) | 0.60 |
| N-acetylcysteine (n=23) | 17.6±7.5 | 15.4±7.2 | −2.2±4.8 | ||||
| SF-36 | |||||||
| Physical | |||||||
| Placebo (n=22) | 35.6±7.5 | 36.2±9.2 | 0.6±7.6 | −1.9 (−6.0, 2.1) | 0.34 | −0.6 (−4.4, 3.2) | 0.75 |
| N-acetylcysteine (n=23) | 38.4±6.5 | 37.1±6.3 | −1.3±5.8 | ||||
| Mental | |||||||
| Placebo (n=22) | 49.5±10.8 | 47.9±13.5 | −1.6±12.3 | 2.7 (−3.5, 8.8) | 0.38 | 3.2 (−2.8, 9.1) | 0.29 |
| N-acetylcysteine (n=23) | 50.2±10.9 | 51.2±12.6 | 1.0±7.6 | ||||
Notes: Results presented as mean ± SD. Adjusted mean differences in change scores estimated in the regression model for change score using intervention, baseline outcome measure, age, sex, and percentage predicted FEV1 as explanatory variables.
Abbreviations: CBSAS, Chronic Bronchitis Symptoms Assessment Scale; FEV1, forced expiratory volume in 1 second; SF-36, Short Form-36 Health Survey; SGRQ, St George’s Respiratory Questionnaire; SD, standard deviation; CI, confidence interval.
Table 3.
Changes in lung function over 8 weeks, according to treatment assignment
| Baseline (mean ± SD) | Study end (mean ± SD) | Change (mean ± SD) | N-acetylcysteine–placebo
|
||||
|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) | P-value | Adjusted mean difference (95% CI) | P-value | ||||
| FEV1 (L) | |||||||
| Placebo (n=21) | 1.18±0.49 | 1.12±0.47 | −0.06±0.11 | 0.06 (0.00, 0.13) | 0.04 | 0.06 (−0.01, 0.12) | 0.10 |
| N-acetylcysteine (n=23) | 1.14±0.42 | 1.14±0.42 | 0.00±0.10 | ||||
| % predicted FEV1 (%) | |||||||
| Placebo (n=21) | 41.1±12.1 | 38.9±12.4 | −2.3±5.6 | 2.1 (−0.9, 5.0) | 0.16 | 1.8 (−1.1, 4.8) | 0.22 |
| N-acetylcysteine (n=23) | 39.1±13.2 | 38.9±13.1 | −0.2±4.0 | ||||
| FVC (L) | |||||||
| Placebo (n=21) | 2.82±0.85 | 2.77±0.84 | −0.05±0.27 | 0.09 (−0.09, 0.27) | 0.31 | 0.08 (−0.08, 0.26) | 0.30 |
| N-acetylcysteine (n=23) | 2.71±0.61 | 2.76±0.57 | 0.04±0.30 | ||||
| FEV1/FVC | |||||||
| Placebo (n=21) | 0.42±0.11 | 0.40±0.10 | −0.02±0.05 | 0.02 (−0.01, 0.05) | 0.14 | 0.02 (−0.003, 0.045) | 0.10 |
| N-acetylcysteine (n=23) | 0.42±0.11 | 0.41±0.10 | 0.00±0.04 | ||||
Notes: Results presented as mean ± SD. Adjusted mean differences in change scores estimated in the regression model for change score using intervention, baseline outcome measure, age, sex, and percentage predicted FEV1 as explanatory variables.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation; CI, confidence interval.
Table 4.
Changes in oxidation and inflammation biomarkers over 8 weeks, according to treatment assignment
| Baseline (mean ± SD) | Study end (mean ± SD) | Change (mean ± SD) |
N-acetylcysteine–placebo
|
||||
|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) | P-value | Adjusted mean difference (95% CI) | P-value | ||||
| GSH (µM) | |||||||
| Placebo (n=15) | 0.88±0.55 | 1.11±0.71 | 0.23±1.00 | −0.10 (−0.99, 0.78) | 0.82 | 0.02 (−0.87, 0.91) | 0.97 |
| N-acetylcysteine (n=14) | 1.13±0.57 | 1.26±1.35 | 0.13±1.32 | ||||
| GSSG (µM) | |||||||
| Placebo (n=15) | 0.08±0.06 | 0.09±0.07 | 0.01±0.08 | 0.02 (−0.07, 0.11) | 0.66 | 0.03 (−0.07, 0.12) | 0.55 |
| N-acetylcysteine (n=14) | 0.10±0.10 | 0.13±0.16 | 0.03±0.16 | ||||
| GSSG/GSH + GSSG (%) | |||||||
| Placebo (n=15) | 8.43±5.64 | 7.52±3.41 | −0.91±5.04 | 3.21 (−1.02, 7.44) | 0.13 | 2.24 (−0.54, 5.40) | 0.10 |
| N-acetylcysteine (n=14) | 7.31±5.58 | 9.61±4.36 | 2.30±6.05 | ||||
| GSH/GSSG redox state (mV) | |||||||
| Placebo (n=15) | −117±16 | −120±18 | −3±19 | 11 (−4, 27) | 0.15 | 8 (−6, 22) | 0.24 |
| N-acetylcysteine (n=14) | −121±12 | −113±15 | 8±22 | ||||
| Cys (µM) | |||||||
| Placebo (n=15) | 1.28±1.50 | 1.23±2.36 | −0.05±1.92 | −0.05 (−1.22, 1.12) | 0.93 | −0.25 (−1.52, 1.02) | 0.69 |
| N-acetylcysteine (n=14) | 0.87±1.03 | 0.77±1.09 | −0.10±0.97 | ||||
| CySS (µM) | |||||||
| Placebo (n=15) | 13.12±8.44 | 15.67±8.95 | 2.55±11.96 | 2.55 (−13.21, 8.11) | 0.63 | −1.44 (−9.74, 6.85) | 0.72 |
| N-acetylcysteine (n=14) | 15.52±10.24 | 15.52±11.59 | 0.00±15.88 | ||||
| CySS/Cys + CySS (%) | |||||||
| Placebo (n=15) | 88.8±13.4 | 94.0±7.9 | 5.2±8.0 | −2.5 (−8.4, 3.4) | 0.39 | −0.1 (−4.1, 3.9) | 0.96 |
| N-acetylcysteine (n=14) | 92.8±9.8 | 95.5±7.1 | 2.7±7.4 | ||||
| Cys/CySS redox state (mV) | |||||||
| Placebo (n=15) | −18.6±39.4 | −3.7±42.5 | 14.9±30.3 | 0.3 (−21.8, 22.4) | 0.98 | 1.6 (−20.4, 23.7) | 0.88 |
| N-acetylcysteine (n=14) | −15.7±35.3 | −0.5±39.9 | 15.2±27.5 | ||||
| 8-Isoprostane (pg/mL) | |||||||
| Placebo (n=13) | 488±413 | 656±807 | 168±919 | 246 (−383, 874) | 0.43 | 144 (−406, 695) | 0.59 |
| N-acetylcysteine (n=14) | 369±305 | 782±543 | 413±654 | ||||
| C-reactive protein (mg/dL) | |||||||
| Placebo (n=20) | 4.8±6.3 | 5.9±10.7 | 1.1±7.2 | −0.3 (−4.7, 4.1) | 0.89 | −0.3 (−4.9, 4.4) | 0.91 |
| N-acetylcysteine (n=21) | 5.8±5.2 | 6.5±7.5 | 0.8±7.1 | ||||
Notes: Results presented as mean ± SD. C-reactive protein in serum; all other measures in plasma. Adjusted mean differences in change scores estimated in the regression model for change score using intervention, baseline outcome measure, age, sex, and percentage predicted FEV1 as explanatory variables.
Abbreviations: Cys, cysteine; CySS, cystine; FEV1, forced expiratory volume in 1 second; GSH, glutathione; GSSG, glutathione disulfide; SD, standard deviation; CI, confidence interval.
After terminating the study, we assessed the conditional probability of having found statistically significant results had the study continued, using the stochastic curtailment method of of Lan and Wittes, as discussed by Lachin.24,25 We calculated the conditional probability of rejecting the null hypothesis for the primary outcome conditional on the data collected from the 45 patients who completed the SGRQ at the beginning and at the end of the 8-week trial. Under the original study design assumptions, the conditional probability that data acquired from the planned completion of 110 patients would have detected the differences in the SGRQ total score, given the findings to date, was 0.096. Using the observed data to estimate the drift parameter central to this calculation, this conditional probability of detecting the differences in the SGRQ total score was <10−6. Had a mid-study futility analysis been incorporated into the protocol, this result would in itself have terminated the study.
High-dose NAC was generally well tolerated, and most adverse events were thought to be unrelated to the study drug. Of the 22 patients in placebo arm, nine reported at least one adverse event with a total of 19 events, three of which were considered serious (Figure 2). Of the 23 patients in NAC arm, 13 reported at least one adverse event with a total of 24 events, three of which were considered serious. Five patients in the NAC arm reported nausea or diarrhea versus only one patient in the placebo arm. These symptoms were mild, and the study drug did not need to be terminated for any of these events. There were six COPD exacerbations reported in the placebo arm with three requiring hospitalization and seven in the NAC arm with two requiring hospitalization. There were no statistically significant treatment-related differences in blood counts, electrolytes, creatinine, alanine aminotransferase, and prothrombin time, when the baseline values were compared with those at the 8-week visit (data not shown). No deaths occurred in either treatment arm.
Figure 2.
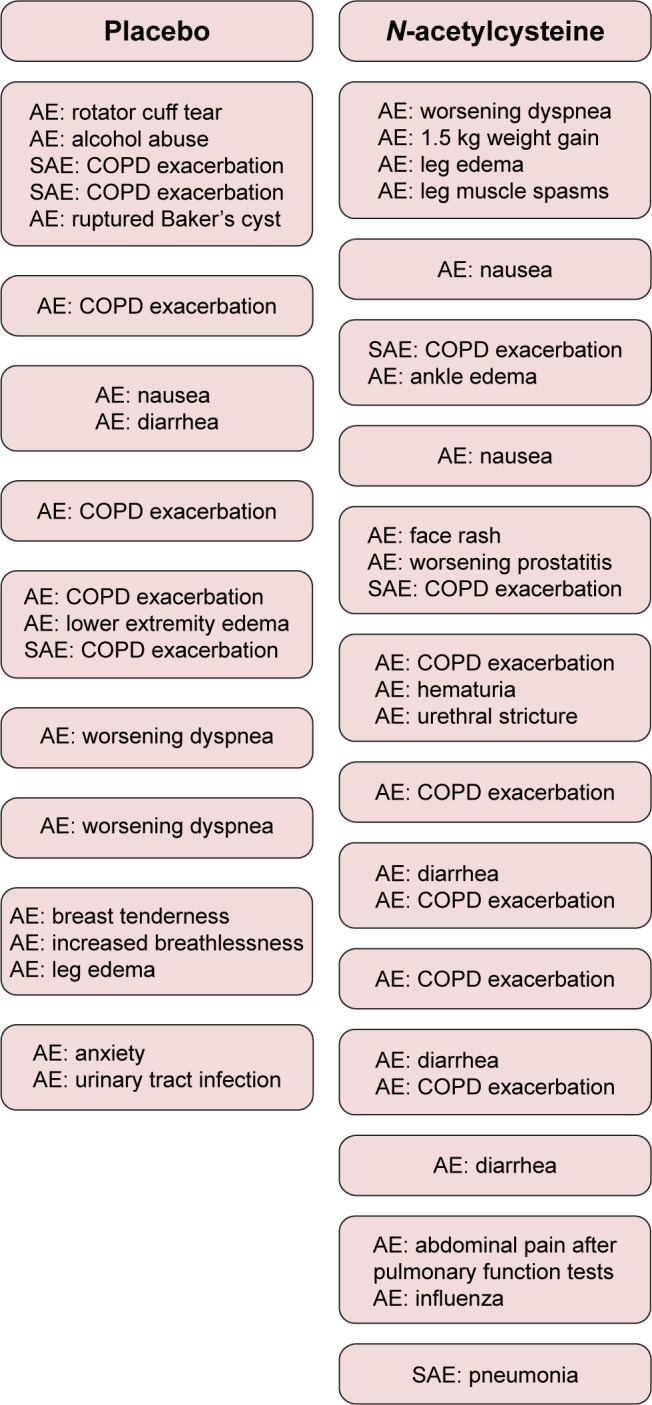
Reported AEs and SAEs, according to treatment assignment.
Note: Each box represents an individual patient.
Abbreviations: AE, adverse event; SAE, serious adverse event.
Discussion
In this randomized controlled trial, we assessed the effects of high-dose oral NAC, 1,800 mg twice daily, for 8 weeks in patients with COPD and chronic bronchitis. This dose of NAC is threefold larger than that tested in any other randomized, placebo-controlled COPD study of which we are aware. Though this dose of NAC was generally well tolerated over an 8-week period, it did not improve the primary outcome of respiratory health status, as assessed by the SGRQ total score. Similarly, NAC did not improve scores on any of the individual domains of the SGRQ, on the CBSAS, or on either component of the SF-36. There was also no substantial improvement in spirometry and no improvement in any of the circulating biomarkers of systemic oxidative stress and inflammation.
There is uncertainty as to how best to assess clinical symptoms associated with chronic bronchitis. We chose to use changes in the SGRQ total score as the primary outcome, as it is the best-validated assessment of overall respiratory health status in patients with COPD, and the clinically meaningful difference of four units has been established. The symptom domain of the SGRQ contains two questions specific to chronic bronchitis, cough and sputum, and additional questions common to both chronic bronchitis and COPD, such as wheezing and dyspnea.13 We did not use the symptom domain as the primary outcome because the clinically meaningful difference has not been established. There was concern that any signal present in the symptom domain might be lost when combined with the activity and impact domains, but we found changes in the symptom domain score to be strongly correlated with changes in the total score (r=0.78). As a secondary outcome, we also administered the CBSAS to assess chronic bronchitis severity. The CBSAS was designed to assess chronic bronchitis in a more specific and comprehensive manner than does the SGRQ, but the instrument has not been fully validated, and the clinically meaningful difference has not been established.16 We did find a moderately strong correlation (r=0.57) between changes in the CBSAS score and changes in the SGRQ total score, providing additional evidence that the use of the SGRQ total score as our primary outcome was appropriate.
Low-molecular thiol/disulfide couples, such as GSH/GSSG and Cys/CySS, have diverse cellular redox signaling functions, some of which occur as responses to a variety of oxidative and inflammatory stresses.26 Measurements of GSH/GSSG and Cys/CySS couples in plasma provide a direct measure of systemic oxidative stress in the extracellular space and an indirect measure of the redox state within the cells.19 It has been demonstrated that plasma GSH/GSSG and Cys/CySS couples in smokers are in a more highly oxidized state when compared to nonsmokers, thus supporting the suggestion that systemic oxidative stress may play an important role in the pathogenesis of COPD and other smoking-related diseases.27,28
By administering a large dose of NAC, we anticipated that measurements of plasma thiol couples might reflect a reduction in systemic oxidative stress. However, within the limitations of our sample size, we were unable to show that NAC had any such effect for reasons that are not understood. When given orally, NAC is readily absorbed in the intestine and rapidly deacylated, mostly in the liver, to produce Cys.29 It has been previously shown that increased levels of plasma Cys can be detected for only 1–2 hours following a single oral dose.30 The availability of Cys is a rate-limiting step in the synthesis of intracellular GSH, but multiple Cys metabolic pathways exist, and it is unclear as to what net effect transient increases in plasma Cys concentrations might have on sustained intracellular and extracellular GSH levels.31 It has been reported that oral NAC, given in daily doses of 1,800 mg for 5 days, increases GSH concentrations in bronchoalveolar lavage fluid by ~50% in patients with fibrotic lung disease.32 However, in another study, the same dosing regimen did not statistically significantly raise GSH levels in plasma or bronchoalveolar lavage fluid from patients with mostly mild to moderately severe COPD.30 We also found that a larger than usual dose of oral NAC did not cause sustained increases in plasma GSH concentrations in our patients with COPD when compared to placebo. We did not attempt to assess GSH levels in bronchoalveolar lavage fluid due to the severity of our patients’ disease.
Oral NAC is frequently described as a mucolytic by virtue of its capability to cleave mucin disulfide bonds. In vitro, equimolar 50 mM concentrations of NAC, Cys, and GSH substantially reduce mucus viscosity to about the same extent.7 This effect is highly concentration dependent, and only small effects are evident with a tenfold dilution. It has been reported that GSH concentrations in the alveolar epithelial lining fluid of smokers may be in the millimolar range, but that methodology has been questioned, and the true values may be substantially lower.33,34 If oral NAC does raise GSH concentrations on alveolar and airway surfaces sufficient to reduce mucus viscosity, it is not apparent whether this imparts clinically important benefits in patients with COPD and chronic bronchitis.
Conclusion
In summary, we were able to show in this randomized, placebo-controlled trial that very large doses of NAC, 3,600 mg daily, are well tolerated over an 8-week period in patients with stable COPD and chronic bronchitis. However, we were unable to show that a high dose of NAC improved overall respiratory health status, lung function, or circulating measures of systemic oxidative stress and inflammation. These conclusions are tempered by the relatively short period of NAC administration and by the small sample size resulting from the early termination of the trial.
Acknowledgments
The Minnesota Veterans Medical Research and Education Foundation, the HealthPartners Institute of Education and Research, and the University of Minnesota Graduate School provided financial support for the study. BioAdvantex Pharma, Inc. donated the study drug and matching placebo.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Lung Association [webpage on the Internet] Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet. [Accessed March 1, 2016]. Available from: http://www.lungusa.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.html.
- 2.Calverley PMA, Anderson JA, Celli B, et al. TORCH Investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C, Peto R, Tinker C, Speizer FE. The Natural History of Chronic Bronchitis and Emphysema. Oxford, England: Oxford University Press; 1976. [Google Scholar]
- 5.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotgreave IA. NAC: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 7.Sheffner AL. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann N Y Acad Sci. 1963;106:298–310. doi: 10.1111/j.1749-6632.1963.tb16647.x. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland ER, Crapo JD, Bowler RP. NAC and exacerbations of chronic obstructive pulmonary disease. J COPD. 2006;3(4):195–202. doi: 10.1080/15412550600977361. [DOI] [PubMed] [Google Scholar]
- 9.Stey C, Steurer J, Bachmann S, Medici TC, Tramèr MR. The effect of oral NAC in chronic bronchitis: a quantitative systematic review. Eur Respir J. 2000;16(2):253–262. doi: 10.1034/j.1399-3003.2000.16b12.x. [DOI] [PubMed] [Google Scholar]
- 10.Decramer M, Rutten-van Mölken M, Dekhuijzen PNR, et al. Effects of NAC on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365(9470):1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J-P, Wen F-Q, Bai C-X, et al. PANTHEON Study Group Twice daily NAC 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2(3):187–194. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 12.De Rosa SC, Zaretsky MD, Dubs JD, et al. NAC replenishes glutathione in HIV infection. Eur J Clin Invest. 2000;30(10):915–929. doi: 10.1046/j.1365-2362.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones PW, Quirk FH, Baveystock CM. The St. George’s respiratory questionnaire. Respir Med. 1991;85(suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim V, Crapo J, Zha H, et al. COPDGene Investigators Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boesgaard S, Aldershvile J, Poulsen HE. Preventive administration of intravenous NAC and development of tolerance to isosorbide dinitrate in patients with angina pectoris. Circulation. 1992;85(1):143–149. doi: 10.1161/01.cir.85.1.143. [DOI] [PubMed] [Google Scholar]
- 16.Au DH, Blough DK, Kirchderfer L, Weiss KB, Udris EM, Sullivan SD. Development of a quantifiable symptom assessment tool for patients with chronic bronchitis: the Chronic Bronchitis Symptoms Assessment Scale. J COPD. 2005;2(2):209–216. [PubMed] [Google Scholar]
- 17.Tarlov AR, Ware JE, Jr, Greenfield S, Nelson EC, Perrin E, Zubkoff M. The medical outcomes study: an application of methods for monitoring the results of medical care. JAMA. 1989;262(7):925–930. doi: 10.1001/jama.262.7.925. [DOI] [PubMed] [Google Scholar]
- 18.Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23(8):360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 19.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47(10):1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahola T, Fellman V, Kjellmer I, Raivio KO, Lapatto R. Plasma 8-isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatr Res. 2004;56(1):88–93. doi: 10.1203/01.PDR.0000130478.05324.9D. [DOI] [PubMed] [Google Scholar]
- 21.Jones PW. St. George’s respiratory questionnaire: MCID. J COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 22.Bjerk SM, Edgington BD, Rector TS, Kunisaki KM. Supplemental vitamin D and physical performance in COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2013;8:97–104. doi: 10.2147/COPD.S40885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221r. doi: 10.1126/scitranslmed.3007653. a15. [DOI] [PubMed] [Google Scholar]
- 24.Lan KKG, Wittes J. The B-value: a tool for data monitoring. Biometrics. 1988;44(2):579–585. [PubMed] [Google Scholar]
- 25.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med. 2005;24(18):2747–2764. doi: 10.1002/sim.2151. [DOI] [PubMed] [Google Scholar]
- 26.Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:26. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriarty SE, Shah JH, Lynn M, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35(12):158215–158288. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 29.Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. NAC – a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7(4):355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bridgeman MME, Marsden M, Selby C, Morrison D, MacNee W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax. 1994;49(7):670–675. doi: 10.1136/thx.49.7.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stipanuk MH, Dominy JE, Jr, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr. 2006;136(6 suppl):1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 32.Meyer A, Buhl R, Magnussen H. The effect of oral NAC on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J. 1994;7(3):431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 33.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 34.Ward C, Thien F, Secombe J, Gollant S, Walters EH. Bronchoalveolar lavage fluid urea as a measure of pulmonary permeability in healthy smokers. Eur Respir J. 2000;15(2):285–290. doi: 10.1034/j.1399-3003.2000.15b11.x. [DOI] [PubMed] [Google Scholar]