Abstract
Catheter-related bloodstream infections are a significant source of morbidity and mortality in the end-stage renal disease population. Although alternative accesses to undergoing renal replacement therapy exist, many patients begin hemodialysis with a dialysis catheter due to logistic and physiologic factors involved in arteriovenous fistula creation and maturation. Colonization of catheters via skin flora leads to the production of biofilm, which acts as a reservoir for virulent bacteria. Preventative therapies center on appropriate catheter maintenance, infection control measures, and early removal of devices as patients transition to other access. Despite best efforts, when conservative measures fail to prevent infections in a high-risk population, antimicrobial lock therapy should be considered as an option to combat catheter-related bloodstream infections.
Keywords: hemodialysis, CRBSI, catheter, end-stage renal disease, ESRD
Video abstract
Introduction
Establishing dialysis access is often one of the most challenging aspects in the life of a patient requiring renal replacement therapy. A number of elements, including anatomic host factors, patient reluctance, and prolonged maturation time, have contributed to a preponderance of dialysis catheter use. National quality improvement programs, including the breakthrough fistula first initiative, have been ineffective and, in many cases, have contributed toward many patients on hemodialysis (HD) initiating renal replacement therapy with a catheter. Data from the Centers for Medicare and Medicaid Services (CMS) show a 29% overall prevalence of catheter use; however, the prevalence is as high as 69% in the first 6 months of dialysis and as high as 41% at the end of the first year.1 The prolonged maturation time inherently required in fistula development has contributed to a longer duration of dialysis catheter use.2 Infections are the second leading cause of death in patients with end-stage renal disease (ESRD).3–7 The antecedent for the majority of these infections is catheter-related bloodstream infection (CRBSI).
Epidemiology of CRBSI
Over 370,000 patients undergo maintenance HD in the United States with up to 80% initiating treatment with a central venous catheter.8 In comparison, catheters are associated with significantly more infections than arteriovenous (AV) fistulae or grafts (Table 1). A Canadian study by Taylor et al9 showed that during the first 6 months of dialysis, there is a high rate of bloodstream infection (BSI). In comparison to the AV fistula, survival analysis yielded a relative risk of BSI of 1.47 (95% confidence interval [CI], 0.36–5.96) for AV grafts, 8.49 (95% CI, 3.03–23.78) for cuffed central venous catheters, and 9.87 (95% CI, 3.46–28.20) for noncuffed central venous catheters.9 The most recent data from the National Healthcare Safety Network (NHSN) of the Centers for Disease Control and Prevention (CDC) revealed a national pooled mean of one CRBSI per 1,000 catheter days; however, incidence rates reported in the literature often range much higher, ranging from 2.5 to 6.6 per 1,000 catheter days.3–7 The CDC reports that CRBSI rates have remained steady in patients with chronic HD with an estimated 37,000 CRBSIs occurring annually in 2008. Infection rates continue to be high as of 2011; 300 hospital admissions were attributed to bacteremia or sepsis per 1,000 patient-years.10,11
Table 1.
Hospitalization and CRBSI rates by vascular access type
| Rate | Fistula | Graft | Tunneled catheter | Nontunneled catheter |
|---|---|---|---|---|
| Hospitalization (Per 100 patient-months) | 7.7 | 9.2 | 15.7 | 34.7 |
| CRBSI (Per 100 patient-months) | 0.5 | 0.9 | 4.2 | 27.1 |
Notes: Pooled mean data from the National Health and Safety Network allows for quantification of the rate of infection as it relates to access type. Adapted with permission from John Wiley and Sons from Klevens RM, Edwards JR, Andrus ML, et al. Special Report: Dialysis surveillance report: national healthcare safety network (NHSN)-data summary for 2006. Semin Dial. 2007;21(1):24–28.5 Copyright © 2007 Blackwell Publishing.5
Abbreviation: CRBSI, catheter-related bloodstream infection.
Defining CRBSIs
CRBSIs are well defined and described in the nondialysis population. It was not until 2009 that the Infectious Diseases Society of America (IDSA) recognized the unique characteristics of HD catheters. The suggested definition relies on obtaining a blood specimen from the dialysis catheter and an additional specimen from a peripheral vein.12 The existence of a similar colony count, differential, and time-to-sensitivity at both sites are the criteria for diagnosis of a CRBSI. Although this might be possible in a hospitalized patient, application of this practice to an outpatient dialysis center is impractical for dialysis centers in remote parts of the country. In most cases, the cultures are transported using mail couriers and cultured in laboratories that are distant from the dialysis unit. This scenario precludes an effective application of the IDSA definition in making the formal diagnosis of CRBSI. Also, obtaining a peripheral venous sample is often difficult in the ESRD population. The common practice in the HD population is to obtain two samples using the dialysis tubing during treatment at two different times. Although this is common practice, the evidence in support of this procedure is lacking and requires further study.
Pathogenesis of CRBSI
All indwelling vascular catheters are colonized by microorganisms within 24 hours after insertion.13 Bacteria are introduced into the lumen through the flora of the surrounding skin or the hands of health care workers during catheter hub manipulation.14 Bacteria migrate from the skin along the outside of the catheter and can seed the bloodstream. A Dacron cuff is incorporated close to the hub of a tunneled dialysis catheter for prevention of bacterial growth. The cuff induces an inflammatory response that creates a fibrotic mechanical barrier against contamination. Infections at the site where the catheter exits the skin at the termination of the tunnel are termed exit site infections. Delayed removal of sutures may also serve as a nidus for localized infection. Ascending infection from the exit site may produce purulence to occur within the tunnel, necessitating removal of the catheter.
A major source of CRBSIs arises from bacterial biofilm that is produced both externally and within catheter lumen. Bacteria may exist in multiple forms (Figure 1).15 Independently, bacteria may cause acute infections; collectively, they form sessile slime-enclosed aggregates that are resistant to extermination. The presence of a biofilm is not limited to catheter-related infections; biofilm can be seen in vivo in relation to nonhematogenous organs, such as pneumonia associated with cystic fibrosis16 as well as contaminated industrial water purification equipment.17 After adhering to artificial surfaces, microorganisms produce extracellular polysaccharides that result in the formation of a matrix that inhibits complete eradication via intravenous antibiotics.3 While the presence of a biofilm does not categorically provide resistance to antibiotics except in the case of charged antimicrobial agents, this slime facilitates persistence of remnant colonies and decreases metabolism that occurs within its milieu. Repeated inocula from biofilm explains persistent and metastatic infections that occur after administration of antibiotic treatment without removal or exchange of the catheter itself.
Figure 1.
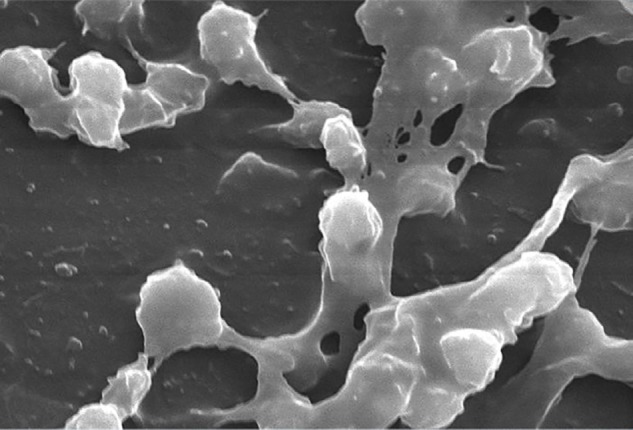
Electron microscopy of Staphylococcus aureus magnified 2,363 times found on the luminal surface of an indwelling catheter.
Notes: The interdigitating substance that connects the round cocci is composed of polysaccharides termed “biofilm”. Photo credit Janice Carr 2005. http://phil.cdc.gov/PHIL_Images/7485/7485_lores.jpg
The majority of CBRSIs are caused by gram-positive organisms, such as coagulase negative staphylococci and Staphylococcus aureus.5 NHSN data were collected from 461 CRBSIs and showed that 19.7% were due to S. aureus, while an additional 46% were due to other gram-positive organisms. Gram-negative rods constituted approximately 23.2% of infections, while 1.7% of cases were due to fungal sources. The latter are typically seen in patients with a compromised immune system as well as the critically ill, who undergo treatment with broad-spectrum antibiotic therapy that alters their normal flora.18,19
S. aureus is commonly associated with significant morbidity and mortality. A retrospective cohort study of 22,130 hospitalizations of dialysis patients with septicemia disclosed a mortality rate from S. aureus bacteremia after 12 weeks of follow-up of 34%.20 The invasive nature of this pathogen is due to its ability to adhere to the surface of endothelial cells and undergo phagocytosis. The intracellular environment protects this organism from host defenses and a variety of antimicrobial agents.21 Methicillin-resistant S. aureus (MRSA) is particularly problematic and has emerged as a bacterium of interest. A report in 2005 estimated the rate of invasive MRSA in dialysis patients to be 45 per 1,000 persons compared to 0.4 infections per 1,000 in the general population.22 Treatment of MRSA infections often calls for a prolonged course of antimicrobial therapy and removal of the catheter to provide source control and prevent widespread organ involvement.12
Sequelae of CBRSI may include infectious endocarditis, osteomyelitis, and septic arthritis as well as spinal and psoas abscesses. A recent retrospective cohort study conducted in Tokyo, Japan, showed that of 73 patients who developed bacteremia, 19.2% developed a metastatic infection. Several predictive factors associated with the development of metastatic infection were identified including a delay in appropriate antimicrobial treatment of >48 hours, persistent fever for >72 hours after starting antibiotic treatment, and low C-reactive protein levels of >3 mg/dL during 2 weeks after the onset of bacteremia.23
S. aureus colonization via nasal carriage has received attention as a source of infection in patients undergoing HD. A study conducted in Taiwan evaluated the nasal carriage of MRSA and subsequent infection among dialysis patients, health care workers, and their families within a single dialysis unit.24 The investigators found that 36% of colonized subjects developed MRSA infections with the same molecular phenotype as the colonizing strain. A recent prospective interventional cohort study conducted in Germany showed that mupirocin ointment eliminated nasal carriage in 26 of 34 patients.25 Of note, the remaining seven patients who were unable to undergo decolonization had an increase in all-cause mortality of >85%. It is debatable whether this finding indicates that resistance to prophylactic eradication is a predictor of disease as opposed to a reflection of the overall burden of comorbid diseases.
Financial impact of CRBSI
Approximately one-third of CRBSIs will require treatment in the hospital26 with significant financial burden. The average cost of each hospitalization is estimated at $22,000 USD per episode of bacteremia.27
While patients with chronic HD account for <1% of the CMS population, they account for a disproportionate percentage (6.7%) of total CMS expenditures. Rates of hospital admissions for infection have increased by >43% than in 1993, consuming an ever increasing fraction of CMS expenditures for the ESRD program.8
Transitioning from the hospital after discharge has become increasingly plagued by readmission. The 2013 USRDS annual data report cites that the dialysis population accounts for a disproportionate number of readmissions, 33.3% versus 17.4% in the general population, across all infection domains.10 Vascular access infections resulted in 31.1% of all-cause rehospitalization in the ESRD population. The high amount of antibiotic use in patients with ESRD confirms the infection rate in this population.
Significance of catheter insertion site
Recently, the location of catheter placement has been called into question. Previous recommendations from the IDSA have advocated avoidance of the femoral vein as an insertion site. Obese patients in particular were found to be at risk for infection because continued sterility of the site was difficult to maintain. The use of jugular access may have a higher risk of infection if there is a concurrent tracheostomy. A meta-analysis that included two randomized, controlled trials and eight cohort studies involving nontunneled central venous catheters was conducted in 2012.28 Overall, 3,230 catheters were placed in the subclavian vein, 10,958 in the internal jugular, and 3,188 in the femoral vein for a total of 113,652 catheter-days. The average CRBSI density was 2.5 per 1,000 catheter-days (range: 0.6–7.2) regardless of location showing no difference in the rate of CRBSIs between the three sites. Despite these findings not being specific to the dialysis population, it does call into question previously held beliefs regarding catheter location and is a topic for further prospective research.
Catheter maintenance procedures
The CDC offers a number of nonpharmacologic recommendations for catheter reduction in the dialysis population.12 Offering appropriate education to technical staff through training and auditing techniques ensures a vested interest on the part of dialysis personnel. Facilities are encouraged to conduct a monthly surveillance for BSIs using the NHSN of the CDC to calculate facility rates and actively share results with clinical staff members. Both monitoring for appropriate hand hygiene behavior and sharing the results with clinical staff members are encouraged. Conducting observations involving catheter care and assessing adherence of staff to aseptic technique when making connections and changing dressings reinforce preventative medicine techniques. Staff should be trained on infection control methods and evaluated with regular competency assessments to maintain skill sets. The introduction of the care bundle, a standardized monitoring system for quality assurance, has been shown to reduce nontunneled CRBSIs in the critical care setting.29 A similar checklist system is advocated by the CDC to allow for uniform exit site care (Figure 2).
Figure 2.
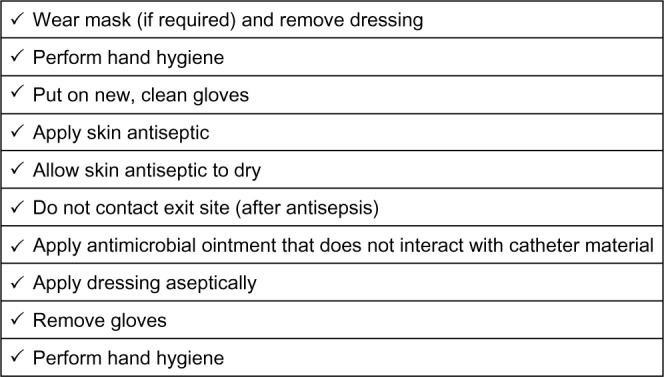
Sample checklist describing conventional catheter exit site care.
Note: Adapted from the Center for Disease Control.30 http://www.cdc.gov/dialysis/PDFs/collaborative/CL-Hemodialysis-Catheter-Exit-Site-Care-508.pdf.
Patient education regarding catheter reduction is also encouraged. Patients should be provided with standardized education regarding prevention topics including vascular access care, hand hygiene, risks related to catheter use, recognition of signs of infection, and instructions for access management when they are away from their dialysis unit.
Efforts should be made to reduce catheter prevalence by identifying barriers to permanent vascular access placement leading to catheter removal. A dedicated vascular access coordinator has been shown to facilitate this process. A retrospective study conducted by Dwyer et al31 showed that implementing a dedicated vascular access coordinator with a corresponding comprehensive access program led to an increase in the prevalent AV fistula rate from 50% to 60% and reduction in dialysis catheters by 50%.
Use of an alcohol-based chlorhexidine solution as the first-line skin antiseptic agent for central line insertion and during dressing changes is recommended to prevent the occurrence of catheter-related infections. Retrospective studies in the pediatric population have compared the use of chlorhexedine versus povidone–iodine solutions and have shown reductions in exit site, tunnel infections, and bloodstream-related infections with the use of chlorhexidine.32
The hub of the catheter is a known source of entry leading to CRBSI. Catheter hub disinfection should involve a thorough scrubbing of catheter hubs with an appropriate antiseptic. This technique should be performed every time a catheter is accessed or disconnected. Topical antibiotics have been shown to decrease bacteremia and exit site infection rates in a large meta-analysis by a ratio rate of 0.22 (CI 0.12–0.40) and 0.17 (CI 0.08–0.38), respectively.33 While concerns for the emergence of resistance to mupirocin have been raised,34 the application of antimicrobial ointment is advocated by the Society of Critical Care Medicine and the IDSA.12 The use of povidone–iodine antiseptic ointment or bacitracin/gramicidin/polymyxin B ointment at the exit site after catheter insertion and at the end of each HD session is recommended provided the ointment does not adversely interact with the catheter substrate materials.
Impact of seasonality
Intensification of prophylactic efforts may be targeted at specific times of the year when patients are at higher risk of catheter-related infection and complications. An increased adjusted hazard ratio was seen when data from the Dialysis Outcomes Practice Patterns study were analyzed with regard to seasonality demonstrating a 46% increase in catheter-related septicemia during summer months when higher heat and humidity cause an increase in perspiration and bacterial growth with biofilm production.35 Protective barriers fail to remain adhesive during these conditions contributing to increased risk. Enhanced vigilance and patient education regarding catheter care should be emphasized during this temporal period.
Dialysis access and the elderly
Lack of alternative access is especially prevalent in the elderly as AV fistulas fail to develop in this patient population due to changes in hemodynamic and endothelial factors as well as atherosclerosis and calcification of surgical conduits.36 Interestingly, a retrospective analysis of a cohort of elderly patients (mean age: 81.9 years) when compared to younger controls (mean age: 54.8 years) with similar microbiology, lock solution, catheter type, and catheter location had a significantly lower rate of CRBSI producing a hazard ratio of 0.33 by multivariate analysis.37 These results could be explained by decreased skin and nasal colonization rates in the elderly. Furthermore, lower physical activity in this age group causes less mechanical stress on the catheter potentiating the infection rate. Further studies concentrating on these proposed age-specific host factor variations may bring more light to this observation.
Antimicrobial-coated catheters
Innovations in catheter material and design to maximize patency and lower infection risk are ongoing. A variety of agents including silver, heparin, and various antibiotics have been incorporated into catheter composition for antimicrobial purposes. A large systematic review conducted in 2009 was unable to show that antimicrobial coating, including chlorhexidine or silver compounds, significantly reduced bloodstream, exit site infections, or all-cause mortality.33 One randomized control trial involving 130 patients with acutely inserted nontunneled catheters did show an 11% reduction in BSIs favoring a minocycline–rifampin impregnated catheter versus standard controls.38 This apparent higher effectiveness likely was a function of the shorter duration of catheter insertion in comparison to long-term, tunneled catheters. Further prospective studies in the dialysis population are needed to justify these devices given their added expense.
Protective barriers
A variety of novel products have been developed to minimize contamination of catheter access ports. The TEGO needleless connector system (Victus Inc., Miami, FL, USA) was designed to decrease catheter hub manipulation and minimize contamination via the use of a silicone barrier system.39 Attachment to blood tubing creates a straight, internal fluid path and reinforces a closed system that avoids the need to cap and recap catheter ports. This apparatus was also designed to obviate the need for heparin-locking solution as an anticoagulant. A retrospective analysis conducted by investigators for DaVita Clinical Research compared 10,652 patients utilizing the TEGO connector system to 6,493 control patients and found a modest reduction in CRBSI rate of 10%–12% as defined by the initiation of antibiotics (adjusted incidence rate ratio 0.92, CI 0.87–0.97) or antibiotic course (incidence rate ratio 0.89, CI 0.84–0.95).
The Curos Disinfecting Port Protector (Ivera Medical Corporation, San Diego, CA, USA) is a plastic threaded device that contains 70% isopropyl alcohol and is designed to be used in conjunction with Luer-lock needleless systems. A quasi-experimental study conducted in a 430-bed tertiary referral trauma center evaluated the rate of CBRSI per 1,000 catheter-days 12 months before and after implementation of these caps. The rate decreased from 1.5±0.37 to 0.88±0.62, yielding a >40% reduction in infection.
Prophylactic intraluminal antimicrobial lock therapy
Antimicrobial lock (AML) therapy involves instillation of a disinfectant solution into the intraluminal portion of a dialysis catheter between treatments to sterilize the interior of the catheter from biofilm. A number of studies have been conducted with different combinations of antimicrobial agents coupled with anticoagulants (Table 2). While treating an established infection has been considered appropriate therapy for catheter salvage, the prophylactic use of AML has been controversial, given concern for the emergence of resistant organisms. This is a major concern for the CDC and the primary reason for the lack of a recommendation for the routine use of any prophylactic antibiotic lock. Most studies evaluating efficacy of antibiotic lock have been of small size or of short duration, making it difficult to evaluate antibiotic resistance over longer periods of time or in a real-world setting. A large study conducted by Landry et al reported a 95% reduction in CRBSIs (17–0.83 per 1,000 catheter days) in >1,400 patients with a prophylactic gentamicin/heparin lock.46 This study reported the presence of gentamicin-resistant organisms during 4 years of AML use. This eventually led to termination of the study protocol. A note should be made that this study lacked susceptibility data for the period before implementing the AML. This makes it difficult to identify whether the emergence of gentamicin resistance patterns changed after initiation of the lock as similar patterns could have existed before AML use. A randomized, controlled trial using the same prophylactic, low-dose citrate gentamicin formulation found stable gentamicin susceptibility patterns over the 5-year course of the study in both treatment groups.43 A prospective, observational cohort study in 555 patients conducted over a 3-year period showed a 73% reduction in CRBSI after a gentamicin citrate locking solution was instituted.47 A unique feature of this study was the decrease in mortality after AML was initiated with an adjusted hazard ratio of 0.32 (CI 0.14–0.75). The rate of gentamicin-resistant organisms actually decreased over the course of the study. It was hypothesized that these findings are related to the lower dose of gentamicin used in the lock solution and the use of citrate as opposed to heparin, which has been shown to promote biofilm formation.53,54
Table 2.
Summary of several clinical trials utilizing antimicrobial lock solution
| Study | Lock solution | N | CD | Outcome | Resistance evaluated | Comments |
|---|---|---|---|---|---|---|
| Al-Hwiesh et al40 | V/G/H (25/40/5,000) vs H (5,000) | 63 | 14,867 | 13.11 vs 4.54 per 1,000 DS, P<0.0001 | No | G levels were detectable but <1 in 6% |
| Dogra et al41 | G/C (40/3.13%) vs H (5,000) | 83 | 5,923 | 4.2 vs 0.3 per 1,000 CD, P=0.003 | No | G levels were detectable in random sample |
| McIntyre et al42 | G/H (5/5,000) vs H (5,000) | 50 | 5,722 | 4 vs 0.3 per 1,000 CD, P=0.02 | No | No detectable G levels were seen |
| Moran et al43 | G/C (0.32/4%) vs H (1,000) | 303 | 72,760 | 0.91 vs 0.28 per 1,000 CD, P=0.003 | Yes | No change in resistance was seen; CRBSI rate was low in the control population |
| Nori et al6 | G/C (4/3.13%) vs M vs H (5,000) | 62 | 6,189 | G 4 vs 0 per 1,000 CD, M 0.4, P=0.008 | No | Two different interventions were compared to control |
| Pervez et al44 | G/C (18/46.7%) vs H (5,000) | 36 | 4,805 | 2.11 vs 0.62 CD, P<0.05 | No | A sterile covering was used as an additional technique to decrease infection |
| Zhang et al45 | G/H (4/5,500) vs H (5,500) | 101 | 9,300 | 0.67 vs 0.06 per 1,000 CD, P=0.014 | No | G concentrations in the serum were low |
| Landry et al46 | G/H (4/5,000) | 1,410 | 142,365 | 17 vs 0.83 per 1,000 CD | Yes | Retrospective cohort trial that did report G resistance; resistance rates prior to lock were not evaluated |
| Moore et al47 | G/C vs H (1,000) | 555 | 71,192 | 1.68 vs 0.45 per 1,000 CD | Yes | No G resistance seen. Trend toward decrease was noted |
| Maki et al48 | C/MB/P vs H | 416 | 49,565 | 0.24 vs 0.82 per 1,000 CD | No | Antiseptic solution was used as opposed to an antibiotic |
| Broom et al49 | E (70%) vs H (5,000) | 49 | 3,614 | 0.85 vs 0.28 per 1,000 CD | No | A trend toward CRBSI reduction was seen but was not statistically significant (P=0.12) |
| Souweine et al50 | E (60%) 2-minute dwell vs H | 1,460 | 12,944 | 2.64 vs 3.83 | No | No difference in CRBSI rate or colonization was seen |
| Solomon et al51 | T–C–H vs T–C vs H (5,000) | 174 | 24,255 | 1.33 vs 1.22 vs 3.25 | No | Heparin was added to AML to decrease the need for thrombolysis |
| Murray et al52 | T–C–H vs H (5,000) | 565 | 135,446 | 0.69 vs 1.59 | No | Only Staphylococcal infections were investigated |
Abbreviations: AML, antimicrobial lock; C, citrate; CRBSI, catheter-related bloodstream infection; CD, catheter-days; DS, dialysis sessions; E, ethanol; G, gentamicin; H, heparin; M, minocycline; MB, methylene blue; P, propylparaben; T, taurolidine; V, vancomycin; vs, versus.
The controversy regarding antibiotic resistance in AML has led to the use of other antiseptic solutions that do not contain antibiotics. Taurolidine, an agent that directly affects bacterial cell walls, has had promising results in the reduction of CRBSIs. While it lacks the stigma of antibiotic resistance, this taurine-like molecule has been linked to an increased need for thrombolytic therapy due to occlusion problems. The incorporation of heparin within the AML solution was shown to obviate this issue.48,51,55
Ethanol has been proposed as an attractive option for AML as it is relatively inexpensive and is effective against a wide variety of microorganisms including fungi. A prospective study involving a high-risk population of 64 patients undergoing chemotherapy via tunneled catheters showed a decrease in infections by four-fold, three in the ethanol group versus eleven in the control group, when ethanol was used as daily prophylaxis.56 Once weekly ethanol instilled for 48 hours in patients with HD was associated with an insignificant 67% decrease in CBRSI.49 Shorter dwell times for this agent have been postulated as prolonged ethanol exposure may lead to catheter dysfunction. A French study involving critically ill patients with nontunneled catheters compared a 2-minute ethanol dwell time to heparinized controls and did not show a significant difference in outcome.50,57 The authors suggested that the short interval of catheter use likely contributed to the results as previous positive studies involved longer durations of catheter use.
Conclusion
A host of resources have been dedicated to combating CRBSIs in patients with HD. Notwithstanding, CBRSIs remain a considerable problem that plague the ESRD community and health care providers due to the consequences of the disease and mortality effects. Early efforts must be directed toward preventative care emphasizing placement of other vascular accesses or initiating an alternative dialysis modality, such as timely peritoneal dialysis (PD) to avert HD catheter placement before it becomes necessary. Initiating PD acutely via “urgent start PD” programs has been shown to be a safe alternative to HD in patients without an established AV fistula or graft.58 Should no options exist outside of HD catheter placement, proper catheter care and infection control procedures are the first step in preventing infections. Auditing and educating both patients and dialysis unit personnel are of the utmost importance. Lastly, AML therapy should be considered in high-risk groups who have utilized all other conservative measures to prevent infection.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Centers for Medicare and Medicaid Services . 2007 Annual Report ESRD Clinical Performance. Baltimore, MD: Department of Health and Human Services, Centers for Medicare and Medicaid Services, Office of Clinical Standards and Quality; 2007. [Google Scholar]
- 2.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46(3):501–508. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. 2004;44(5):779–791. [PubMed] [Google Scholar]
- 4.Hannah EL, Stevenson KB, Lowder CA, et al. Outbreak of hemodialysis vascular access site infections related to malfunctioning permanent tunneled catheters: making the case for active infection surveillance. Infect Control Hosp Epidemiol. 2002;23(9):538–541. doi: 10.1086/502103. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Andrus ML, et al. Special Report: Dialysis surveillance report: national healthcare safety network (NHSN)-data summary for 2006. Semin Dial. 2007;21(1):24–28. doi: 10.1111/j.1525-139X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 6.Nori US, Manoharan A, Yee J, et al. Comparison of low-dose gentamicin with minocycline as catheter lock solutions in the prevention of catheter-related bacteremia. Am J Kidney Dis. 2006;48(4):596–605. doi: 10.1053/j.ajkd.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Saad TF. Central venous dialysis catheters: catheter-associated infection. Semin Dial. 2001;14(6):446–451. doi: 10.1046/j.1525-139x.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- 8.US Renal Data System . USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 9.Taylor G, Gravel D, Johnston L, et al. Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patients. Am J Infect Control. 2004;32(3):155–160. doi: 10.1016/j.ajic.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 10.US Renal Data System . USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 11.Centers for Disease Control and Prevention Vital signs: central line-associated blood stream infections – United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243–248. [PubMed] [Google Scholar]
- 12.O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):1087–1099. doi: 10.1093/cid/cir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raad I, Costerton W, Sabharwal U, et al. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993;168(2):400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]
- 14.Marr KA, Sexton DJ, Conlon PJ, et al. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med. 1997;127(4):275–280. doi: 10.7326/0003-4819-127-4-199708150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;(136):1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann M, Michaud G, Visini R, et al. Multivalency effects on Pseudomonas aeruginosa biofilm inhibition and dispersal by glycopeptide dendrimers targeting lectin LecA. Org Biomol Chem. 2016;14(1):138–148. doi: 10.1039/c5ob01682g. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Givskov M. Chemical biology strategies for biofilm control. Microbiol Spectr. 2015;3(4) doi: 10.1128/microbiolspec.MB-0019-2015. [DOI] [PubMed] [Google Scholar]
- 18.Sychev D, Maya ID, Allon M. Clinical outcomes of dialysis catheter-related candidemia in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1102–1105. doi: 10.2215/CJN.01610309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira L, Graham J, Lok C, et al. Risk factors for yeast superinfection in the treatment of suspected exit site infections: a case-control study. J Vasc Access. 2008;9(1):35–38. [PubMed] [Google Scholar]
- 20.Danese MD, Griffiths RI, Dylan M, et al. Mortality differences among organisms causing septicemia in hemodialysis patients. Hemodial Int. 2006;10(1):56–62. doi: 10.1111/j.1542-4758.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients – United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(9):197–199. [PubMed] [Google Scholar]
- 23.Horino T, Sato F, Hosaka Y, et al. Predictive factors for metastatic infection in patients with bacteremia caused by methicillin-sensitive Staphylococcus aureus. Am J Med Sci. 2015;349(1):24–28. doi: 10.1097/MAJ.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu PL, Tsai JC, Chiu YW, et al. Methicillin-resistant Staphylococcus aureus carriage, infection and transmission in dialysis patients, healthcare workers and their family members. Nephrol Dial Transplant. 2008;23(5):1659–1665. doi: 10.1093/ndt/gfm806. [DOI] [PubMed] [Google Scholar]
- 25.Schmid H, Romanos A, Schiffl H, et al. Persistent nasal methicillin-resistant Staphylococcus aureus carriage in hemodialysis outpatients: a predictor of worse outcome. BMC Nephrol. 2013;14:93. doi: 10.1186/1471-2369-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokars JI, Light P, Anderson J, et al. A prospective study of vascular access infections at seven outpatient hemodialysis centers. Am J Kidney Dis. 2001;37(6):1232–1240. doi: 10.1053/ajkd.2001.24527. [DOI] [PubMed] [Google Scholar]
- 27.Engemann JJ, Friedman JY, Reed SD, et al. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol. 2005;26(6):534–539. doi: 10.1086/502580. [DOI] [PubMed] [Google Scholar]
- 28.Marik PE, Flemmer M, Harrison W. The risk of catheter-related bloodstream infection with femoral venous catheters as compared to subclavian and internal jugular venous catheters: a systematic review of the literature and meta-analysis. Crit Care Med. 2012;40(8):2479–2485. doi: 10.1097/CCM.0b013e318255d9bc. [DOI] [PubMed] [Google Scholar]
- 29.Entesari-Tatafi D, Orford N, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;203(3):138. doi: 10.5694/mja15.00440. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention [webpage on the Internet] Check List: Hemodialysis Exit Site Care. [Accessed December 30, 2015]. Available from: http://www.cdc.gov/dialysis/PDFs/collaborative/CL-Hemodialysis-Catheter-Exit-Site-Care-508.pdf.
- 31.Dwyer A, Shelton P, Brier M, et al. A vascular access coordinator improves the prevalent fistula rate. Semin Dial. 2012;25(2):239–243. doi: 10.1111/j.1525-139X.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 32.Paglialonga F, Consolo S, Biasuzzi A, et al. Reduction in catheter-related infections after switching from povidone-iodine to chlorhexidine for the exit-site care of tunneled central venous catheters in children on hemodialysis. Hemodial Int. 2014;18(suppl 1):S13–S18. doi: 10.1111/hdi.12218. [DOI] [PubMed] [Google Scholar]
- 33.Rabindranath KS, Bansal T, Adams J, et al. Systematic review of antimicrobials for the prevention of haemodialysis catheter-related infections. Nephrol Dial Transplant. 2009;24(12):3763–3774. doi: 10.1093/ndt/gfp327. [DOI] [PubMed] [Google Scholar]
- 34.Deshpande LM, Fix AM, Pfaller MA, et al. Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY antimicrobial surveillance program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagn Microbiol Infect Dis. 2002;42(4):283–290. doi: 10.1016/s0732-8893(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 35.Lok CE, Thumma JR, McCullough KP, et al. Catheter-related infection and septicemia: impact of seasonality and modifiable practices from the DOPPS. Semin Dial. 2014;27(1):72–77. doi: 10.1111/sdi.12141. [DOI] [PubMed] [Google Scholar]
- 36.Ponce P. Vascular access for dialysis in the elderly. Int Urol Nephrol. 2001;33(3):571–573. doi: 10.1023/a:1019550307514. [DOI] [PubMed] [Google Scholar]
- 37.Murea M, James KM, Russell GB, et al. Risk of catheter-related bloodstream infection in elderly patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9(4):764–770. doi: 10.2215/CJN.07710713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatzinikolaou I, Finkel K, Hanna H, et al. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med. 2003;115(5):352–357. doi: 10.1016/s0002-9343(03)00367-x. [DOI] [PubMed] [Google Scholar]
- 39.Brunelli SM, Njord L, Hunt AE, et al. Use of the Tego needlefree connector is associated with reduced incidence of catheter-related bloodstream infections in hemodialysis patients. Int J Nephrol Renovasc Dis. 2014;7:131–139. doi: 10.2147/IJNRD.S59937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Hwiesh AK, Abdul-Rahman IS. Successful prevention of tunneled, central catheter infection by antibiotic lock therapy using vancomycin and gentamycin. Saudi J Kidney Dis Transpl. 2007;18(2):239–247. [PubMed] [Google Scholar]
- 41.Dogra GK, Herson H, Hutchison B, et al. Prevention of tunneled hemodialysis catheter-related infections using catheter-restricted filling with gentamicin and citrate: a randomized controlled study. J Am Soc Nephrol. 2002;13(8):2133–2139. doi: 10.1097/01.asn.0000022890.29656.22. [DOI] [PubMed] [Google Scholar]
- 42.McIntyre CW, Hulme LJ, Taal M, et al. Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int. 2004;66(2):801–805. doi: 10.1111/j.1523-1755.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 43.Moran J, Sun S, Khababa I, et al. A randomized trial comparing gentamicin/citrate and heparin locks for central venous catheters in maintenance hemodialysis patients. Am J Kidney Dis. 2012;59(1):102–107. doi: 10.1053/j.ajkd.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 44.Pervez A, Ahmed M, Ram S, et al. Antibiotic lock technique for prevention of cuffed tunnel catheter associated bacteremia. J Vasc Access. 2002;3(3):108–113. doi: 10.1177/112972980200300305. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P, Yuan J, Tan H, et al. Successful prevention of cuffed hemodialysis catheter-related infection using an antibiotic lock technique by strictly catheter-restricted antibiotic lock solution method. Blood Purif. 2009;27(2):206–211. doi: 10.1159/000197560. [DOI] [PubMed] [Google Scholar]
- 46.Landry DL, Braden GL, Gobeille SL, et al. Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol. 2010;5(10):1799–1804. doi: 10.2215/CJN.01270210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore CL, Besarab A, Ajluni M, et al. Comparative effectiveness of two catheter locking solutions to reduce catheter-related bloodstream infection in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9(7):1232–1239. doi: 10.2215/CJN.11291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maki DG, Ash SR, Winger RK, et al. A novel antimicrobial and anti-thrombotic lock solution for hemodialysis catheters: a multi-center, controlled, randomized trial. Crit Care Med. 2011;39(4):613–620. doi: 10.1097/CCM.0b013e318206b5a2. [DOI] [PubMed] [Google Scholar]
- 49.Broom JK, Krishnasamy R, Hawley CM, et al. A randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients – the HEALTHY-CATH trial. BMC Nephrol. 2012;13:146. doi: 10.1186/1471-2369-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souweine B, Lautrette A, Gruson D, et al. Ethanol lock and risk of hemodialysis catheter infection in critically ill patients. A randomized controlled trial. Am J Respir Crit Care Med. 2015;191(9):1024–1032. doi: 10.1164/rccm.201408-1431OC. [DOI] [PubMed] [Google Scholar]
- 51.Solomon LR, Cheesbrough JS, Bhargava R, et al. Observational study of need for thrombolytic therapy and incidence of bacteremia using taurolidine-citrate-heparin, taurolidine-citrate and heparin catheter locks in patients treated with hemodialysis. Semin Dial. 2012;25(2):233–238. doi: 10.1111/j.1525-139X.2011.00951.x. [DOI] [PubMed] [Google Scholar]
- 52.Murray EC, Deighan C, Geddes C, et al. Taurolidine-citrate-heparin catheter lock solution reduces staphylococcal bacteraemia rates in haemodialysis patients. QJM. 2014;107(12):995–1000. doi: 10.1093/qjmed/hcu128. [DOI] [PubMed] [Google Scholar]
- 53.Shanks RM, Sargent JL, Martinez RM, et al. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21(8):2247–2255. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 54.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 55.Solomon LR, Cheesbrough JS, Ebah L, et al. A randomized double-blind controlled trial of taurolidine-citrate catheter locks for the prevention of bacteremia in patients treated with hemodialysis. Am J Kidney Dis. 2010;55(6):1060–1068. doi: 10.1053/j.ajkd.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 56.Sanders J, Pithie A, Ganly P, et al. A prospective double-blind randomized trial comparing intraluminal ethanol with heparinized saline for the prevention of catheter-associated bloodstream infection in immunosuppressed haematology patients. J Antimicrob Chemother. 2008;62(4):809–815. doi: 10.1093/jac/dkn284. [DOI] [PubMed] [Google Scholar]
- 57.Vercaigne LM, Takla TA, Raghavan J. Long-term effect of an ethanol/sodium citrate locking solution on the mechanical properties of hemodialysis catheters. J Vasc Access. 2010;11(1):12–16. doi: 10.1177/112972981001100103. [DOI] [PubMed] [Google Scholar]
- 58.Alkatheeri AM, Blake P, Gray D, et al. Success of urgent-start peritoneal dialysis in a large Canadian renal program. Perit Dial Int. 2015 Sep 15; doi: 10.3747/pdi.2014.00148. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]


