Abstract
Non-functional parathyroid carcinoma is an exceedingly rare disease with 31 reported cases since 1909. Because of the scarce number of cases of non-functional parathyroid carcinoma, there are no evidence-based recommendations for its optimal treatment. Surgery, including en bloc resection of the carcinoma, ipsilateral thyroid lobe and isthmus together with a neck dissection only in case of lymph node involvement, is the main treatment for non-functioning parathyroid carcinoma. The patient usually has a poorer prognosis because of detection at advanced stages, the relative ineffectiveness of adjuvant treatment modalities and the lack of adequate parameters for clinical follow-up. In this report, we present a case of non-functional parathyroid carcinoma at our institution, and we review the previous literature to discuss the latest advances in the diagnosis and treatment of this rare disease.
Keywords: carcinoma, non-functional, parathyroid, surgery
Introduction
Parathyroid carcinoma is a rare malignant endocrine cancer, which accounts for 0.55% of all parathyroid tumors.1 Less than 10% of cases of parathyroid carcinoma are non-functional,2 presenting with normal levels of parathyroid hormone. De Quervain,3 described the first known case in 1909, and to date, few cases have been reported in the literature. The clinical detection of non-functioning parathyroid malignancies preoperatively is primarily based on symptoms, including an expanding mass on the neck. Difficulties in its diagnosis arise because of the absence of symptoms of hyperparathyroidism and because a positive pathological diagnosis is difficult to obtain. In this report, we present a case of non-functional parathyroid carcinoma at our institution, and we discuss recent and new advances in the diagnosis and treatment of this rare disease
Case Report
The patient was a 49-year-old married woman with a firm mass, which had gradually grown on the right side of the neck over a period of 7 months. She had previously undergone surgery because of urinary stones in 1999 and had undergone a partial thyroidectomy 11 y ago on the right side of the neck; pathology revealed a thyroid adenoma. Six months ago, she was diagnosed with breast hyperplasia but has not been treated. She does not smoke or drink. She denied a family history of cancer
A review of her symptoms revealed nothing else. Physical examination revealed a 3-cm firm mass on the right side of the neck. The mass was located where the thyroid had undergone the partial thyroidectomy. Ultrasound was performed at another hospital 6 months previously and showed right thyroid lobe volume reduction (after surgery). The sonographic findings were not homogeneous and showed multiple solid hypoechoic nodules located at the right lobe of the thyroid. After admission, her blood tests indicated that the serum thyroid function panel and the levels of thyroid-stimulating hormone, triiodothyronine, thyroxine, thyroglobulin, anti-thyroglobulin antibody, and calcitoninwere within normal limits (NLs). Of note, the parathyroid hormone level was 40.02pg/ml (NL=15-65 pg/ml), and the calcium level was 2.33mmol/L (NL=2.03-2.54 mmol/L). Upon reassessment, the ultrasound showed a solid mass located on the right lobe of the thyroid area (Fig. 1). A CT scan revealed thyroid adenoma recurrence (Fig. 2).
The patient underwent surgery on November 19, 2014. During the surgical procedure, a large cervical tumor was found on the right side, which was involved intimately with the surrounding tissue, including the cervical strap muscles, and adhered to the internal jugular vein and the carotid artery. The right recurrent laryngeal nerve had been invaded. The tumor was resected en bloc with the surrounding tissue, including the right recurrent laryngeal nerve. We separated the internal jugular vein and the carotid artery from the tumor tissue. The specimen was diagnosed as a rare malignancy, such as a medullary thyroid carcinoma, by frozen section analysis during the procedure. Then, we performed a total thyroidectomy and central lymph node dissection
Postoperatively, her physical condition was stable. On the second day after surgery, her parathyroid hormone level was 22.31pg/ml (NL=15-65 pg/ml), and her calcium level was 2.15mmol/L (NL=2.03-2.54 mmol/L). The pathological results suggested parathyroid carcinoma with invasion of the surrounding tissues. No metastasis (0/7) was detected in the central lymph nodes. Some of the broad-spectrum CK, Syn, and CgA tests and approximately 58%- of the Ki-67 tests were positive.The TG, TTF-1, S-100, HMB45, CEA, Bcl−2 and calcitonin tests were negative (Fig. 3). On the sixth day after the operation, her parathyroid hormone level was 30.57pg/ml (NL=15-65 pg/ml), and her blood calcium level was 2.16mmol/L (NL=2.03-2.54 mmol/L). She was discharged from the hospital on the ninth postoperative day, and at 2 months after surgery she has no evidence of recurrent tumor
Discussion
Non-functional parathyroid carcinoma is a rare malignant endocrine cancer. To date, 32 cases (Table-1) have been reported in the literature.2,4-33 An additional 6 cases were mentioned in the literature but were not accessible for this review.3,34-38 Patients with non-functional parathyroid carcinoma, as the name implies, have normal serum PTH levels but do not present with symptoms of hypercalcemia. In the 32 cases, the mean age at the time of diagnosis was 52.75 y (range: 27-71 years). Because the tumor does not exhibit excessive parathyroid hormone production or hypercalcemia, it is difficult to detect early; most patients come to the hospital because of a neck mass or a mediastinal mass on radiographic imaging. When patients discover the tumor, the disease is already at an advanced stage. In patients with non-functional parathyroid carcinoma, the tumor size varies; however, approximately half of cases have tumors between 5 and 11 cm in size. In comparison, the NCDB case series reported a median tumor size of approximately3.3 cm.39 Non-functioning parathyroid carcinoma lacks specific signs, and effective diagnostic methods are lacking. The diagnosis of non-functional parathyroid carcinoma is challenging because patients often present with signs and symptoms of local growth and invasion, such as a neck mass, hoarseness, and dysphagia.40 As shown in Table-1, more than half of cases come to the hospital because of a neck mass
Table 1.
Reported cases of non-functional parathyroid carcinoma
| References |
Basic Information |
Serum hormone levels |
Cancer basics |
Adjuvant therapy |
Prognosis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Publication date | Sex | Age | Ca (mg/dl) | PTH (pg/ml) | Clinical presentation | Tum or size (cm) | Local invasion | Surgery | Radiation therapy | Chemotherapy | Local recurrence | Metastasis | Survival | |
| Current study | 2014 | F | 45 | 9.3 | 40 | Neck mass | 1.1×1.0×1.0 | Yes | en bloc resection, total thyroidectomy, cervical lymph node dissection | No | No | No | No | Alive at 2 months | |
| Piciu D[4] | 2013 | F | 51 | 9.1 | 54 | Neck mass | 2.5 | No | en bloc resection, total thyroidectomy, cervical lymph node dissection | No | No | No | No | Alive at 15 months | |
| Zeljko Kotromanovi[5] | 2012 | M | 65 | 9.1 | 47.2 | Neck mass | 8.0×4.0 | Yes | R selective lymphadenectomy | No | No | Yes | Yes | Alive at 54 months | |
| Yitong Xu[6] | 2012 | F | 54 | 9.7 | NR | Neck mass | 3.5×3.0 | NR | R thyroid lobectomy, neck dissection | No | No | Yes | NR | NR | |
| Chao Chen[7] | 2011 | M | 44 | 10.1 | NR | Neck mass, hoarseness | 1.0×1.0×0.8 | Yes | R thyroid lobectomy + neck dissection | No | No | No | Yes | Alive at 72 months | |
| Krvavica A[8] | 2011 | M | 60 | Normal | Normal | Neck mass | 6.0×6.0×10 | Yes | total thyroidectomy, en bloc resection, L thyroid lobectomy, cervical lymph node dissection | No | No | No | No | Dead at 36 months | |
| Benjamin J [2] | 2009 | M | 59 | 10.1 | 20 | Dysphagia, hoarseness, neck mass | 9.5×6.5×6.5 | Yes | en bloc resection mass | Yes | No | No | No | Alive at 7 months | |
| W.C.Gao[9] | 2009 | M | 47 | 10.5 | 42.8 | Neck mass, hoarseness | 4.0, 2.5 | Yes | L thyroid lobectomy + neck dissection (levels II-VI) | No | No | No | No | Alive at 8 months | |
| Mazeh H[10] | 2008 | F | 44 | 10.1 | NR | Neck mass | 1.5 | NR | L thyroid lobectomy | No | No | No | No | Alive at 60 months | |
| Grodski[11] | 2008 | M | 46 | 9.4 | NR | Neck mass | 12 | No | total thyroidectomy, neck exploration | No | No | No | No | NR | |
| Fernandez-Ranvier [12] | 2007 | F | 67 | 9.1 | 19 | Multinodular goiter | 4.5×2.0×0.8 | Yes | total thyroidectomy | Yes | No | Yes | Yes | Alive at 31 months | |
| Ashkenazi D[13] | 2006 | F | 54 | 10-10.8 | 62(with parathyroid adenoma) | mild osteopenia, nonspecific renal pain | NR | No | L hemithyroidectomy | No | No | No | No | Alive at 12 months | |
| Kirkby-Bott [14] | 2005 | M | 66 | Normal | NR | Neck mass, tracheal obstruction | NR | Yes | neck exploration, incomplete exc | Yes | No | Yes | Yes | Dead at 98 months | |
| Eurelings M[15] | 2002 | F | 45 | Normal | NR | Dysphagia, hoarseness | NR | Yes | hemithyroidectomy | Yes | Yes | No | Yes | Dead at 45 months | |
| Yamashita[16] | 1992 | F | 56 | 9.2 | Normal | Sore throat, neck mass | Diameter: 3.0 | Yes | mass+ thyroid lobe | Yes | No | No | No | Alive at 10 months | |
| Klink[17] | 1991 | M | 39 | 9.2 | 0.4 (0.3–2.9) | Neck mass, hoarseness, nephrolithiasis | 7.5×5.5×5.0 | Yes | en bloc resection | No | No | No | No | Alive at 4 months | |
| Collins[18] | 1986 | M | 65 | 9.4 | NR | MNG, dyspnea, dysphagia | 11.0×5.0×5.0 | Yes | L thyroid lobectomy | No | No | No | No | Alive at 24 months | |
| Murphy[19] | 1986 | M | 51 | Normal | Normal | Hoarseness, dyspnea, mediast. Mass | Extensive | Yes | incomplete resection | Yes | Yes | Yes | No | Alive at 11 months | |
| Baba, H[20] | 1986 | F | 64 | 9.3 | 200 (NL<300pg/ml) | Neck mass | 5.0×4.0 | Yes | en bloc resection* | NR | NR | Yes | NR | NR | |
| Merlano[21] | 1985 | M | 59 | Normal | Normal | Neck mass, VCP, dysphagia, axillary LN | 8.0×6.0×4.0 | Yes | biopsies | Yes (Preoperative R+R) | No | Yes | Yes | Alive at 48 months | |
| Yamashita[22] | 1984 | F | 69 | 9.3 | NR | Neck mass | 5.0×4.0×3.0 | Yes | L thyroid lobectomy | Yes | No | No | No | Alive at 20 months | |
| Hickey et al[23-25] | 1982-1983 | M | 27 | 9.2-9.8 | NR | Neck mass | 5.5 | Yes | simple excision | Yes | Yes | Yes | Yes | Dead at 28 months | |
| M | 49 | NR | NR | Neck mass | NR | Yes | No | No | Yes | Yes | Yes | Alive at 9 months | |||
| M | 59 | 9.5 | NR | Neck mass | 2.5 | Yes | total thyroidectomy, L radical neck dissection | No | Yes | Yes | Yes | Dead at 20 months | |||
| Chahinian[26] | 1981 | F | 69 | Normal | Normal | Neck mass, pleural effusion | 2.0×2.0 | Yes | nodule excision | No | Yes | Yes | Yes | Alive at 96 months | |
| Dhom G[27] | 1980 | M | 38 | Normal | NR | Neck mass | 2.5×1.5 | Yes | radical thyroidectomy | No | No | NR | NR | NR | |
| Altenahr [28] | 1973 | M | 50 | Normal | NR | Neck mass | NR | Yes | L thyroid lobectomy | Yes | No | Yes | Yes | Alive at 84 months | |
| Pachter MR[29] | 1963 | F | 50 | Normal | NR | Chest pain | Diffuse | Yes | partial excision | No | No | Yes | Yes | Alive at 60 months | |
| Sieracki[30] | 1960 | F | 43 | 9.1 | NR | Neck mass, pain, dysphagk VCP | 5.0×4.0 | Yes | subtotal resection | Yes | No | Suspicious | Suspicious | Alive at 36 months | |
| McQuillan [31] | 1938 | F | 53 | Normal | NR | Neck mass | Golf ball | Yes | R thyroid lobectomy | Yes | No | Yes | No | Alive at 28 months | |
| Armstrong [32] | 1938 | F | 71 | 11.9 | NR | Neck mass | 11.0×11.0×4.0 | Yes | en bloc resection | No | No | No | No | Alive at 2 months | |
| Guy [33] | 1929 | F | 29 | 8 | NR | Neck mass, dysphagia, pain | 8.0×6.0×4.0 | Yes | simple excision | Yes | No | Yes | Yes | Alive at 28 months | |
NR: not reported. *: L thyroid lobectomy and neck dissection was performed in 1981 because of functional parathyroid carcinoma.
The non-secretory state, including the decreased or impaired synthesis of hormone, the production and secretion of an abnormalinactive hormone, or the impaired secretion of hormone, is one cause of non-functional PTC.2 Based on the hypothesis of impaired hormone secretion, studies on the ultrastructure of nonfunctioning PTCs have found that the number of cytoplasmic organellesis increased, especially in the Golgi apparatus. Moreover, the amounts of cytoplasmic secretory granules, occasionallipid vacuoles, and glycogen are increased. The impaired secretion of hormone results in the accumulation of secretory granules.26,28 In addition, a large number of mitochondria with fewer and smaller rough endoplasmic reticula and Golgi apparatus, a large amount of cytoplasmic glycogen and a large number of liposomes, rather than specific secretory granules, have been observed.27 Pre-pro-PTH, a precursor of PTH, is encoded by mRNA, and the presence of mRNA coding for pre-pro-PTH(PTH mRNA) has been detected in PTCs, indicating that the synthesis of PTH is intermittent.20 In addition, studies have demonstrated that cells are full of cytoplasmic glycogen particles but have few organelles and secretory granules and no lipid vacuoles.22 More studies are needed to determine the actual molecular mechanisms involved in non-functioning PTC in the absence of hyperparathyroidism
TNM staging criteria are currently unavailable for non-functional parathyroid carcinoma because of the rarity of this disease. Schulte reported that the low-risk (invasion limited to the capsule and soft tissue) versus high-risk (vascular invasion, lymph node metastasis or invasion of the trachea, esophagus or major cervical vessels) classification was an excellent predictor of disease-specific mortality and recurrence in a multicenter validation study on parathyroid carcinoma.41 Using the information provided by various reviews on parathyroid carcinoma, Shaha and Shah proposed a staging system based on the size of the tumor, extent of local invasion, and presence of regional nodal disease and distant metastasis.42
Ultrasonography, radionuclide scanning, computed tomography (CT) and magnetic resonance imaging (MRI) have been used for the initial diagnosis and the detection of recurrence in patients with parathyroid carcinoma. Non-functional parathyroid carcinoma is rare; however, this disease is noteworthy for ultrasound. Hara.43 reported that ultrasound, which can reliably assess tumor size with no risk and at a low cost, has significant value for patients with parathyroid carcinoma. Parathyroid carcinomas are typically lobulated, hypoechoic, and relatively large, with ill-defined borders compared with adenomas.44 Sidhu PS,45 reported a high positive predictive value (PPV) for infiltration (PPV 100%) and calcification (PPV 100%) in the diagnosis of cancer, whereas a high negative predictive value (NPV) was found for the absence of suspicious vascularity (NPV 97.6%), a thick capsule (NPV 96.7%), and in homogeneity (NPV 100%), which could mostly exclude cancer. Additionally, the study concluded that ultrasonography for a specific feature is a valuable tool to identify parathyroid cancers before surgery, especially parathyroid lesions larger than 15 mm. However, ultrasound has limitations. First, this approach may easily miss tumors less than 500mg, especially in patients with thyroid nodules. Second, distinguishing a large adenoma from a carcinoma is difficult. Third, because of the interference caused by the sternum and clavicle, ultrasound is restricted for the lower position of parathyroid carcinoma. Technecium-99 m sestamibi scanning has largely replaced parathyroid imaging by 201Tl and is widely used to localize parathyroid tumors. However, no specific characteristics exist for distinguishing benign disease from parathyroid carcinoma. 46 CT and MRI are relatively limited in value for patients with parathyroid carcinoma, and these modalities have less diagnostic value than ultrasound. CT and MRI scans may be used to assess lymph node status in the centrocervical compartment.47 In addition, CT or MRI may be used to differentially diagnose parathyroid carcinoma from neck and chest diseases, detect ectopic parathyroid carcinoma, and assess preoperative risks. However, because non-functional parathyroid carcinoma lacks specificity, even if a tumor is found, these imaging modalities need to be combined with other laboratory examinations
Fine-needle aspiration (FNA) biopsy of the primary lesion cannot be recommended because this diagnostic method has been associated with tumor seeding of the biopsy tract.48 In addition, FNA biopsy may not be used to distinguish benign from malignant parathyroid tumors of the primary lesion.49 However, FNA biopsy maybe used to distinguish thyroid from parathyroid tissue or to identify metastatic parathyroid carcinoma.50 Frozen section analysis is of little value and unreliable because the histopathological features of parathyroid carcinoma may overlap with those of parathyroid adenoma.40 During our operation, the frozen section analysis of resected material indicated rare malignant cells, and medullary thyroid carcinoma was considered for the following reasons: 1)non-functional parathyroid carcinoma is a rare malignant endocrine cancer, 2)patients have a history of thyroid adenoma, and 3)non-functioning parathyroid carcinoma and medullary thyroid cancer are both endocrine tumors and have similar pathology
In pathology, an unequivocal diagnosis of parathyroid carcinoma is defined by the presence of infiltrative growth or histological proof of vascular invasion with/without invasion of vital organs or major blood vessels, or the presence of locoregional or distant metastasis.51 Parathyroid carcinoma is poorly circumscribed because this condition often appears adherent to the surrounding tissue.52 Carcinoma is firm and grayish white compared with adenomas, which are usually soft and tan colored.53 In microscopy, parathyroid carcinoma cells are more uniform in size and shape: round or oval and tightly packed. The tumor cells have round to oval hyperchromatic nuclei, granular chromatin with inconspicuous nucleoli, and a small amount of eosinophilic cytoplasm. Solid nests of tumor cells have been observed with tumor cells accounting for approximately one-quarter of the tissue examined, which is separated by a dense fibrotic stroma.2 In 1973, Schantz and Castleman,52 reported the histological criteria for the diagnosis of parathyroid carcinoma, and these criteria remain valid today, which include sheets or lobules of tumor cells separated by dense fibrous bands, mitotic figures, necrosis, capsular invasion, or vascular invasion. However, the specificity of these criteria is not particularly high because these characteristics can be observed in certain benign tumors. Recent studies have suggested that mutations of HRPT2 (also known as CDC73), a tumor suppressor gene on chromosome 1q25 that encodes the protein parafibromin, are an important contributor to the pathogenesis of parathyroid carcinoma.54 Immunohistochemical staining for parafibromin, the function of which involves the regulation of gene expression and inhibition of cell proliferation,55 is a powerful tool to diagnose parathyroid cancer and may help avoid diagnostic errors.56 Additionally, the over expression of cyclin D1 was encountered in up to 91% of the parathyroid carcinoma specimens studied by Vasef and colleagues.57 Other recurrent genetic aberrations in parathyroid cancer include the abnormal expression of cell cycle regulators, such as retinoblastoma (Rb), breast carcinoma susceptibility (BRCA2), and p53.58 However, there is no definitive evidence for a primary role of these genes in parathyroid carcinoma, although the altered expression of these gene products may participate in the process of malignant transformation.
Surgery is the main treatment for non-functional parathyroid carcinoma. Clinical suspicion of the tumor alone is insufficient to guide the surgical approach beyond the indication for surgery. The surgical approach for parathyroid carcinoma, which is closely related to the approach for deep cervical fascia,59 may be categorized into local excision alone, en bloc resection of the cancer with surrounding tissue, and oncological resection, including the field of lymphatic drainage.60 Among these surgical treatments, en bloc resection of the carcinoma and the adjacent involved structures is the standard treatment for parathyroid carcinoma.42,50,67 A selective neck dissection is necessary when cervical lymph nodes are involved. There seems to be dispute over the need for prophylactic central compartment lymphadenectomy.59 It is more cautious about recommending prophylactic central compartment lymph node resection in the absence of suspicious findings because prophylactic neck dissection has not shown to improve survival and is associated with increased morbidity.61 Usually, parathyroid carcinoma recurs 2 to 5 y after initial operation, and it is associated with a local recurrence rate of 33% to 82% within 5 y61 There are 2 main factors that influence patient outcomes: the nature of the tumor, which is reflected by its prognostic classification, and the chosen surgical approach 62 incomplete resection of the tumor or tumor spillage and the high rate of nodal metastasis. Therefore, when parathyroid cancer is proven or suspected, local excision alone should not be recommend. Distant recurrence is equally common, and the majority of distant metastases occur in the lungs, mediastinum, and bone.1 Prophylactic central neck dissection may be beneficial from an aggressive surgical approach when feasible in patients with recurrent or metastatic parathyroid carcinoma.63 The purpose of the surgical operation is to remove all gross disease in the neck, mediastinum, and distant sites if possible. However, the symptomatic relief and biochemical normalization are temporary in most cases
Demonstrating the efficacy of radiotherapy has been difficult because of the rarity of parathyroid carcinoma. However, radiotherapy may be a valuable adjunct to surgical treatment,64 especially in patients with positive surgical margins. Adjuvant radiation with 40-50 Gy is recommended in patients at high risk of local relapse, such as patients with microscopic residual disease after surgery.65 Munson has used a radiation dosage as high as 70 Gy in studies.66 No reports on the efficacy of chemotherapy in patients with non-functional parathyroid carcinoma have been published. It can be only found in the case reports which described the role of chemotherapy in the management of non-functional parathyroid carcinoma. Eurelings suggested that treatment with a combination of radiotherapy and chemotherapy resulted in a good response and prolonged survival in patients with non-functional parathyroid carcinoma.15 More studies of chemotherapy in non-producing parathyroid carcinoma are necessary to develop a treatment strategy
The prognosis of nonfunctional parathyroid carcinoma is usually poor because of detection at advanced stages, the relative ineffectiveness of adjuvant treatment modalities and the lack of adequate parameters for clinical follow-up.67 It is necessary to follow-up patients with parathyroid cancer for the duration of their lives. The local invasion of non-functional parathyroid carcinoma is significant; therefore, relapse and distant metastasis commonly occur, especially in the lungs, bone and lymph nodes. Non-functioning parathyroid carcinoma is regarded as an adverse prognostic factor for survival in addition to initial management with simple parathyroidectomy alone and the presence of nodal or distant metastatic disease at presentation.68 The overall prognosis of the nonfunctioning parathyroid carcinoma is not favorable.17 Death in patients with nonfunctional parathyroid tumors is primarily due to the volume of regional disease and metastases.25
Conclusions
As a rare malignant tumor of the head and neck, non-functional parathyroid carcinoma often manifests as a palpable mass in the neck without any other distractions. Surgery is the main treatment for non-functioning parathyroid carcinoma, which consists of en bloc resection of the carcinoma, ipsilateral thyroid lobe and isthmus together with a neck dissection only in case of lymph node involvement.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figure 1.

Ultrasound of non-functional parathyroid carcinoma: a lobulated hypoechoic lesion with ill-defined borders, 3.6 cm × 2.9 cm, in where was no scintigraphy of right lobe of the thyroid.
Figure 2.
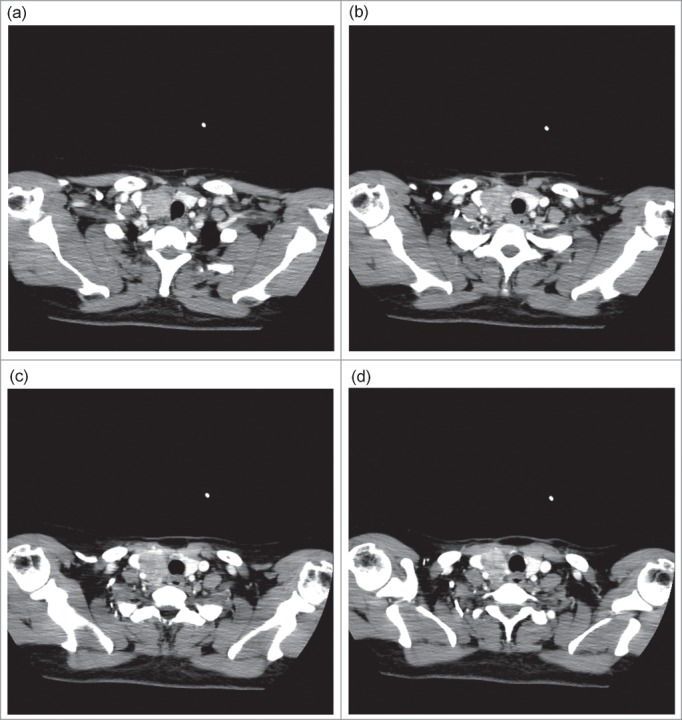
CT of non-functional parathyroid carcinoma: a lobulated enhancing lesion, 3.0 cm × 3.1 cm, in where was no normal thyroid structure in the right of thyroid area extending around the internal jugular vein and pushed it to the outside.
Figure 3.

Pathology of non-functional parathyroid carcinoma. (A-B) Hematoxylin and eosin-stained sections a intermediate (100x), b mitotic figures (200x); c-d representative high-magnification images (200x) of immunohistochemical stains for (C) CGA, (d) TTF.
References
- 1.Talat N, Schulte KM. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol 2010; 17(8): p:2156-74; PMID:20221704; http://dx.doi.org/ 10.1245/s10434-010-1003-6 [DOI] [PubMed] [Google Scholar]
- 2.Wilkins BJ, Lewis JS Jr, Non-functional parathyroid carcinoma: a review of the literature and report of a case requiring extensive surgery. Head Neck Pathol 2009; 3(2): p:140-9; PMID:19644546; http://dx.doi.org/ 10.1007/s12105-009-0115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quervain FD. Parastruma maligna aberrata[J]. Langenbeck s Archives of Surgery 1909; 100:334-53. [Google Scholar]
- 4.Piciu D, Irimie A, Kontogeorgos G, Piciu A, Buiga R. Highly aggressive pathology of non-functional parathyroid carcinoma. Orphanet J Rare Dis 2013; 8: p:115; PMID:23915575; http://dx.doi.org/ 10.1186/1750-1172-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotromanovic Z, Birtić D, Vceva A, Medić D, Zubcić Z, Mihalj H, Kotromanović Z, Erić S, Dmitrović B, Stefanić M. Non-functional parathyroid gland carcinoma, a rare malignant tumor of the head and neck. Coll Antropol 2012; 36 Suppl 2: p:23-5; PMID:23397750 [PubMed] [Google Scholar]
- 6.Yitong Xu, Guanyu Zhang, Lili Xu, et al.. Nonfunctional parathyroid carcinoma. A case report and review of the literature. Chin J Clinicians 2012; 6(10): p: 2807-08. [Google Scholar]
- 7.Chao Chen, Hong Chen, Baorui Liu, et al.. Nonfunctional parathyroid carcinoma. Chin Clin Oncol 2011; 16(10): p:959-60. [Google Scholar]
- 8.Krvavica A, Kovacić M, Baraka I, Rudić M, Non-functioning parathyroid gland carcinoma: case report. Acta Clin Croat 2011; 50(2): p:233-7. [PubMed] [Google Scholar]
- 9.Gao WC, Ruan CP, Zhang JC, Liu HM, Xu XY, Sun YP, Wang Q. Nonfunctional parathyroid carcinoma. J Cancer Res Clin Oncol 2010; 136(7): p:969-74; PMID:19967413; http://dx.doi.org/ 10.1007/s00432-009-0740-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazeh H, Prus D, Frend HR. Freund, Incidental non-functional parathyroid carcinoma identified during thyroidectomy. Isr Med Assoc J 2008; 10(8-9): p:659; PMID:18847176 [PubMed] [Google Scholar]
- 11.Grodski S, Gill A, Robinson BG, Sidhu S. Nonfunctioning parathyroid cancer presenting as a cervical mass. Thyroid 2008; 18(4): p:473-4; PMID:18346007; http://dx.doi.org/ 10.1089/thy.2006.0323 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Ranvier GG, Jensen K, Khanafshar E, Quivey JM, Glastonbury C, Kebebew E, Duh QY, Clark OH. Nonfunctioning parathyroid carcinoma: case report and review of literature. Endocr Pract 2007; 13(7): p:750-7; PMID:18194932; http://dx.doi.org/ 10.4158/EP.13.7.750 [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi D, Elmalah I, Rakover Y, Luboshitzky R. Concurrent nonfunctioning parathyroid carcinoma and parathyroid adenoma. Am J Otolaryngol 2006; 27(3): p:204-6; PMID:16647986; http://dx.doi.org/ 10.1016/j.amjoto.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Kirkby-Bott J, Lewis P, Harmer CL, Smellie WJ. One stage treatment of parathyroid cancer. Eur J Surg Oncol 2005; 31(1): p:78-83; PMID:15642430; http://dx.doi.org/ 10.1016/j.ejso.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 15.Eurelings M, Frijns CJ, Jeurissen FJ. Painful ophthalmoplegia from metastatic nonproducing parathyroid carcinoma: case study and review of the literature. Neuro Oncol 2002; 4(1): p:44-8; PMID:11772432; http://dx.doi.org/ 10.1215/15228517-4-1-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita H, Noguchi S, Murakami N, Toda M, Adachi M, Daa T. Immunohistological study of nonfunctional parathyroid carcinoma. Report of a case. Acta Pathol Jpn 1992; 42(4): p:279-85; PMID:1609615 [DOI] [PubMed] [Google Scholar]
- 17.Klink BK, Karulf RE, Maimon WN, Peoples JB. Nonfunctioning parathyroid carcinoma. Am Surg, 1991; 57(7): p:463-7; PMID:1647716 [PubMed] [Google Scholar]
- 18.Collins FD, Warren MC, Palmer FJ, Rankin DR. Nonfunctioning parathyroid carcinoma: a case history. J Surg Oncol, 1986; 31(1): p:60-1; PMID:3945079; http://dx.doi.org/ 10.1002/jso.2930310114 [DOI] [PubMed] [Google Scholar]
- 19.Murphy MN, Glennon PG, Diocee MS, Wick MR, Cavers DJ. Nonsecretory parathyroid carcinoma of the mediastinum. Light microscopic, immunocytochemical, and ultrastructural features of a case, and review of the literature. Cancer 1986; 58(11): p:2468-76; PMID:3533247; http://dx.doi.org/ 10.1002/1097-0142(19861201)58:11%3c2468::AID-CNCR2820581120%3e3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 20.Baba H, Kishihara M, Tohmon M, Fukase M, Kizaki T, Okada S, Matsuzuka F, Kobayashi A, Kuma K, Fujita T. Identification of parathyroid hormone messenger ribonucleic acid in an apparently nonfunctioning parathyroid carcinoma transformed from a parathyroid carcinoma with hyperparathyroidism. J Clin Endocrinol Metab 1986; 62(2): p:247-52; PMID:2867104; http://dx.doi.org/ 10.1210/jcem-62-2-247 [DOI] [PubMed] [Google Scholar]
- 21.Merlano M, Conte P, Scarsi P, Tatarek R, Barbieri A, Santelli A, Scarpati D, De Angelis P. Non-functioning parathyroid carcinoma: a case report. Tumori 1985; 71(2): p:193-6; PMID:4002350 [DOI] [PubMed] [Google Scholar]
- 22.Yamashita H, Noguchi S, Nakayama I, Togon H, Moriuchi A, Yokoyama S, Mochizuki Y, Noguchi A. Light and electron microscopic study of nonfunctioning parathyroid carcinoma. Report of a case with a review of the literature. Acta Pathol Jpn 1984; 34(1): p:123-32; PMID:6730960 [DOI] [PubMed] [Google Scholar]
- 23.Anderson BJ, Samaan NA, Vassilopoulou-Sellin R, Ordonez NG, Hickey RC. Parathyroid carcinoma: features and difficulties in diagnosis and management. Surgery 1983; 94(6): p:906-15; PMID:6648803 [PubMed] [Google Scholar]
- 24.Ordonez NG, Ibañez ML, Samaan NA, Hickey RC. Immunoperoxidase study of uncommon parathyroid tumors. Report of two cases of nonfunctioning parathyroid carcinoma and one intrathyroid parathyroid tumor-producing amyloid. Am J Surg Pathol 1983; 7(6): p:535-42; PMID:6353951; http://dx.doi.org/ 10.1097/00000478-198309000-00004 [DOI] [PubMed] [Google Scholar]
- 25.Aldinger KA. Hickey RC, Ibanez ML, Samaan NA. Parathyroid carcinoma: a clinical study of seven cases of functioning and two cases of nonfunctioning parathyroid cancer. Cancer 1982; 49(2): p:388-97; PMID:7053835; http://dx.doi.org/ 10.1002/1097-0142(19820115)49:2%3c388::AID-CNCR2820490230%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 26.Chahinian AP, Holland JF, Nieburgs HE, Marinescu A, Geller SA, Kirschner PA. Metastatic nonfunctioning parathyroid carcinoma: ultrastructural evidence of secretory granules and response to chemotherapy. Am J Med Sci 1981; 282(2): p:80-4; PMID:7325189; http://dx.doi.org/ 10.1097/00000441-198109000-00005 [DOI] [PubMed] [Google Scholar]
- 27.Dhom G, Hohbach C. The value of electron microscopy in diagnostic pathology. Case 12. Ultrastruct Pathol 1980; 1(1): p:141-50.PMID:7233574 [DOI] [PubMed] [Google Scholar]
- 28.Altenahr E, Saeger W. Light and electron microscopy of parathyroid carcinoma. Report of three cases. Virchows Arch A Pathol Pathol Anat 1973; 360(2): p:107-22; PMID:4200384; http://dx.doi.org/ 10.1007/BF00543222 [DOI] [PubMed] [Google Scholar]
- 29.Pachter MR, Lattes R. Uncommon mediastinal tumors. Report of two parathyroid adenomas, one nonfunctional parathyroid carcinoma and one “bronchial-type-adenoma. Dis Chest, 1963; 43: p:519-28; http://dx.doi.org/ 10.1378/chest.43.5.519; PMID:13940962 [DOI] [PubMed] [Google Scholar]
- 30.Sieracki JC, Horn RC Jr. Nonfunctional carcinoma of the parathyroid. Cancer 1960; 13: p:502-6; PMID:14446529; http://dx.doi.org/ 10.1002/1097-0142(196005/06)13:3%3c502::AID-CNCR2820130312%3e3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 31.McQuillan AS. Parathyroid tumor: report of two cases. Ann Surg. 1938; 108:464-8; PMID:17857245; http://dx.doi.org/ 10.1097/00000658-193809000-0000817857245 [DOI] [Google Scholar]
- 32.Armstrong HG. Primary carcinoma of the parathyroid gland with report of a case. Bull Acad Med Tor 1938; 11:105-10. [Google Scholar]
- 33.Guy CC. Tumors of the parathyroid glands. Surg Gynecol Obstet 1929; 149:522-7. [Google Scholar]
- 34.Mendiola R. Parathyroid carcinoma. Rev. Mex. Cir 1942; 10:387-394. [Google Scholar]
- 35.Hall EM, ChafŽn L. Malignant tumor of the parathyroid gland. West J Surg 1934; 42:578-86. [Google Scholar]
- 36.Price LW, Mowat GT. A case of rapidly-growing carcinoma in the neck, arising in a parathyroid rest. Br J Surg 1932; 19:645-650; http://dx.doi.org/ 10.1002/bjs.1800197615 [DOI] [Google Scholar]
- 37.Toland CG. Tumors of the parathyroid gland. J Am Med Assoc 1931; 96:741-4; http://dx.doi.org/ 10.1001/jama.1931.02720360011003 [DOI] [Google Scholar]
- 38.Roffo AH, Landivar Y. A.F. Case report. Prensa Med Argent 1914; 2:177-81. [Google Scholar]
- 39.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the US between 1985-1995: a national cancer data Base report. The American college of surgeons commission on cancer and the American cancer society. Cancer, 1999; 86(3): p:538-44; PMID:10430265; http://dx.doi.org/ 10.1002/(SICI)1097-0142(19990801)86:3%3c538::AID-CNCR25%3e3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 40.Shane E. Clinical review 122: parathyroid carcinoma. J Clin Endocrinol Metab 2001; 86(2): p:485-93; PMID:11157996; http://dx.doi.org/ 10.1210/jcem.86.2.7207 [DOI] [PubMed] [Google Scholar]
- 41.Schulte KM, Gill AJ, Barczynski M, Karakas E, Miyauchi A, Knoefel WT, Lombardi CP, Talat N, Diaz-Cano S, Grant CS. Classification of parathyroid cancer. Ann Surg Oncol 2012; 19(8): p:2620-8; PMID:22434247; http://dx.doi.org/ 10.1245/s10434-012-2306-6 [DOI] [PubMed] [Google Scholar]
- 42.Shaha AR, Shah JP, Parathyroid carcinoma: a diagnostic and therapeutic challenge. Cancer 1999; 86(3): p:378-80; PMID:10430243; http://dx.doi.org/ 10.1002/(SICI)1097-0142(19990801)86:3%3c378::AID-CNCR3%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 43.Hara H, Igarashi A, Yano Y, Yashiro T, Ueno E, Aiyoshi Y, Ito K, Obara T. Ultrasonographic features of parathyroid carcinoma. Endocr J 2001; 48(2): p:213-7; PMID:11456270; http://dx.doi.org/ 10.1507/endocrj.48.213 [DOI] [PubMed] [Google Scholar]
- 44.Edmonson GR, Charboneau JW, James EM, Reading CC, Grant CS. Parathyroid carcinoma: high-frequency sonographic features. Radiology 1986; 161(1): p:65-7; PMID:3532184; http://dx.doi.org/ 10.1148/radiology.161.1.3532184 [DOI] [PubMed] [Google Scholar]
- 45.Sidhu PS, Talat N, Patel P, Mulholland NJ, Schulte KM. Ultrasound features of malignancy in the preoperative diagnosis of parathyroid cancer: a retrospective analysis of parathyroid tumours larger than 15 mm. Eur Radiol 2011; 21(9): p:1865-73; PMID:21556910; http://dx.doi.org/ 10.1007/s00330-011-2141-3 [DOI] [PubMed] [Google Scholar]
- 46.Rodgers S.E, Perrier ND. Parathyroid carcinoma. Curr Opin Oncol 2006; 18(1): p:16-22; PMID:16357559; http://dx.doi.org/ 10.1097/01.cco.0000198019.53606.2b [DOI] [PubMed] [Google Scholar]
- 47.Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf) 2012; 76(1): p:131-6; PMID:21722150; http://dx.doi.org/ 10.1111/j.1365-2265.2011.04162.x [DOI] [PubMed] [Google Scholar]
- 48.Spinelli C, Bonadio AG, Berti P, Materazzi G, Miccoli P. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J Endocrinol Invest 2000; 23(4): p:255-7; PMID:10853713; http://dx.doi.org/ 10.1007/BF03343718 [DOI] [PubMed] [Google Scholar]
- 49.Kassahun WT, Jonas S. Focus on parathyroid carcinoma. Int J Surg 2011; 9(1): p:13-9; PMID:20887820; http://dx.doi.org/ 10.1016/j.ijsu.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 50.Owen RP, Silver CE, Pellitteri PK, Shaha AR, Devaney KO, Werner JA, Rinaldo A, Ferlito A. Parathyroid carcinoma: a review. Head Neck 2011. 33(3): p:429-36; PMID:20310041 [DOI] [PubMed] [Google Scholar]
- 51.Bondeson L, Grimelius L, Delellis RA. World Health Organisation Classification of Tumours, Pathology and Genetics: Tumour of Endocrine Organs(IARC, Lyon) 2004 [Google Scholar]
- 52.Schantz A, Castleman B. Parathyroid carcinoma: a study of 70cases. Cancer 1973; 31:600-5; PMID:4693587; http://dx.doi.org/ 10.1002/1097-0142(197303)31:3%3c600::AID-CNCR2820310316%3e3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 53.Smith JF, Coombs RR. Histological diagnosis of carcinoma of the parathyroid gland. J Clin Pathol 1984; 37(12): p:1370-8; PMID:6511982; http://dx.doi.org/ 10.1136/jcp.37.12.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharretts JM, Simonds WF. Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab 2010; 24(3): p:491-502; PMID:20833339; http://dx.doi.org/ 10.1016/j.beem.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Kurd A, Mekel M, Mazeh H. Parathyroid carcinoma. Surg Oncol 2014; 23(2): p:107-14; PMID:24742584; http://dx.doi.org/ 10.1016/j.suronc.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 56.Lim S, Elston MS, Gill AJ, Marsh DJ, Conaglen JV. Metastatic parathyroid carcinoma initially misdiagnosed as parathyroid adenoma: the role of parafibromin in increasing diagnostic accuracy. Intern Med J 2011; 41(9): p:695-9; PMID:21899683; http://dx.doi.org/ 10.1111/j.1445-5994.2011.02545.x [DOI] [PubMed] [Google Scholar]
- 57.Vasef MA, Brynes RK, Sturm M, Bromley C, Robinson RA. Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: a paraffin immunohistochemical study. Mod Pathol 1999; 12(4): p:412-6; PMID:10229506 [PubMed] [Google Scholar]
- 58.Fernandez-Ranvier GG, Khanafshar E, Tacha D, Wong M, Kebebew E, Duh QY, Clark OH. Defining a molecular phenotype for benign and malignant parathyroid tumors. Cancer 2009; 115(2): p:334-44; PMID:19107770; http://dx.doi.org/ 10.1002/cncr.24037 [DOI] [PubMed] [Google Scholar]
- 59.Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat 2012; 25(1): p:19-31; PMID:21800365; http://dx.doi.org/ 10.1002/ca.21220 [DOI] [PubMed] [Google Scholar]
- 60.Schulte KM, Talat N, Miell J, Moniz C, Sinha P, Diaz-Cano S. Lymph node involvement and surgical approach in parathyroid cancer. World J Surg 2010; 34(11): p:2611-20; PMID:20640422; http://dx.doi.org/ 10.1007/s00268-010-0722-y [DOI] [PubMed] [Google Scholar]
- 61.Kebebew E. Parathyroid carcinoma. Curr Treat Options Oncol 2001; 2(4): p:347-54; PMID:12057115; http://dx.doi.org/ 10.1007/s11864-001-0028-2 [DOI] [PubMed] [Google Scholar]
- 62.Schulte KM, Talat N. Diagnosis and management of parathyroid cancer. Nat Rev Endocrinol 2012; 8(10): p:612-22; PMID:22751344; http://dx.doi.org/ 10.1038/nrendo.2012.102 [DOI] [PubMed] [Google Scholar]
- 63.Mohebati A, Shaha A, Shah J, Parathyroid carcinoma: challenges in diagnosis and treatment. Hematol Oncol Clin North Am, 2012; 26(6): p:1221-38; PMID:23116578; http://dx.doi.org/ 10.1016/j.hoc.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 64.Wynne AG. van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992; 71(4): p:197-205; PMID:1518393; http://dx.doi.org/ 10.1097/00005792-199207000-00002 [DOI] [PubMed] [Google Scholar]
- 65.Chow E, Tsang RW, Brierley JD, Filice S. Parathyroid carcinoma-the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys 1998; 41(3): p:569-72; PMID:9635703; http://dx.doi.org/ 10.1016/S0360-3016(98)00098-4 [DOI] [PubMed] [Google Scholar]
- 66.Munson ND, Foote RL, Northcutt RC, Tiegs RD, Fitzpatrick LA, Grant CS, van Heerden JA, Thompson GB, Lloyd RV. Parathyroid carcinoma: is there a role for adjuvant radiation therapy? Cancer 2003; 98(11): p:2378-84; PMID:14635072; http://dx.doi.org/ 10.1002/cncr.11819 [DOI] [PubMed] [Google Scholar]
- 67.Giessler GA, Beech DJ. Nonfunctional parathyroid carcinoma. J Natl Med Assoc 2001; 93(7-8): p:251-5; PMID:11491274 [PMC free article] [PubMed] [Google Scholar]
- 68.Koea JB, Shaw JH. Parathyroid cancer: biology and management. Surg Oncol 1999; 8(3): p:155-65; PMID:11113666; http://dx.doi.org/ 10.1016/S0960-7404(99)00037-7 [DOI] [PubMed] [Google Scholar]


