Abstract
Anaplastic thyroid carcinoma (ATC) is a rare, poorly differentiated type of thyroid cancer occurring in less than 5% of all thyroid cancers. Patients typically have a poor prognosis with very few options for treatment.2 With current therapy of surgery, chemotherapy, and radiation, median survival is only 6 months from the time of diagnosis. Several mutations in cell cycle regulation have been discovered in ATC that contribute to its undifferentiated state, one of which is the BRAF kinase mutation. This mutation results in activation of the MAPK pathway and uncontrolled cell proliferation. In this case report, a 51 y old male presented with a 2-week history of hoarseness and was diagnosed with ATC. Genetic analysis revealed a mutation in BRAF kinase; the patient subsequently began therapy with vemurafenib, a BRAF kinase inhibitor indicated for melanoma. After an initial response, the patient quickly declined and consequently died from his disease. Anaplastic thyroid carcinoma is a deadly cancer without an effective treatment. Inhibiting mutated enzymes that drive the development of this cancer is a potential drug target that may improve outcomes in patients with ATC.
Keywords: Anaplastic thyroid cancer, BRAF inhibitors, vemurafenib
Background
Cancers of the thyroid are typically well differentiated and have favorable outcomes with surgery and radioactive iodine therapy.12 Anaplastic thyroid carcinoma is a rare variant that displays poor differentiation and resistance to standard treatment of chemotherapy and radiation.1 It comprises less than 5% of thyroid malignancies, but has a mortality rate of 90% with a median survival of 6 months. This rare cancer typically harbors several oncogenic mutations, most commonly in the MAPK pathway.2 This pathway is responsible for cell growth, differentiation, and survival.3 A mutation in the BRAF serine/threonine kinase, an integral part of the MAPK pathway, has been identified in up to 20% of ATC cases.6 This mutation results in constitutive cell activation allowing for uncontrolled growth and survival.
Case Report
A fifty one year old Caucasian male presented to his primary care provider with a 2-week history of voice hoarseness and was subsequently referred to an Ear, Nose, and Throat (ENT) specialist. The patient had no significant past medical, surgical or family history. His review of symptoms other than hoarseness was benign. Upon exam, he was found to have a thyroid nodule, which resulted in fine needle aspiration. The sample was suspicious for malignancy and a total thyroidectomy was performed (Fig. 1). Pathology of the resected tissue suggested a diagnosis of anaplastic thyroid carcinoma with positive margins. He was diagnosed with T4aN0M0, Stage IVA Anaplastic Thyroid Carcinoma
Figure 1.
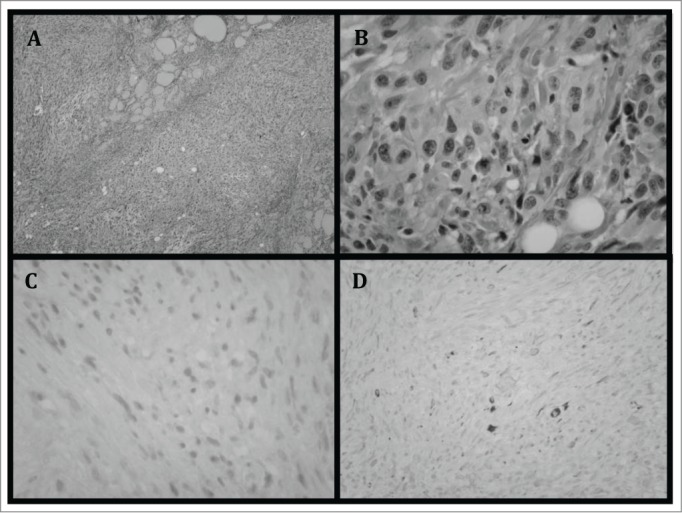
(A) Thyroid specimen demonstrating infiltration of tumor cells with lack of normal thyroid follicle arrangement. (B) Magnified view of the tumor cells demonstrating cells with atypical nuclei and lack of organization. (C) Tumor cells showing lack of staining with TTF-1. TTF-1 is expressed in follicular derived cells and is typically absent in poorly differentiated tumors.14 (D) Cells also demonstrate lack of staining with CK7. CK7 is expressed in more differentiated thyroid tumors, including papillary carcinomas, as well as some anaplastic carcinomas. Poorly differentiated tumors show lack of staining.14
.
The patient underwent adjuvant chemotherapy with weekly Taxol and Carboplatin and radiation for 7 weeks. A PET/CT obtained 2 months after completion of treatment was positive for multiple lung nodules as well as subcutaneous metastases (Fig. 2). Subsequent lung biopsies confirmed metastasis of the ATC. Molecular testing demonstrated a BRAF kinase mutation (Table 1). The patient then began therapy with vemurafenib, a BRAF kinase inhibitor FDA approved for use in melanoma. He received 960 mg orally twice daily with no drug related toxicities noted. There was an early clinical response, demonstrating a decrease in the subcutaneous metastasis, but follow up CT 2 months after initiation of vemurafenib showed rapid progression of disease with metastases to the CNS and esophagus with progression of his pulmonary metastasis. The patient's mental status soon began to decline. He was referred to hospice, and he subsequently died from his disease a few months later.
Figure 2.
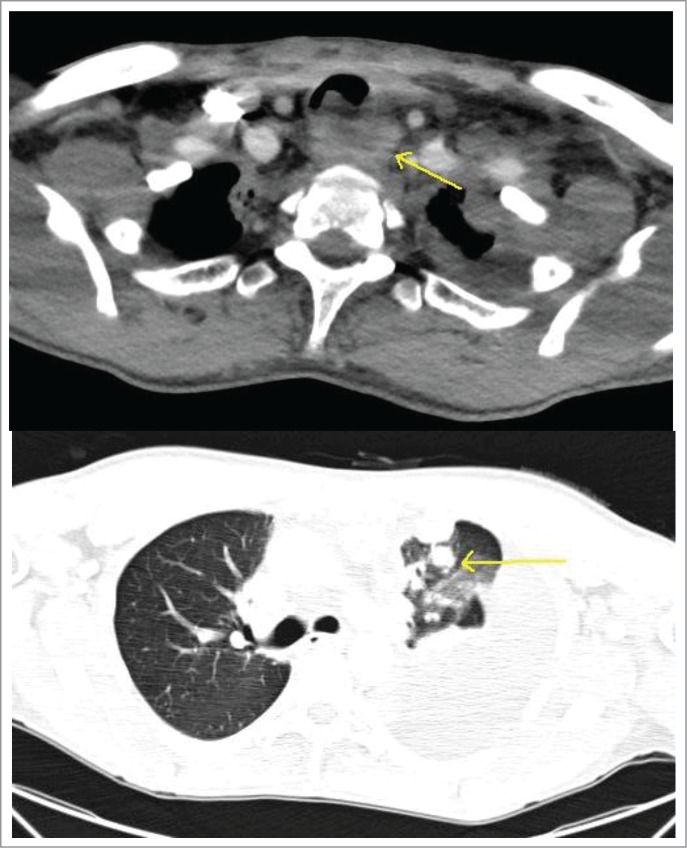
Above – CT scan of the chest demonstrating metastatic disease compressing the esophagus. Below - worsening pulmonary nodules with a massive left sided pleural effusion.
Table 1.
This table represents the genetic mutations discovered in our patient's tumor, completed through Foundation ONE, a DNA sequencing assay
| Mutation Detected | Function of Mutated Gene | Approved Therapy for ATC | Approved Therapy for other cancers |
|---|---|---|---|
| BRAFV600E | Activates the MAPK pathway resulting in cell division and differentiation | None | Dabrafenib Regorafenib Trametinib Vemurafenib |
| MITF | Encodes a protein required for pigment cell development in embryogenesis | None | Carbozantinib Crizotinib |
| MYC | Oncogene involved in cell-cycle regulation and cell growth. Mutation results in deregulation of target genes. | None | None |
| TP53 | Tumor suppressor involved in gene regulation, apoptosis, and DNA repair | None | None |
Discussion
Anaplastic thyroid carcinomas arise from a collection of oncogenic mutations, contributing to their poorly differentiated structure. Current hypotheses suggest that anaplastic cancers may arise from previously differentiated thyroid carcinomas that accumulate additional mutations within the cell cycle.4 Ultimately, the tissue undergoes unregulated growth and dedifferentiation.
Differentiated thyroid cancers, including follicular and papillary carcinomas, are successfully treated with thyroidectomy and radioactive iodine therapy. Unfortunately, this is not the case for anaplastic thyroid carcinomas.12 Retrospective studies suggest that current treatment with chemotherapy and radiation typically result in a median survival of only 6 months.5 Newer treatments are necessary in order to improve the prognosis of ATC.
Common mutations seen in ATC involve the BRAF, PTEN, and PI3KCA genes, as well as RAS and TP53, all of which are involved in cell cycle regulation. The BRAF kinase mutation is seen in 20% of cases of ATC.6 This mutation results in constitutive activation of the MAPK pathway and subsequent uncontrolled cell proliferation and growth.3 The specific mutation noted in this cancer was the BRAFV600E mutation, which is a glutamine for valine substitution. This mutation results in a 500-fold increase in activation of the MEK pathway (Fig. 3)
Figure 3.
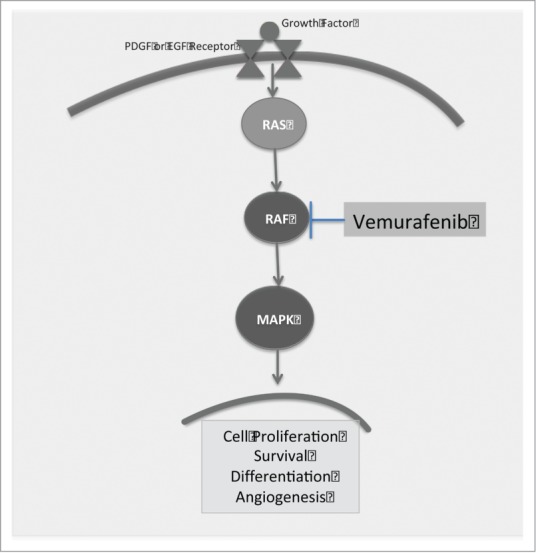
A simplified depiction of the MAPK pathway. Binding to the PDGF or EGF receptor results in activation of the RAS/RAF pathway and subsequent cell proliferation and differentiation. Blockade of the BRAF kinase with vemurafenib halts this process seen in the progression of many cancers.
.15
Vemurafinib, an inhibitor of the BRAF serine threonine kinase, is successful in treating melanoma patients with a BRAF mutation. In melanoma patients with this mutation that were treated with vemurafenib, there was a 63% reduction in the risk of death compared to standard therapy.7 To the authors' knowledge, vemurafenib has only been used in one other patient with ATC. After a poor response to chemotherapy, this patient was treated with vemurafenib and went into remission after 38 d.8 It is unknown as to how long he remained cancer free.
In many cases of anaplastic thyroid carcinoma with BRAF mutations, responses to BRAF inhibitors are of limited duration.9 Sorafenib, a tyrosine kinase inhibitor used in ATC has shown a partial response in patients with a history of papillary thyroid carcinoma that then converted to ATC. This drug targets several tyrosine kinases involved in cell cycle regulation, whereas Vemurafenib targets the BRAFV600E mutation specifically.16 The question remains as to why the response is not more extensive. It is hypothesized that subsequent mutations occur in pathways that bypass the BRAF blockade. In many anaplastic thyroid carcinomas, there is an additional mutation in another MAPK activator, the RAS oncogene; this additional trigger renders the blockade of BRAF kinase ineffective.9 To overcome this resistance in melanoma patients, a study was done with the BRAF inhibitor, dabrafenib, and a MEK inhibitor, trametinib. Combination therapy resulted in prolonged progression free survival.10 It has been hypothesized that by inhibiting the pathway further downstream, there will be less activation of alternate pathways.15 There is currently a clinical trial studying the response of combination therapy with dabrafenib and trametinib in anaplastic thyroid carcinoma.11 Unfortunately, our patient died before the advent of this combination therapy.
Anaplastic thyroid carcinoma represents a cancer that cannot be adequately treated with the standard therapies of thyroidectomy and chemoradiation. With the detection of specific mutations and the introduction of targeted therapies, the dedifferentiation process may be controlled or even prevented. Further research is necessary, though, to better understand the aberrant pathways and overcome resistance to current therapy. With additional insight, the hope is that patients with anaplastic thyroid carcinoma may have better outcomes in the future.
Summary
Anaplastic thyroid carcinoma is a poorly differentiated cancer with a dismal prognosis. Current therapy with surgery, chemotherapy, and radiation only has a median survival of 6 months. With the detection of specific mutations and targeted drug therapies, there is hope for improved survival in patients with ATC. Blockade of the BRAF kinase pathway with vemurafenib resulted in a successful, yet short-lived, response. Research on mechanisms of resistance to BRAF inhibitors as well additional activating pathways will promote the discovery of improved treatments for patients with anaplastic thyroid carcinoma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
Our sincere gratitude to the patient and his family for their permission to use the pictures and slides for this paper.
References
- 1.Smallridge R, Copland J. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol 2010. 22(6):486-97; PMID:20418080; http://dx.doi.org/ 10.1016/j.clon.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. “Update on anaplastic thyroid carcinoma: Morphological, molecular, and genetic features of the most aggressive thyroid cancer.” Int J Endocrinol 2014; 2014:790834; PMID:25214840; http://dx.doi.org/ 10.1155/2014/790834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G1 cell cycle progression and cancer. Cancer Sci 2006; 97(8):697-702; PMID:16800820; http://dx.doi.org/ 10.1111/j.1349-7006.2006.00244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikiforova M, Kimura E, Gandhi M, Biddinger P, Knauf J. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metabol 2003; 88(11):5399-404; PMID:14602780; http://dx.doi.org/ 10.1210/jc.2003-030838 [DOI] [PubMed] [Google Scholar]
- 5.Haymart M, Banerjee M, Yin H, Worden F. Marginal treatment benefit in anaplastic thyroid cancer. Cancer 2013; 119(17):3133-9; PMID:23839797; http://dx.doi.org/ 10.1002/cncr.28187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takano T, Ito Y, Hirokawa M, Yoshida H, Miyauchi A. BRAF V600E mutation in anaplastic thyroid carcinomas and their accompanying differentiated carcinomas. Br J Cancer 2007; 96:1549-53; PMID:17453004; http://dx.doi.org/ 10.1038/sj.bjc.6603764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. “Improved survival with vemurafenib in melanoma with BRAF V600E mutation.” N Engl J Med 2011; 364:2507-516; PMID:21639808; http://dx.doi.org/ 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosove MH, Peddi PF, Glaspy JA. “BRAF V600E inhibition in anaplastic thyroid cancer.” N Engl J Med 2013; 368:684-85; PMID:23406047; http://dx.doi.org/ 10.1056/NEJMc1215697 [DOI] [PubMed] [Google Scholar]
- 9.Guerra A, Di Crescenzo V, Garzi A, Cinelli M, Carlomagno C, Tonacchera M, Zeppa P, Vitale M. Genetic mutations in the treatment of anapestic thyroid cancer: a systematic review. BMC Surg 2013; 13(Suppl 2):S44; PMID:24267151; http://dx.doi.org/ 10.1186/1471-2482-13-S2-S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichenitser M, Dummer R, Grange F, Mortier L, et al.. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372(1):30-9; PMID:25399551; http://dx.doi.org/ 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 11.Efficacy and Safety of the Combination Therapy of Dabrafenib and Trametinib in Subjects With BRAF V600E- Mutated Rare Cancers (2013, December 5). Retrieved March17, 2015, from https://clinicaltrials.gov/ct2/show/NCT02034110 [Google Scholar]
- 12.Mazzaferri E, Jhiang S. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994; 97(5):418-28; PMID:7977430; http://dx.doi.org/ 10.1016/0002-9343(94)90321-2 [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey R. Foundation Medicine, Inc. 2011 [Google Scholar]
- 14.Fischer S, Asa S. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med 2008; 132:359-273; PMID:18318579 [DOI] [PubMed] [Google Scholar]
- 15.Cantwell-Dorris E, O'Leary J, Sheils O. BRAFV600E: implications for carcinogenesis and molecular thearpy. Mol Cancer Ther 2011; 10:385-94; PMID:21388974; http://dx.doi.org/ 10.1158/1535-7163.MCT-10-0799 [DOI] [PubMed] [Google Scholar]
- 16.Savvides P, Nagajah G, Lavertu P, Fu P, Wright J, Chapman R, Wasman J, Dowlati A, Remick SC. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013; 23:600-4; PMID:23113752; http://dx.doi.org/ 10.1089/thy.2012.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]


