Abstract
Objectives:
To quantitatively evaluate the relationship of vascularity of tongue cancer as demonstrated on intraoral ultrasonography images and tumour thickness with pathological grade of malignancy and the presence of cervical lymph node metastases.
Methods:
18 patients with tongue cancer were enrolled in this retrospective study. Using Doppler ultrasonography images of the invasion front of the cancers along the length of their tumour boundaries, three vascular indexes were analysed quantitatively, namely ratio of blood flow signal area within the cancer to whole tumour area (BAR), blood flow signal number ratio (BNR) and blood flow signal width ratio (BWR). The associations between these three indexes and occurrence of cervical lymph node metastasis and pathological grade of malignancy [Yamamoto–Kohama (YK) classification] were assessed. Furthermore, the relationship between tumour thickness and occurrence of cervical lymph node metastasis was evaluated on B-mode intraoral ultrasonography images.
Results:
There was no significant association between BAR and tumour thickness or occurrence of cervical lymph node metastasis. The BNRs and BWRs of patients with cervical lymph node metastasis were significantly higher than those of patients without nodal involvement. The BWRs of patients with high-grade malignancy (YK-4C) were significantly higher than those of patients with low-grade malignancy (YK-2 or 3).
Conclusions:
BNR and BWR on the invasion front of the tongue cancer are predictors of pathological grade of malignancy and cervical lymph node metastasis.
Keywords: Doppler ultrasonography, tongue cancer, metastasis
Introduction
Intraoral ultrasonography is a non-invasive and easy and effective means of evaluating the thickness of tongue cancer.1–5 Tumour thickness on intraoral ultrasonography images is reportedly an important predictor of cervical lymph node metastasis and patient outcome.4–8 Invasion front ratio calculated from the length of the invasive front on intraoral ultrasonography images is related to the histopathological grade.3 However, most studies have reported the morphological characteristics of tongue cancer, as assessed on B-mode intraoral ultrasonography images.
Tumour angiogenesis has an important effect on tumour growth and lymph node metastasis.9 Strong immunohistochemical expression of vascular endothelial growth factor, which causes tumour angiogenesis, is associated with higher clinical stage and worse overall survival in patients with head and neck squamous-cell carcinoma.10 High blood vessel density and histological grade of malignancy are significantly related to cervical lymph node metastasis in patients with oral squamous-cell carcinoma.11–13 Shinozaki et al5 reported that the presence of blood vessel infiltration is a determinant of prognosis of cervical lymph node metastasis and that Doppler-identified blood vessel infiltration and lymph duct infiltration have significantly different associations with prognosis. However, no studies have used Doppler intraoral ultrasonography images to quantitatively evaluate vascularity within tumours and in their invasion fronts.
The purpose of this study was to use intraoral ultrasonography images to quantitatively analyse the relationship of vascular findings on the invasion front and within tongue cancers and tumour thickness with pathological grade of malignancy and cervical lymph node metastasis.
Methods and materials
Patients
The subjects for this study were drawn retrospectively from 21 consecutive patients with tongue cancer (19 patients with squamous-cell carcinomas and 2 patients with verrucous carcinomas), who had undergone pre-operative Doppler intraoral ultrasonography and tumour resection at our hospital between April 2012 and October 2014. Because this was a retrospective study, we could not obtain prior informed consent from the study subjects. Instead, we published the study protocol on our hospital's website and stated that patients who did not agree to the use of their data would not be included. However, all patients agreed to use of their data. Two patients with superficial verrucous carcinomas (tumour thickness, 2–3 mm) were excluded from this analysis because the amount of noise in the blood flow signals on their Doppler intraoral ultrasonography images precluded evaluation. An additional patient was excluded because of failure to attend for follow-up. Thus, 18 patients with squamous-cell carcinoma were enrolled in this study. They comprised 16 males and 2 females, ranging in age from 26 to 90 years (median, 60.5 years) (Table 1).
Table 1.
Summary of characteristics of patients in this study
| Patient |
TNM classification | Pathological findings |
Follow-up period |
Ultrasonography findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Sex | Age (years) | Diagnosis | YK classification | Duration (months) | Subsequent metastasis | Recurrence of primary lesion | Tumour thickness (mm) | BAR (%) | BNR | BWR (%) | |
| 1 | M | 49 | cT1N0M0 | SCC | 2 | 41 | No | No | 6 | 0.30 | 0.0114 | 4.3 |
| 2 | M | 61 | cT1N0M0 | SCC | 2 | 40 | No | No | 5 | 55.23 | 0.0120 | 6.8 |
| 3 | F | 26 | cT1N0M0 | SCC | 2 | 39 | No | No | 3 | 0 | 0 | 0 |
| 4 | M | 90 | cT1N0M0 | SCC | 2 | 37 | No | No | 5 | 19.90 | 0.0091 | 9.1 |
| 5 | M | 82 | pT2N0M0 | SCC | 2 | 36 | No | No | 12 | 10.12 | 0 | 0 |
| 6 | M | 72 | pT4aN2bM0 | SCC | 4C | 35 | No | No | 17 | 5.36 | 0.0117 | 15.8 |
| 7 | M | 73 | pT2N1M0 | SCC | 3 | 34 | No | No | 10 | 3.81 | 0.0124 | 9.6 |
| 8 | M | 45 | pT3N1M0 | SCC | 3 | 30 | Yes (6)a | Yes (6)b | 19 | 21.11 | 0.0169 | 19.3 |
| 9 | M | 60 | cT1N0M0 | SCC | 4C | 28 | Yes (4)a | Yes (15)b | 3 | 9.36 | 0.0050 | 12.4 |
| 10 | M | 57 | cT1N0M0 | SCC | 3 | 24 | Yes (14)a | No | 5 | 52.28 | 0.0185 | 10.2 |
| 11 | M | 73 | cT1N0M0 | SCC | 2 | 22 | Yes (12)a | No | 5 | 57.72 | 0.0170 | 25.5 |
| 12 | M | 46 | cT2N0M0 | SCC | 3 | 19 | No | No | 5 | 1.49 | 0 | 0 |
| 13 | M | 53 | pT2N2bM0 | SCC | 4C | 19 | Yes (1)a | Yes (6)b | 10 | 41.70 | 0.0272 | 27.9 |
| 14 | M | 79 | pT4aN2bM0 | SCC | 3 | 17 | Yes (3)a | No | 18 | 9.34 | 0.0094 | 7.5 |
| 15 | M | 69 | pT3N1M0 | SCC | 3 | 16 | No | No | 17 | 27.45 | 0.0095 | 6.6 |
| 16 | M | 77 | cT1N0M0 | SCC | 3 | 16 | No | No | 2 | 36.62 | 0 | 0 |
| 17 | F | 57 | cT2N0M0 | SCC | 3 | 13 | No | No | 19 | 89.49 | 0.0067 | 14.3 |
| 18 | M | 26 | cT1N0M0 | SCC | 3 | 12 | No | No | 6 | 0 | 0 | 0 |
BAR, ratio of blood flow signal area within the cancer to whole tumour area; BNR, blood flow signal number ratio; BWR, blood flow signal width ratio; F, female; M, male; SCC, squamous-cell carcinoma; TNM, tumour–node–metastasis; YK, Yamamoto–Kohama.
Number indicates a period (months) from first operation when subsequent metastasis occurred.
Number indicates a period (months) from first operation when recurrence of primary lesion occurred.
The Yamamoto–Kohama (YK) classification was used for pathological grade of malignancy.6,14 This classification focuses on the shape of tumour-cell cords at the tumour–host interface and has five grades (1, 2, 3, 4C and 4D). The criteria for these five categories are as follows: grade 1, well-defined border; grade 2, cells in cords, less marked border; grade 3, groups of cells, no distinct border; grade 4C, involving a cord-like type of diffuse invasion; and grade 4D, involving a widespread type of diffuse invasion. According to the YK classification, no patients had YK-1 tumours, six patients had YK-2, nine patients had YK-3, three patients had YK-4C and none had YK-4D.
On pre-operative cervical lymph node staging by careful clinical examination, CT and ultrasonography, 7 of 18 patients had suspected cervical lymph node metastases and therefore underwent neck dissection (Patients 5–8 and 13–15). Nodal involvement was confirmed pathologically in six of these seven patients. There was no pre-operative evidence suggestive of metastases in the remaining 11 patients (Patients 1–4, 9–12 and 16–18).
The duration of follow-up ranged from 12 to 41 months (median, 26 months). During follow-up, additional cervical lymph node metastases developed in six patients (Patients 8–11, 13 and 14). Three of these patients had been staged as N0, when they underwent surgery for their primary lesions (Patients 9–11). The remaining three patients had undergone neck dissection during their first surgeries and were staged pathologically as pN1 or pN2b (Patients 8, 13 and 14). These three patients developed contralateral neck node metastases during follow-up.
No patients received pre-operative radiotherapy or chemotherapy.
Doppler intraoral ultrasonography
Intraoral ultrasonography was performed with a Sequoia 512 (Acuson, Mountain View, CA). The tongue cancers were imaged using a wide bandwidth linear transducer, 15L8w (Acuson, Maintain View, CA). The aperture of the transducer was 6-cm long and 0.5-cm wide. The common settings in B-mode were a central frequency of 12 MHz and gain of 80 dB. The settings in power Doppler mode were a central frequency of 10 MHz, velocity scale of 0.013 m s−1 and Colour Doppler gain of 40 dB.
Intraoral ultrasonography of the tongue was performed by coating a probe with echo jelly and covering it with disposable cling wrap. The probe was then placed parallel to the long axis of the tongue, which was gently held using a gauze; the operator used minimal pressure to achieve adhesion of the probe. Both B-mode and power Doppler ultrasonography images of the maximum cross-sectional area of the tumour were obtained (Figure 1). Furthermore, 3-s Doppler cine images consisting of about 40 still images were stored. The intraoral ultrasonography examinations were performed by three radiologists (CY, TS and KM).
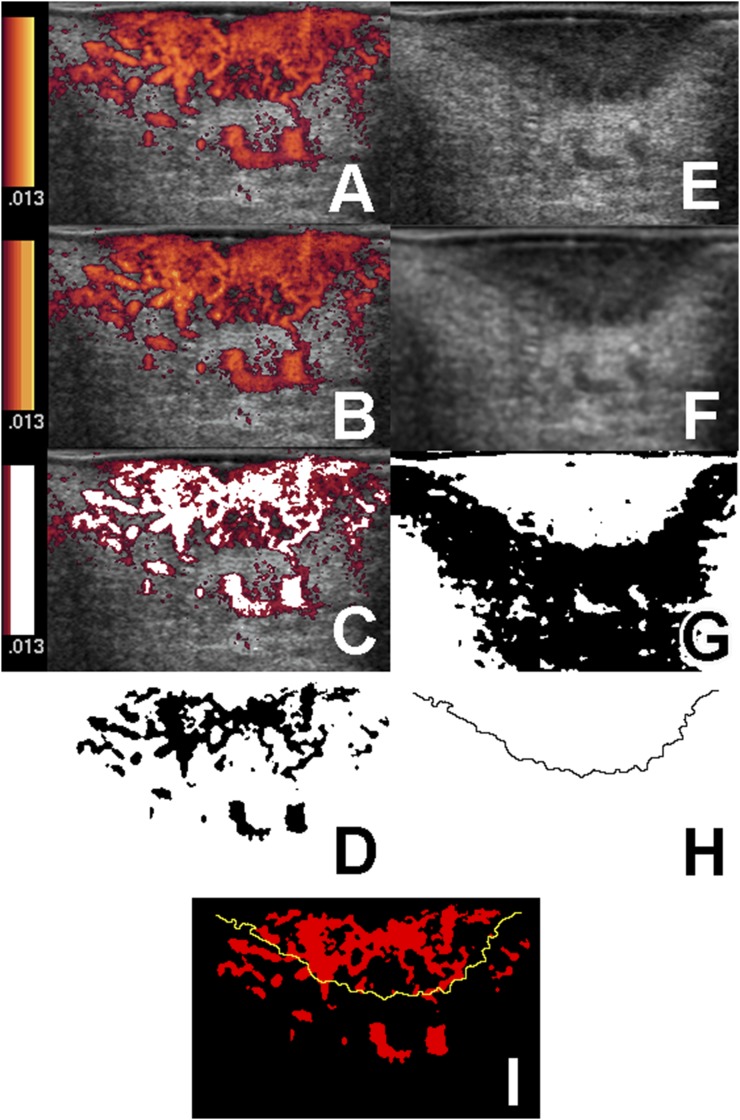
Figure 1.
Procedure for extracting genuine blood flow signals and tumour contours from intraoral ultrasonography images. (a) Original Doppler ultrasonography image (256 colours). The velocity range scale is shown to the left of the image. (b) The original Doppler ultrasonography image (a) has been converted into 64 colours. Colour reduction processing has been used to show the velocity range scale in nine stages of yellow–red. (c) The noise signals of three of nine stages in the velocity range scale. The noise signals are dark red, whereas genuine blood flow signals are shown in white. (d) Extracted blood flow signals. (e) An original B-mode ultrasonography image. (f) Median, mean and low-path filters have been applied to the original B-mode ultrasonography image (e) for noise reduction. (g) The image (f) was binarized by discriminant function analysis. (h) The tumour boundaries extracted using a Laplacian filter. (i) The extracted blood flow signals image (d) and tumour contour extracted tumour invasion front from image (h) have been superimposed.
Intraoral ultrasonography image analysis
All data were acquired in digital imaging and communications in medicine (DICOM) and transferred to the server of a picture archiving and communication system (Centricity PACS SE-J; GE Healthcare, Milwaukee, WI). The data were converted from (DICOM) to tagged image file formate (TIFF) format and transferred to a personal computer using CD-R. The TIFF images were analysed with Image J v. 1.48 software (National Institutes of Health, Bethesda, MD).15–17
The thickness of the maximum cut surface of the tongue cancers was measured on B-mode intraoral ultrasonography images.
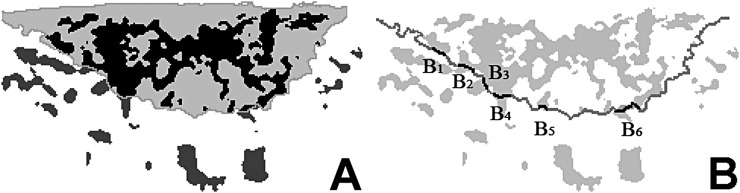
Power Doppler intraoral ultrasonography images of the maximum cut surface of the tongue cancers were analysed quantitatively using three vascular indexes that reflect the amount of tumour angiogenesis. These three indexes were obtained as follows (Figures 1, 2):
Figure 2.
Procedure for quantitative analysis of the cancers' blood flow signals. (a) The ratio of blood flow signal area within the cancer to whole tumour area defined as percentage of blood flow signals area within the cancer (black area) to those of the whole cancer area. (b) The blood flow signal number ratio defined as the ratio of the number of blood flow signals penetrating the tumour boundary divided by the full length of the tumour boundary. The blood flow signal width ratio defined as the sum of blood flow signal widths on the tumour boundary (B1+B2+B3+…+Bn) divided by the full length of the tumour boundary.
1. Extraction of genuine blood flow signals by removing noise signals from blood flow signals on Doppler intraoral ultrasonography images (Figure 1a–d).
Noise was defined as signals in the Doppler cine images that flashed on and off irregularly; velocity colours corresponding to these blinking signals were removed from the blood flow signals on the Doppler ultrasonography still images. However, because the velocity range scale of the original Doppler still images changed continuously from yellow to dark red, it was difficult to separate noise from genuine blood flow signals. Next, the original Doppler images (256 colours) were converted into images with 64 colours (Figure 1a,b). Colour reduction processing was used to display the velocity range scale in these 64-colour images with 8–12 stages of yellow to red, the noise signals being in two to four stages. This process resulted in extraction of genuine blood flow signals (white area in Figure 1c, black area in Figure 1d).
2. Extraction of boundary line of invasion front of tumours on B-mode intraoral ultrasonography images (Figure 1e–h).
The method for extracting the boundaries of tumour invasion on B-mode intraoral ultrasonography images was according to the processing procedure with the computer-aided diagnosis system described by Yamane et al.2 First, noise was reduced by applying median, mean and low-path filters to the original B-mode images (Figure 1e,f). We then binarized the images by discriminant function analysis (Figure 1g).18 Finally, the boundaries of the tumours were extracted using a Laplacian filter (Figure 1h).
3. Superimposition (Figure 1i) of extracted genuine blood flow signals (Figure 1d) and tumour boundaries (Figure 1h).
4. Quantitative analysis of tongue cancer blood flow signals.
The following three vascular indexes were calculated on the invasion fronts of the cancers along the lengths of the tumour boundaries: ratio of blood flow signal area within the cancer (BAR) to whole tumour area, blood flow signal number ratio (BNR) and blood flow signal width ratio (BWR).
BAR was defined as percentage of blood flow signals area within the cancer to whole cancer area (Figure 2a). BNR was defined as the ratio of the number of blood flow signals penetrating the tumour boundary divided by the full length of the tumour boundary according to the following equation: BNR = n/L, where n is the number of blood flow signals penetrating the tumour boundary and L is the full length of the tumour boundary. BWR was defined as the width ratio of blood flow signals penetrating the tumour boundary divided by the full length of the tumour boundary according to the following equation:
where B is the width on the tumour boundary (Figure 2b).
Associations of tumour thickness and these three indexes of cancer vascularity with pathological grade of malignancy and occurrence of cervical lymph node metastasis were then assessed.
Pathological grade of malignancy
Pathological grade of malignancy was classified using the YK classification6,14 and evaluated on haematoxylin-eosin-stained sections of the resected specimens by two examiners (CY and KO), including an oral pathology specialist. Because patients with YK-4C or 4D grade reportedly have a higher risk of tumour recurrence and cervical lymph node metastasis than patients with other grades (YK-1, 2 or 3),6,14 the study subjects were allocated to two groups according to YK classification (YK-4C and other grades).
Statistical analysis
All statistical analyses were performed on a personal computer using JMP® 11(SAS Institute, Cary, NC). Statistical significance was assessed using the Mann–Whitney U test. A p-value <0.05 was considered to indicate statistical significance. Receiver-operating characteristic (ROC) analysis was also performed using JMP 11. Accuracy of prediction of poor outcomes such as tumour recurrence and subsequent cervical lymph node metastasis during follow-up using BNR and BWR was evaluated by ROC analysis. The area under curve and asymptotic 95% confidence intervals were calculated by ROC curve analysis.
Results
Table 1 shows a summary of relevant patient characteristics and pathological and intraoral ultrasonography findings.
Relationships between tumour thickness and occurrence of cervical lymph node metastasis and pathological grade of malignancy
Tumour thickness in the nine patients with cervical lymph node metastases was 3–19 mm (median, 10 mm) and in the nine patients without cervical lymph node metastases, 2–19 mm (median, 5.5 mm); this difference was not statistically significant (p = 0.2104).
The tumour thickness of YK-4C grade tumours ranged from 3–17 mm (median, 10 mm) and of YK-2 or 3 grade tumours from 2–19 mm (median, 6 mm); this difference was not statistically significant (p = 1.0000).
Relationships between BAR and occurrence of cervical lymph node metastasis and pathological grade of malignancy
The BAR of nine patients with cervical lymph node metastases was 3.82–57.72% (median, 21.11%) and of nine patients without cervical lymph node metastasis, 0–89.49% (median, 10.12%); this difference was not statistically significant (p = 0.4265).
The BAR of patients with YK-4C grade tumours ranged from 5.36 to 41.70% (median, 9.36%) and those of patients with YK-2 or 3 grade tumours 0–89.49% (median, 19.90%); this difference was not statistically significant (p = 1.0000).
Relationships between BNR and occurrence of cervical lymph node metastases and pathological grade of malignancy
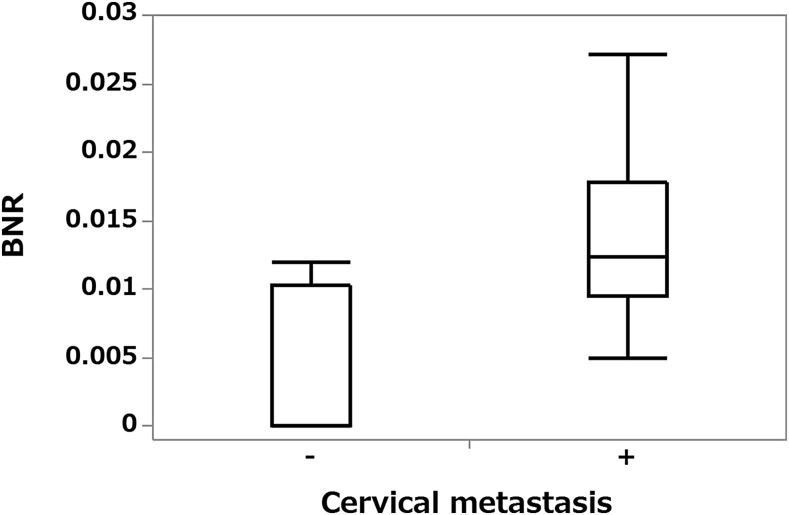
The BNR of nine patients with metastases at operation on their primary lesions or during follow-up or both was 0.005–0.0272 (median, 0.0124) and of nine patients without metastases 0–0.012 (median, 0). The BNR of patients with metastases was significantly higher than that of those without metastases (p = 0.0057) (Figure 3).
Figure 3.
Box plot graph showing the relationship between cervical lymph node metastases and blood flow signal number ratio (BNR). The BNR is significantly higher for patients with metastases than for those without them (p < 0.01).
The BNR of patients with YK-4C grade tumours ranged from 0.005 to 0.0272 (median, 0.0117) and of those with YK-2 or 3 grade tumours from 0 to 0.0185 (median, 0.00945); this difference was not statistically significant (p = 0.4020).
Relationships between BWR and occurrence of cervical lymph node metastases and pathological grade of malignancy
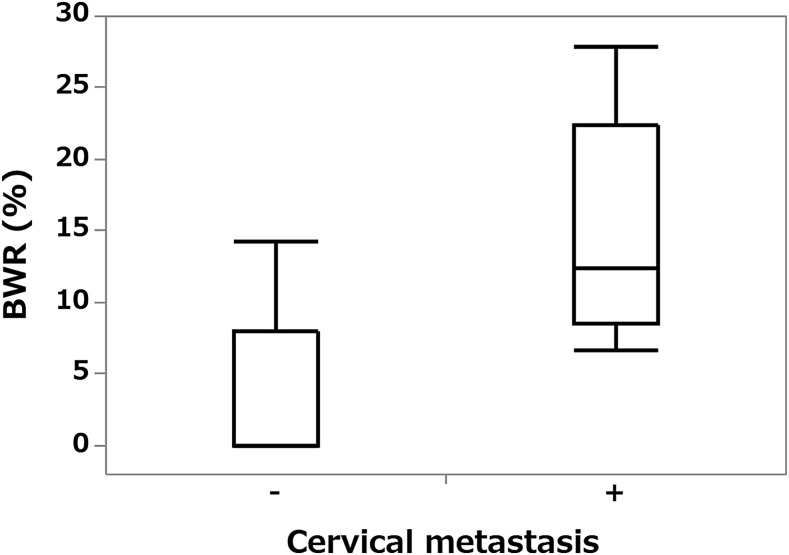
The BWR of nine patients with metastases at operation on primary lesion or during follow-up or both was 6.6–27.9% (median, 12.4%) and of nine patients without metastases 0–14.3% (median, 0%); this difference was statistically significant (p = 0.0043) (Figure 4).
Figure 4.
Box plot graph showing the relationship between cervical lymph node metastases and blood flow signal width ratio (BWR). The BWR is significantly higher for patients with metastases than for those without them (p < 0.01).
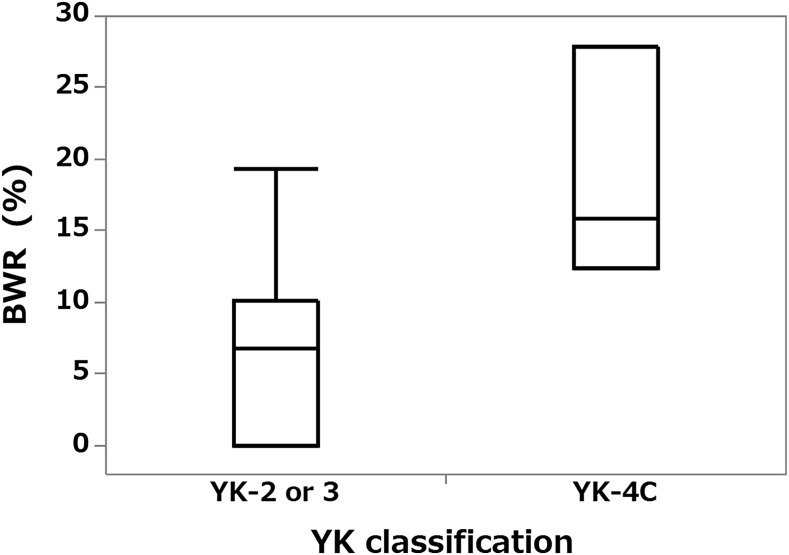
The BWR of patients with YK-4C grade tumours ranged from 12.4 to 27.9% (median, 15.8%) and of those with YK-2 or 3 grade tumours from 0 to 25.5% (median, 6.8%); this difference was statistically significant (p = 0.0418) (Figure 5).
Figure 5.
Box plot graph showing relationship between Yamamoto–Kohama (YK) classification and blood flow signal width ratio (BWR). The BWR was significantly higher for YK-4C grade tumours than for YK-2 or 3 tumours (p < 0.05).
Receiver-operating characteristic curve analyses
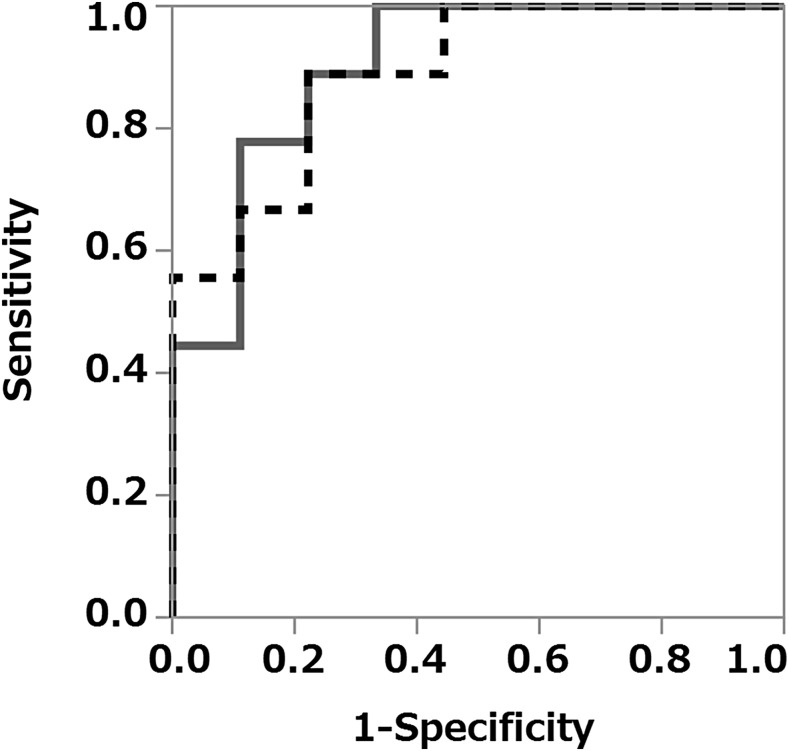
ROC analyses using BNR and BWR were performed to predict metastasis. The area under the curve of the BNR and BWR were 0.889 and 0.901, respectively (Figure 6). The cut-off value for the BNR was 0.00943, the sensitivity and specificity for predicting metastasis being 88.9% and 77.8%, respectively. Using a BWR cut-off of 6.6%, the sensitivity and specificity for predicting metastasis were 100% and 66.7%, respectively.
Figure 6.
Receiver-operating characteristic curves (ROC) for predicting cervical lymph node metastases using the blood flow signal number ratio (BNR) and blood flow signal width ratio (BWR). ROC using BNR (dashed line): the area under the curve (AUC) is 0.889, and the sensitivity and specificity for predicting metastases were 88.9% and 77.8%, respectively. ROC using BWR (solid line): the AUC was 0.901, and the sensitivity and specificity were 100% and 66.7%, respectively.
Discussion
Tumour angiogenesis affects tumour growth and metastasis progression;9 Okada reported that this is a predictor of metastasis.13 A high vascular density is reportedly associated with advanced tumour stage and a poor prognosis in oral cancers.11–13 We therefore postulated that quantitative evaluation of the vascularity of tongue cancers on Doppler intraoral ultrasonography images predict the grade of malignancy and occurrence of cervical lymph node metastasis and accordingly devise three quantitative indexes of vascularity, the BAR, BNR and BWR. The BAR, which is a measure of the quantity of blood flow within a cancer, did not differ significantly between patients with and without cervical lymph node metastases. However, both the BNR and BWR, which are measures of the quantity of blood flow at a cancer's invasion front, were significantly higher in patients with nodal involvement than in those without nodal involvement (Figures 3, 4).
High vascularity is considered to indicate a hypernutritional state that supports the high proliferative activity of cancer cells around the invasion front of a tumour, whereas such high vascularity is not necessary for nourishing cells within a carcinoma, such cells having a lower proliferative rate than those at the invasion front.
We assessed the ability of the BNR and BWR to predict cervical lymph node metastases. The sensitivity and specificity of the BNR were 88.9% and 77.8%, respectively, and of the BWR 100% and 66.7%, respectively. These values are better than those previously reported for tumour thickness, which had a sensitivity and specificity for predicting metastases of 64% and 100%, respectively, using a cut-off value of 5 mm and 100% and 61.5%, respectively, using a cut-off value of 3 mm.5,8 In the present study, there was no significant difference in tumour thickness between patients with and without nodal involvement.
Highly malignant tumours such as YK-4 tumours can cause cervical lymph node metastases and recurrence even when small (<5 mm) at initial diagnosis. We speculate that the prognosis is affected more strongly by the grade of malignancy than tumour thickness.
To evaluate the pathological grade of malignancy, we used the YK classification,6,14 which is widely used in Japan for oral cancers. The YK classification is a modified version of the Jakobsson et al19 and Willen et al20 classifications, which focus on the pattern of invasion at tumour margins, and is a predictor of cervical lymph node metastases and prognosis.2,3,5,6,14 Many studies have reported that patients with tumours of grade of YK-4C or 4D have significantly higher risks of tumour recurrence and cervical lymph node metastases than those with tumours of lesser grades (YK-1–3).6,14
The BWR of grade YK-4C tumours was significantly higher than that of tumour with lesser grades (YK-2 or 3), whereas the BAR and BNR did not differ significantly between these two groups. Therefore, we consider the BWR to be a useful index for predicting the pathological grade of malignancy.
The sensitivity of the BWR was high at 100%, but specificity was low at 66.7%, and the false-positive ratio was high. This is because not only the number of new blood vessels but also the width of these vessels should increase in the site where angiogenesis is prevalent; therefore, the BNR, which is an index used to indicate the number of blood vessels, more accurately reflects angiogenesis. Moreover, we consider that this may be the factor that raised a false-positive ratio; circulatory diseases such as hyperaemia or congestion that are unrelated to angiogenesis can easily increase blood vessel diameters. Further study is necessary.
In this study, the duration of follow-up ranged from 12 to 41 months, within 5 years. Post-operative development of cervical lymph node metastases reportedly occurs within 1 year after first surgery in 77–91% of all patients with oral cancer.21–26 Therefore, our duration of follow-up was long enough to evaluate the occurrence of subsequent cervical lymph node metastases.
In intraoral ultrasonography, an ultrasound probe designed for intraoral use which is compact and easy to use in scanning should be employed; however, deployment of these probes is not widespread, and many hospitals do not have one. The ultrasound device that we used did not have a specifically designed intraoral probe, and we chose a small linear-array probe for superficial imaging instead. It was similar in shape (T-shape) and size to the probe that Shinozaki et al5 employed for intraoral ultrasonography, and we could deploy it using a technique similar to that employed by these authors. We can substitute a probe that is not specifically designed for intraoral use, if we can clearly capture tumour boundaries and the blood flow signals around the invasion front of the tumour.
A limitation of our study was the small number of patients enrolled; we consider that a further larger study is necessary. If more subjects had been included in the study, it would follow that the statistical significance of the study results would be strengthened. The prediction of pathological grade of malignancy and the cervical lymph node metastasis using intraoral ultrasonography enables the choice of improved treatment for oral cancer and appropriate follow-up after treatment; we expect that the prediction of the pathological grade of malignancy and cervical lymph node metastasis using intraoral ultrasonography will enable the choice of improved treatments.
In conclusion, the indexes that we developed for this study reflect angiogenesis and tumour growth. Quantitative measures of vascularity around the invasion front of tongue cancers can predict the pathological grade of malignancy and cervical lymph node metastasis.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the institutional and national committees on human experimentation and the Helsinki Declaration of 1964 and later versions. Because this was a retrospective study, we could not obtain prior informed consent from the study subjects. The Ethics Committee of Fukuoka Dental College reviewed and approved the study protocol (No. 245).
Acknowledgments
Acknowledgments
We would like to express our gratitude to our colleagues in the Department of Oral and Maxillofacial Surgery at Fukuoka Dental College.
References
- 1.Helbig M, Flechtenmacher C, Hansmann J, Dietz A, Tasman AJ. Intraoperative B-mode endosonography of tongue carcinoma. Head Neck 2001; 23: 233–7. doi: http://dx.doi.org/10.1002/1097-0347(200103)23:3<233::AID-HED1024>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- 2.Yamane M, Ishii J, Izumo T, Nagasawa T, Amagasa T. Noninvasive quantitative assessment of oral tongue cancer by intraoral ultrasonography. Head Neck 2007; 29: 307–14. doi: http://dx.doi.org/10.1002/hed.20523 [DOI] [PubMed] [Google Scholar]
- 3.Kaneoya A, Hasagawa S, Tanaka Y, Omura K. Quantitative analysis of invasive front in tongue cancer using ultrasonography. J Oral Maxillofac Surg 2009; 67: 40–6. doi: http://dx.doi.org/10.1016/j.joms.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 4.Chammas MC, Macedo TAA, Moyses RA, Gerhard R, Durazzo MD, Cernea CR, et al. Relationship between the appearance of tongue carcinoma on intraoral ultrasonography and neck metastasis. Oral Radiol 2011; 27: 1–7. [Google Scholar]
- 5.Shinozaki Y, Jinbu Y, Ito H, Noguchi T, Kusama M, Matsumoto N, et al. Relationship between appearance of tongue carcinoma on intraoral ultrasonography and histopathologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 634–9. doi: http://dx.doi.org/10.1016/j.oooo.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck 1984; 6: 938–47. doi: http://dx.doi.org/10.1002/hed.2890060508 [DOI] [PubMed] [Google Scholar]
- 7.Byers RM, EI-Naggar AK, Lee YY, Rao B, Fornage B, Terry NHA, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck 1997; 20: 138–44. doi: http://dx.doi.org/10.1002/(SICI)1097-0347(199803)20:2<138::AID-HED7>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Ito J, Taira S, Katsura K. The relationship of primary tumor thickness in carcinoma of the tongue to subsequent lymph node metastasis. Dentomasillofac Radiol 2001; 30: 242–5. doi: http://dx.doi.org/10.1038/sj/dmfr/4600615 [DOI] [PubMed] [Google Scholar]
- 9.Ellis LM, Fidler IJ. Angiogenesis and metastasis. Eur J Cancer 1996; 32: 2451–60. [DOI] [PubMed] [Google Scholar]
- 10.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 2005; 131: 624–30. doi: http://dx.doi.org/10.1007/s00432-005-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannen EJM, Riediger D. The quantification of angiogenesis in relation to metastasis in oral cancer: a review. Int J Oral Maxillofac Surg 2004; 33: 2–7. doi: http://dx.doi.org/10.1054/ijom.2003.0433 [DOI] [PubMed] [Google Scholar]
- 12.Schimming R, Reusch P, Kushnierz J, Schmelzeisen R. Angiogenic factors in squamous cell carcinoma of the oral cavity: do they have prognostic relevance? J Craniomaxillofac Surg 2004; 32: 176–81. doi: http://dx.doi.org/10.1016/j.jcms.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Okada Y. Relationships of cervical lymph node metastasis to histopathological malignancy grade, tumor angiogenesis, and lymphatic invasion in tongue cancer. Odontology 2010; 98: 153–9. doi: http://dx.doi.org/10.1007/s10266-010-0131-6 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto E, Kohama G, Sunakawa H, Iwai M, Hiratsuka H. Mode of invasion, Bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer 1983; 51: 2175–80. doi: http://dx.doi.org/10.1002/1097-0142(19830615)51:12<2175::AID-CNCR2820511205>3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 15.Rasband WS. ImageJ 1997–2013. Bethesda, MD: U. S. National Institutes of Health. Available from: http://imagej.nih.gov/ij/ [Google Scholar]
- 16.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–5. doi: http://dx.doi.org/10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 2004; 11: 36–42. [Google Scholar]
- 18.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 1979; 9: 62–6. doi: http://dx.doi.org/10.1109/TSMC.1979.4310076 [Google Scholar]
- 19.Jakobsson PA, Eneroth CM, Killander D, Moberger G, Martensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol 1973; 12: 1–8. doi: http://dx.doi.org/10.3109/02841867309131085 [DOI] [PubMed] [Google Scholar]
- 20.Willen R, Nathanson A, Moberger G, Anneroth G. Squamous cell carcinoma of the gingiva. Histological classification and grading of malignancy. Acta Otolaryngol 1975; 79: 146–54. [DOI] [PubMed] [Google Scholar]
- 21.Tewari M, Rai P, Singh GB, Kumar M, Shukla HS. Long-term follow-up results of Nd: YAG laser treatment of premalignant and malignant (stage I) squamous cell carcinoma of the oral cavity. J Surg Oncol 2007; 95: 281–5. doi: http://dx.doi.org/10.1002/jso.20677 [DOI] [PubMed] [Google Scholar]
- 22.Takada K, Endo K, Nakamura K, Sakamoto T, Moroyama T, Yoshiga K. Clinicostatistical investigation on the cervical lymph node metastasis of oral malignant tumor. Jpn J Oral Maxillofac Surg 1988; 34: 872–8. doi: http://dx.doi.org/10.5794/jjoms.34.872 [Google Scholar]
- 23.Umeda M, Omori A, Yokoo S, Teranobu O, Nakanishi K, Shimada K. A clinicopathological study of secondary neck metastasis of oral squamous cell carcinoma. Jpn J Oral Maxillofac Surg 1991; 37: 143–51. doi: http://dx.doi.org/10.5794/jjoms.37.143 [Google Scholar]
- 24.Kurokawa H, Murata T, Yamashita Y, Miura K, Tokudome S, Yoshikawa T, et al. Clinicopathological evaluation of secondary cervical lymph node metastasis in oral squamous cell carcinoma. Jpn J Oral Maxillofac Surg 1997; 43: 661–6. doi: http://dx.doi.org/10.5794/jjoms.43.661 [Google Scholar]
- 25.Kirita T, Okabe S, Yagihara K, Matsuki K, Endoh T, Matsuki S, et al. Secondary cervical lymph node metastases of squamous cell carcinoma of the head and neck. Jpn J Oral Maxillofac Surg 1993; 39: 1320–9. doi: http://dx.doi.org/10.5794/jjoms.39.1320 [Google Scholar]
- 26.Harada H, Tei K, Makino S, Satoh A, Hanzawa M, Yamashita T, et al. Mode of metastasis and treatment outcome of patients with secondary cervical lymph node metastases. Jpn J Oral Maxillofac Surg 1998; 44: 508–10. doi: http://dx.doi.org/10.5794/jjoms.44.508 [Google Scholar]