Abstract
Objectives:
Bisphosphonate-associated osteonecrosis of the jaw (BP-ONJ) is a side effect of antiresorptive treatment that is increasingly prescribed for patients with osteoporosis or malignant diseases with bone metastases. Surgical treatment of BP-ONJ requires adequate pre-operative imaging. To date, CT is the imaging standard in clinical routine; however, defining the extent of the pathological area is difficult and soft tissues are poorly displayed. MRI with zero echo time (ZTE-MRI) to display hard tissues enables a precise display of calcified structures and soft tissues for the delineation of bone necrosis and soft-tissue reactions.
Methods:
BP-ONJ was induced in eight sheep by extraction of two premolars in the left mandible and zoledronate (ZOL) administration. Eight sheep without ZOL administration served as the control group. Four sheep of each main group underwent osteopenia induction via ovariectomy, glucocorticoid administration and a calcium-free diet. After sacrifice, the area of tooth extraction was harvested and scanned with micro-CT (µCT) and ZTE-MRI. Two trained dentists analyzed digital imaging and communications in medicine data sets using three-dimensional imaging software. The periosteal reaction and the remaining extraction sockets were measured.
Results:
BP-ONJ was evident, and the remaining extraction sockets were observed in all animals treated with ZOL. Periosteal reactions were more pronounced in animals treated with ZOL, and they appeared broader in ZTE-MRI.
Conclusions:
BP-ONJ lesions in the sheep mandible can be detected using µCT and ZTE-MRI. Although illustration of sequester was more consistent using the µCT, ZTE-MRI was advantageous in evaluation of periosteal reaction.
Keywords: bisphosphonate-associated osteonecrosis of the jaw, magnetic resonance imaging, x-ray microtomography, periosteum, animal model
Introduction
Bisphosphonate-associated osteonecrosis of the jaw (BP-ONJ) is a side effect of antiresorptive treatment. BP-ONJ was firstly described in 2003 in relation to bisphosphonate treatment [zoledronate (ZOL) and pamidronate] of bone metastases. Its growing prevalence is due to an increase of the prescription of antiresorptive medication in different indications including osteoporosis and myeloma. Regarding a growing number of patients who develop similar bone necrosis after treatment with the receptor activator of nuclear factor kappa-B ligand antibody Denosumab and several cases of bone necroses in patients receiving tyrosin kinase inhibitors, the hypernym medication-related osteonecrosis of the jaw has been proposed.1
Even though most lesions occur after invasive dental procedures such as tooth extractions, the exact pathomechanism is not elucidated yet. In patients with progressive periodontal lesions, exposed bone can be seen before tooth removal. Also, non-exposed variants of the condition that can be visualized using single-photon emission tomography with low dose single slice CT or MRI have been published.2,3 As bisphosphonates are mainly enriched in the bone that underlies remodeling and the remodeling rates in the alveolar processes seem to be higher than in other bones, it is most likely that the local concentration of bisphosphonates in the alveolar processes is elevated.4,5 High levels of bisphosphonates have been shown to lead to apoptosis of osteoclasts.6 Given that especially in bones with a high turnover, a certain level of osteoclastic activity is necessary to maintain the tissue's integrity, a lack of osteoclasts leads to osteonecrosis. Otto et al7 describe an interaction of different factors: an oral infection or dentoalveolar surgery may lower the local pH value of the bone and lead to release and activation of toxic levels of bisphosphonates; following which the influences on osteoclasts (suppressed bone remodeling), immune cells (specific infection), angiogenesis (ischaemia) and mucosa (soft-tissue toxicity) result in BP-ONJ.
Osteolytic lesions with cortical bone destruction, osteolysis, sequestrum formation and sclerotic regions with periosteal bone proliferation are typical for BP-ONJ.8–12 Surgical procedures include the resection of necrosis in advanced stages (American Associations of Oral and Maxillofacial Surgeons; AAOMS) and the early surgical approach and safe wound closure.13 Surgical procedures require precise pre-operative imaging to define necrotic areas and delineate resection margins.
Conventional two-dimensional radiography does not deliver information on the spatial dimensions of necrotic areas. However, panoramic radiography together with three-dimensional CT is the most frequently used imaging techniques in clinical routine.14 Other imaging modalities for the detection of BP-ONJ are multislice CT,15 CBCT10,11,16 and MRI.12,15–17 Hybrid single-photon emission tomography with low-dose single-slice CT and scintigraphy enables detection of BP-ONJ in pre-clinical stages;3 however, scintigraphy has a low spatial resolution and does not allow for metric analysis of lesions.12
MRI is a non-ionizing method with superior soft-tissue contrast compared with radiographic techniques. However, in conventional MRI with T1 and T2 weighted contrast, BP-ONJ lesions show a variable signal.18 Areas of exposed bone are associated with low signal in T1 and T2 weighted images, whereas soft tissues with concomitant oedema and inflammation cause a high-signal T2 contrast.15 Compact bone and teeth are as well associated with a low signal due to fast signal decay and are poorly displayed. By reducing the time between signal excitation and detection to a few microseconds, MRI with zero echo time (ZTE-MRI),19 it was possible to depict dentin, cementum and enamel of extracted teeth with a voxel volume of 150 µm3 using small-bore animal MR systems at 7.0 T and 9.4 T.20,21 Therefore, ZTE-MRI was chosen as an imaging tool for the detection of soft- and hard-tissue alterations in BP-ONJ.
Hitherto, numerous groups have used small animal models to study the pathogenesis of BP-ONJ. However, because rodents have distinct differences in bone architecture and biology, large animal models are needed. Sheep have been proposed and used as animal models for in vivo studies of bone metabolism. The induction of osteopenia with morphological changes of the spine and jawbone has been documented.22,23 The occurrence of BP-ONJ after tooth extraction and spontaneous exposition of bone was demonstrated in healthy and osteopenic ewes under ZOL administration.24 The protocol for induction of osteopenia in sheep suggested by Veigel et al23 was adopted in this study.
The aim of the study was to display morphological changes in sheep with BP-ONJ using ZTE-MRI and micro-CT (µCT) and to compare the diagnostic abilities of both imaging methods with regard to hard- and soft-tissue alterations. The hypothesis was that with both imaging modalities, the morphological changes were displayed in the same way. Control groups without osteopenia and with bisphosphonate administration as well as with osteopenia and without bisphosphonate administration were established.
Methods and materials
Animal model
All procedures within the study were carried out in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility according to the Swiss laws of animal welfare and were approved by the local animal welfare commission of the official veterinary authorities (authorization number 2012_10).
16 adult female Swiss mountain sheep were randomly divided into 2 groups of equal size (Group 1 and Group 2). Inclusion criteria were adulthood, weight between 55 and 70 kg and general health, assessed using clinical and blood examinations. All animals had a 2-week acclimatization period prior to the experimental phase. The weight of the animals was assessed at entry and on a weekly basis during the experimental phase. During post-operative care, an everyday score for behaviour, breathing, feeding, temperature and wound healing was measured. Animals were fed a maintenance ratio of hey and had water ad libitum.
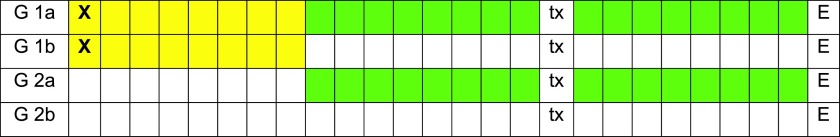
In Group 1, eight animals underwent ovariectomy, followed by 16 weeks of glucocorticoid induction with weekly injections of 54 mg of dexamethasone and 1.5 ml of dexamethasone sodium phosphate and a low calcium diet. In Group 2, eight animals served as controls. These two main groups were again randomly divided into subgroups of four animals (Groups 1a, 1b, 2a and 2b). In Group 1a and 2a, ZOL (75 μg kg−1) was administered intravenously every third week for ten times. In Group 1b and 2b, no ZOL was administered. After the fifth ZOL infusion, or at a corresponding time point in the control groups, two premolars of the left mandible were extracted. The extraction sites of the second premolars were investigated. The treatment schedule in each group is displayed in Figure 1.
Figure 1.
Overview of the time schedule of interventions in each group [Group 1a (G1a), Group 1b (G1b), Group 2a (G2a) and Group 2b (G2b)] in which each square represents 2 weeks (yellow = low calcium diet, dexamethasone infusion; X = ovariectomy, green = zoledronate infusion, tx = tooth extraction, E = euthanasia). For colour image see online.
In Group 1a, two animals had to be excluded from the study because of severe mastitis with systemic illness and excessive bone loss, respectively. The first animal was excluded during the induction of osteopenia before the first ZOL injection; the second animal was excluded prior to tooth extraction after having received five ZOL injections. One animal in Group 1b received an accidental ZOL infusion and was therefore excluded from the study. Two animals remained in Group 1a, and three animals remained in Group 1b.
18 weeks after tooth extraction, all animals were euthanized and the region of tooth extraction was harvested. After removal of the outer skin and underlying tissues, the region mesially of the second premolar and distally of the incisors was cut with a saw. Specimens were fixed in a buffered 4% formaldehyde solution.
Clinical examination
An experienced animal caretaker and a veterinarian did a weekly clinical evaluation during the course of the study. At euthanasia, clinical images were taken and evaluated by two maxillofacial surgeons using the BP-ONJ stage description by Ruggiero et al.1
Micro-CT
All 13 specimens were scanned using cone beam µCT (Viva CT 40; Scanco Medical AG, Brüttisellen, Switzerland). The specimens were removed from the fixation solution for scanning and positioned perpendicular to the X-ray beam, hence the sagittal axis of the mandible was oriented along the axis of rotation of µCT. The size of the image matrix was 1024 × 1024 pixels, resulting in an isotropic voxel size of 30 µm (slice thickness 30 µm, distance 30 µm) and a field of view with a diameter of 38 mm. The acquisition time was 250 ms with 500 projections at 70 kV and 114 µA.
Zero echo time MRI
Specimens were removed from the fixation solution and placed on a mount for MRI measurements. All MR experiments were performed on a 9.4-T small-bore animal system (BioSpec® 94/20; Bruker BioSpin, Ettlingen, Germany) using a 1H quadrature bird-cage whole volume mouse coil with 35 mm inner diameter for excitation and detection. Three-dimensional ZTE imaging was performed with an isotropic resolution of 117 µm in 30 min. Additional MR parameters are: delay between excitation and detection Δ = 10.9 μs at echo time = 0, repetition time = 5 ms, field of view = (30 mm)3, matrix size (256)3, bandwidth = 200,000 Hz, averages = 9, polar undersampling = 5.28, flip angle = 5.2° and projections = 39,134.
Fusion of zero echo time MRI and μCT data
Data derived from ZTE-MRI and μCT were available in a digital imaging and communications in medicine format and were imported into the software VoXim® (IVS Solutions, Germany). Corresponding data sets of each dissected jaw were aligned according to anatomical landmarks using a manual alignment tool. The aligned data sets were positioned within an individual co-ordinate system for further measurement (x-axis = axial trough the mandible (Figures 2 and 3); y-axis = sagittal through the mandible; z-axis = transversal through the mandible). The origin of the co-ordinate system was located at the most caudal point of the mandible (Figure 4).
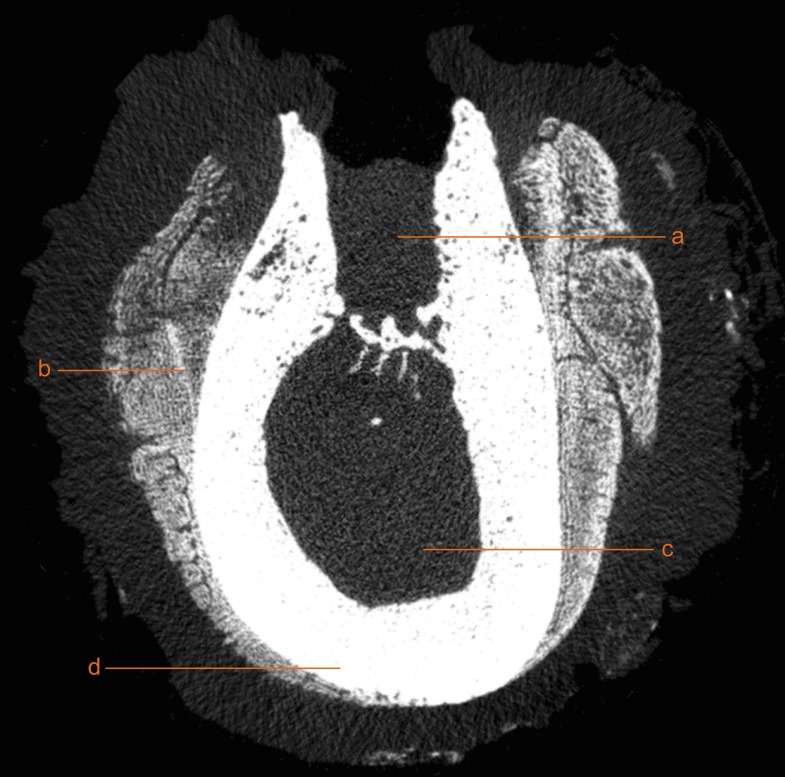
Figure 2.
Coronal cross-section of a specimen scanned using micro-CT with anatomical and pathological structures. a, remaining extraction socket; b, periostal reaction; c, bone marrow; and d, cortical bone.
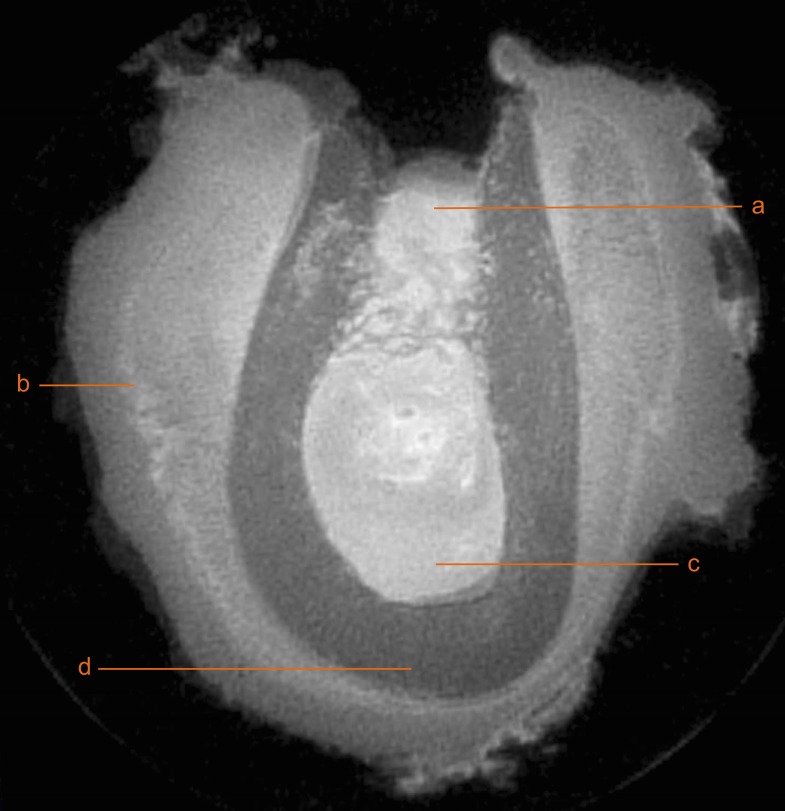
Figure 3.
Coronal cross-section scanned using zero echo time MRI. a, remaining extraction socket; b, periostal reaction; c, bone marrow; and d, cortical bone.
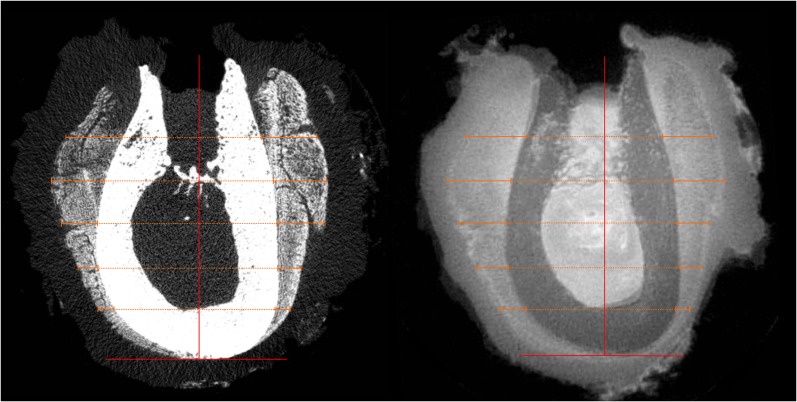
Figure 4.
Alignment of the specimen scanned within an individual co-ordinate system. The x-axis is oriented axial to the mandible at the most caudal point of the mandible, the y-plane is oriented sagittal to the mandible and located in the centre of the extraction socket (vertical lines). The periosteal reaction is measured in the coronal cross-sections of micro-CT (left) and MRI (right) with a distance of 3 mm between each measuring plane (horizontal lines).
Measurement of the periosteal reaction
The periosteal reaction was measured at different levels of the specimen. Parallel lines to the axial plane were drawn from the most caudal part of the jaw to the most crestal point with a distance of 3 mm in one slice of the extraction socket. At each parallel line, the vestibular and oral periosteal reaction was measured (Figure 4). Two examiners (E1 and E2) trained in medical image diagnostics performed measurements. Each of the examiners repeated the measurements three times (t1, t2 and t3) at least 2 weeks subsequent to the previous measurement.
Statistical evaluation
The periosteal reaction measured in MR and CT images are stated as estimated marginal means with 95% confidence intervals (CIs) to summarise the measurements of each examiner at each time point.
Measurements of periosteal reaction on the buccal and oral aspect of the alveolar bone were correlated with Bland–Altman plots for the two examiners (E1 and E2) and for the two imaging modalities MRI and μCT. The arithmetic mean of the differences is stated with 95% CI. The oral and vestibular measurements were considered as dependent variables and were therefore evaluated separately. The interclass correlation coefficient with 95% CI was calculated for the measurements of each examiner at the different time points (t1, t2 and t3).
Results
Clinical findings
In all animals treated with ZOL (Groups 1a and 2a), BP-ONJ with exposed and necrotic bone, infection and purulent drainage was observed. In the control groups, the extraction sites healed uneventfully.
Imaging findings
The extraction sites in osteopenic and healthy sheep without bisphosphonate medication (Groups 1b and 2b) showed a complete bony regeneration and an intact cortical bone layer after regeneration. Radiography and MRI of extraction sockets in sheep treated with ZOL showed remaining extraction sockets and a periosteal reaction. In osteopenic sheep without BP-ONJ, a periosteal reaction was observed.
The interclass correlation coefficient calculated for repeated measurements and for the two examiners showed values between 0.972 (CI 0.93–0.991) and 0.996 (CI 0.99–0.999) for MRI and between 0.929 (CI 0.758–0.989) and 0.997 (CI 0.988–1) showing a high agreement between examiners and time points of measurement.
The estimated marginal means of the repeated measurements by two examiners were calculated for the comparison of specimens. In Group 1b, only MRI measurements were available. In μCT cross-sections, the periosteal reaction was not assessable because it was not visible. In Group 2b, the periosteal reaction was not detectable in all μCT images of four specimens and on MR images of one specimen. All estimated marginal means are stated in Table 1.
Table 1.
Estimated marginal means and confidence intervals on vestibular (vest) and oral aspects in Groups 1a, 1b, 2a and 2b stated in millimeters
| Image | Group 1a |
Group 1b |
Group 2a |
Group 2b |
||||
|---|---|---|---|---|---|---|---|---|
| vest | oral | vest | oral | vest | oral | vest | oral | |
| MRI | 2.37 (1.69 to 3.04) | 1.58 (0.48 to 2.67) | 0.4 (−0.15 to 0.95) | 0.42 (−0.48 to 1.3) | 2.36 (1.89 to 2.84) | 1.96 (1.19 to 2.74) | 0.33 (−0.22 to 0.88) | 0.26 (0.63 to 1.16) |
| Micro-CT | 1.7 (0.38 to 2.96) | 1.23 (−0.36 to 2.82) | – | – | 1.83 (0.92 to 2.74) | 1.65 (0.53 to 2.77) | – | – |
Periosteal reaction was not significantly different between Groups 1a and 2a, as well as Groups 1b and 2b. However, there was a significant difference between the periosteal reaction in Groups 2a and 2b. The difference of periosteal reaction in Groups 1a and 1b was only detected on the vestibular aspect but not on the oral aspect.
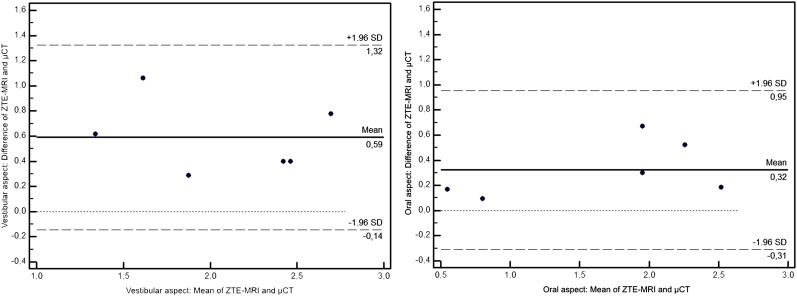
The difference between mean measurements in MRI and μCT images was 0.59 mm (CI 0.46–0.72 mm) on the vestibular aspect and 0.32 mm (CI 0.21–0.43 mm) on the oral aspect. The measurements for periostal thickness in μCT were lower than in MR images (Figure 5a,b).
Figure 5.
Bland–Altman plots of the difference between the mean MRI and mean micro-CT (μCT) measurements on the vestibular and oral aspects. Each dot represents a measurement of one examiner at one time point (overall two examiners at each three time points). SD, standard deviation; ZTE-MRI, zero echo time MRI.
The difference of the mean measurements made by E1 and E2 were 0.05 mm (CI ×0.03–0.13 mm) for the oral aspect in MRI and 0.03 mm (CI −0.07–0.13 mm) for the vestibular aspect in MRI. For μCT measurements, the difference was −0.07 mm (CI −0.16–0.01 mm) for vestibular measurements and −0.05 mm (CI −0.16–0.06 mm) oral measurements (Figure 5).
Discussion
The present study used μCT and ZTE-MRI to quantify and compare morphological alterations in corresponding cross-sections of identical specimens with BP-ONJ. μCT was previously described as an adequate technique to display morphological changes of the jawbone;23 however, it is limited by a poor soft-tissue contrast. ZTE-MRI was successfully used by Weiger et al25 to depict the trabecular microstructure of bone after dehydration and removal of soft tissue. This study examines the display of hard and soft tissues with ZTE-MRI and μCT.
BP-ONJ was successfully induced in sheep treated with ZOL, and the clinical picture of BP-ONJ could be correlated with the image of non-mineralized extractions sockets in μCT and MRI. These findings are in accordance with Farias et al9 and Bisdas et al,18 who described remaining extraction sockets among other radiological findings such as destruction of cancellous bone, sequestration, erosion of cortical bone in their patient collectives. The specimens were segmented in μCT with the help of gray values to detect other morphological changes than the remaining extraction sockets. Owing to the small specimen size with a focus on the extraction socket, sequestra formation and osteolysis could not be outlined to their full extent. Therefore, a quantification of these findings was not possible.
ZTE-MRI and µCT data displayed a reaction of the periosteum around tooth extraction sites in osteopenic sheep after the administration of bisphosphonates. To the knowledge of the authors, this is the first study that quantified and compared the periosteal reaction in BP-ONJ between two imaging modalities.
The periosteal reaction is characterized by the deposition of newly formed bone on the cortical bone surface in response to local insult or generalized processes. Therefore, it is only visible in CT after the mineralization of the newly formed bone.26
Previously, several authors8,11,18 detected periosteal reactions with CT, characterized by new periosteal bone formation but did not quantify their findings. The number of patients with periosteal reactions was relatively low in relation to the study collective.8 The infrequent documentation of periosteal reaction could be due to the effect that only mineralized parts of the newly formed bone were visible.26 In a patient collective with late stages of the disease, a higher number of periosteal reactions was documented.18
The findings of this study confirm differences in the visibility of periosteal reactions between μCT and ZTE-MRI. A periosteal reaction was not visible in μCT and MRI cross-sections of all specimens. In the control groups, a periosteal reaction could not be assessed in seven specimens in μCT and in one specimen in MRI, respectively. The periosteal reaction measured in μCT had a minimum width of 0.5 mm. Therefore, the extent of periosteal bone formation might have been underestimated and less often detected in μCT compared with MRI. The threshold for detection of alterations was not regarded as a result of the spatial image resolution and voxel size, respectively, as it was higher for μCT (30 μm) than was for MRI (117 μm). However, ZTE-MRI was not only able to display hard-tissue but also soft-tissue alterations such as reactive periosteal hypertrophy and newly formed bone in mineralized and non-mineralized stages.
Previous studies on imaging of BP-ONJ used CBCT,10,16 CT,8,15,18 positron emission tomography CT16 and MRI12,15–18 and focused on the display of osteolytic areas, osteosclerosis, sequestra formation and erosion of the cortical bone.8,9,11,12,16 As a result, the extent of morphological changes in BP-ONJ may not be reliably assessed using the current imaging methods.8,15 The heterogeneity of imaging protocols, the differing stages of the disease and the individual and subjective assessment protocols in the mentioned studies do not allow a general conclusion for the radiographic visibility of BP-ONJ.
BP-ONJ in osteopenic and healthy sheep, resulted in comparable periosteal reactions. In healthy sheep with and without BP-ONJ, a significant difference of the periosteal reaction was observed. In osteopenic sheep with and without BP-ONJ, a statistical difference of the periosteal reaction was only calculated for the vestibular aspects of the specimens. This effect was observed despite deviating means and could be due to the small sample size in Group 1a that was caused by an early loss of two animals with major infections after osteopenia induction using ovariectomy and glucocorticoid treatment.
In all healthy and osteopenic sheep without ZOL administration, uneventful wound healing was observed. However, a periosteal reaction was detected in osteopenic sheep. Induced osteopenia followed by periosteal thickening is in accordance with Bisseret et al,26 who suggested periosteal apposition as a compensation for endocortical bone loss in pre-menopausal females. Even though the periosteal reaction in all specimens was studied in μCT and ZTE-MRI, a limitation of this study is the missing histomorphometric evaluation. Therefore, the actual value of periosteal bone formation could not be assessed. Nonetheless, a quantitative evaluation of the periosteal reaction was possible with identical specimens examined using both imaging modalities. Another limitation is that specimens were scanned after sacrificing of the animals which is not directly transferable to the clinical setting in patients. However, with the additional information that is supplied with contrast agents, the use of ZTE-MRI may be even more advantageous. The clinical use of ZTE-MRI described by Weiger et al27 gives a perspective for the use of this imaging modality for in vivo examination of BP-ONJ. Further studies should focus on different stages of the disease to determine the visibility of early pathological alterations.
Conclusions
A periosteal reaction was observed in ZTE-MRI and μCT in all sheep diagnosed with BP-ONJ. A broader and more frequent periosteal reaction was measured in ZTE-MRI compared with μCT. Therefore, ZTE-MRI proved a reliable imaging modality to display hard- and soft-tissue structures of the mandible and might be applied for the display of earlier stages of BP-ONJ.
Acknowledgments
Acknowledgments
The authors thank Gerda Siebert, Dipl.-Math., for help with statistical analysis of data.
Contributor Information
Pit Voss, Email: pit.voss@uniklinik-freiburg.de.
Ute Ludwig, Email: ute.ludwig@uniklinik-freiburg.de.
Philipp Poxleitner, Email: philipp.poxleitner@uniklinik-freiburg.de.
Veronika Bergmaier, Email: veronika.bergmaier@gmx.net.
Nora El-Shafi, Email: nora_el_shafie@hotmail.com.
Dominik von Elverfeldt, Email: dominik.elverfeldt@uniklinik-freiburg.de.
Vincent Stadelmann, Email: vincent.stadelmann@aofoundation.org.
Jan-Bernd Hövener, Email: hoevener@gmail.com.
Tabea Flügge, Email: tabea.fluegge@uniklinik-freiburg.de.
References
- 1.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. ; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg 2014; 72: 1938–56. doi: http://dx.doi.org/10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 2.Patel S, Choyee S, Uyanne J, Nguyen AL, Lee P, Sedghizadeh PP, et al. Non-exposed bisphosphonate-related osteonecrosis of the jaw: a critical assessment of current definition, staging, and treatment guidelines. Oral Dis 2012; 18: 625–32. doi: http://dx.doi.org/10.1111/j.1601-0825.2012.01911.x [DOI] [PubMed] [Google Scholar]
- 3.Dore F, Filippi L, Biasotto M, Chiandussi S, Cavalli F, Di Lenarda R. Bone scintigraphy and SPECT/CT of bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med 2009; 50: 30–5. doi: http://dx.doi.org/10.2967/jnumed.107.048785 [DOI] [PubMed] [Google Scholar]
- 4.Dixon R, Tricker N, Garetto L, eds. Bone turnover in elderly canine mandible and tibia. J Dent Res, ALEXANDRIA, VA: AMER ASSOC DENTAL RESEARCH; 1997. [Google Scholar]
- 5.Van den Wyngaert T, Huizing MT, Fossion E, Vermorken JB. Scintigraphic evaluation of mandibular bone turnover in patients with solid tumors receiving zoledronic acid. Oral Oncol 2010; 46: 214–8. doi: http://dx.doi.org/10.1016/j.oraloncology.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 6.Schindeler A, Little DG. Osteoclasts but not osteoblasts are affected by a calcified surface treated with zoledronic acid in vitro. Biochem Biophys Res Commun 2005; 338: 710–6. doi: http://dx.doi.org/10.1016/j.bbrc.2005.09.198 [DOI] [PubMed] [Google Scholar]
- 7.Otto S, Hafner S, Mast G, Tischer T, Volkmer E, Schieker M, et al. Bisphosphonate-related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? J Oral Maxillofac Surg 2010; 68: 1158–61. doi: http://dx.doi.org/10.1016/j.joms.2009.07.079 [DOI] [PubMed] [Google Scholar]
- 8.Elad S, Gomori MJ, Ben-Ami N, Friedlander-Barenboim S, Regev E, Lazarovici TS, et al. Bisphosphonate-related osteonecrosis of the jaw: clinical correlations with computerized tomography presentation. Clin Oral Investig 2010; 14: 43–50. doi: http://dx.doi.org/10.1007/s00784-009-0311-3 [DOI] [PubMed] [Google Scholar]
- 9.Farias DS, Zen Filho EV, de Oliveira TF, Tinôco-Araujo JE, Sampieri MB, Antunes HS, et al. Clinical and image findings in bisphosphonate-related osteonecrosis of the jaws. J Craniofac Surg 2013; 24: 1248–51. doi: http://dx.doi.org/10.1097/SCS.0b013e3182902b91 [DOI] [PubMed] [Google Scholar]
- 10.Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Santos EC, et al. Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 695–703. doi: http://dx.doi.org/10.1016/j.oooo.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 11.Wilde F, Heufelder M, Lorenz K, Liese S, Liese J, Helmrich J, et al. Prevalence of cone beam computed tomography imaging findings according to the clinical stage of bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 804–11. doi: http://dx.doi.org/10.1016/j.oooo.2012.08.458 [DOI] [PubMed] [Google Scholar]
- 12.Chiandussi S, Biasotto M, Dore F, Cavalli F, Cova MA, Di Lenarda R. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol 2006; 35: 236–43. doi: http://dx.doi.org/10.1259/dmfr/27458726 [DOI] [PubMed] [Google Scholar]
- 13.Lesclous P, Grabar S, Abi Najm S, Carrel JP, Lombardi T, Saffar JL, et al. Relevance of surgical management of patients affected by bisphosphonate-associated osteonecrosis of the jaws. A prospective clinical and radiological study. Clin Oral Investig 2014; 18: 391–9. doi: http://dx.doi.org/10.1007/s00784-013-0979-2 [DOI] [PubMed] [Google Scholar]
- 14.Leite AF, Ogata Fdos S, de Melo NS, Figueiredo PT. Imaging findings of bisphosphonate-related osteonecrosis of the jaws: a critical review of the quantitative studies. Int J Dent 2014; 2014: 784348. doi: http://dx.doi.org/10.1155/2014/784348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockmann P, Hinkmann FM, Lell MM, Fenner M, Vairaktaris E, Neukam FW, et al. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin Oral Investig 2010; 14: 311–7. doi: http://dx.doi.org/10.1007/s00784-009-0293-1 [DOI] [PubMed] [Google Scholar]
- 16.Guggenberger R, Fischer DR, Metzler P, Andreisek G, Nanz D, Jacobsen C, et al. Bisphosphonate-induced osteonecrosis of the jaw: comparison of disease extent on contrast-enhanced MR imaging, [18F] fluoride PET/CT, and conebeam CT imaging. AJNR Am J Neuroradiol 2013; 34: 1242–7. doi: http://dx.doi.org/10.3174/ajnr.A3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Ferrer L, Bagan JV, Martinez-Sanjuan V, Hernandez-Bazan S, Garcia R, Jimenez-Soriano Y, et al. MRI of mandibular osteonecrosis secondary to bisphosphonates. AJR Am J Roentgenol 2008; 190: 949–55. doi: http://dx.doi.org/10.2214/AJR.07.3045 [DOI] [PubMed] [Google Scholar]
- 18.Bisdas S, Chambron Pinho N, Smolarz A, Sader R, Vogl TJ, Mack MG. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin Radiol 2008; 63: 71–7. doi: http://dx.doi.org/10.1016/j.crad.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 19.Weiger M, Pruessmann KP, Hennel F. MRI with zero echo time: hard versus sweep pulse excitation. Magn Resonance Med 2011; 66: 379–89. doi: http://dx.doi.org/10.1002/mrm.22799 [DOI] [PubMed] [Google Scholar]
- 20.Hovener JB, Zwick S, Leupold J, Eisenbeiβ AK, Scheifele C, Schellenberger F, et al. Dental MRI: imaging of soft and solid components without ionizing radiation. J Magn Reson Imaging 2012; 36: 841–6. doi: http://dx.doi.org/10.1002/jmri.23712 [DOI] [PubMed] [Google Scholar]
- 21.Weiger M, Pruessmann KP, Bracher AK, Köhler S, Lehmann V, Wolfram U, et al. High-resolution ZTE imaging of human teeth. NMR Biomed 2012; 25: 1144–51. doi: http://dx.doi.org/10.1002/nbm.2783 [DOI] [PubMed] [Google Scholar]
- 22.Kimmel DB. Animal models for in vivo experimentation in osteoporosis research. Osteoporosis 1996; 2: 29–47. [Google Scholar]
- 23.Veigel E, Moore RJ, Zarrinkalam MR, Schulze D, Sauerbier S, Schmelzeisen R, et al. Osteopenia in the maxillofacial area: a study in sheep. Osteoporos Int 2011; 22: 1115–21. doi: http://dx.doi.org/10.1007/s00198-010-1289-z [DOI] [PubMed] [Google Scholar]
- 24.Voss PJ, Stoddart MJ, Bernstein A, Schmelzeisen R, Nelson K, Stadelmann V, et al. Zoledronate induces bisphosphonate-related osteonecrosis of the jaw in osteopenic sheep. Clin Oral Investig 2016; 20: 31–8. doi: http://dx.doi.org/10.1007/s00784-015-1468-6 [DOI] [PubMed] [Google Scholar]
- 25.Weiger M, Stampanoni M, Pruessmann KP. Direct depiction of bone microstructure using MRI with zero echo time. Bone 2013; 54: 44–7. doi: http://dx.doi.org/10.1016/j.bone.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 26.Bisseret D, Kaci R, Lafage-Proust MH, Alison M, Parlier-Cuau C, Laredo JD, et al. Periosteum: characteristic imaging findings with emphasis on radiologic-pathologic comparisons. Skeletal Radiol 2015; 44: 321–38. doi: http://dx.doi.org/10.1007/s00256-014-1976-5 [DOI] [PubMed] [Google Scholar]
- 27.Weiger M, Brunner DO, Dietrich BE, Müller CF, Pruessmann KP. ZTE imaging in humans. Magn Reson Med 2013; 70: 328–32. doi: http://dx.doi.org/10.1002/mrm.24816 [DOI] [PubMed] [Google Scholar]