Abstract
Background
17β-Estradiol (E2) has been reported to protect annulus fibrosus (AF) cells in vitro against interleukin-1β (IL-1β)-induced apoptosis in a concentration-dependent manner. However, its time-response effect remains unexplored. In addition, integrin α2/collagen II interaction has been reported to influence the apoptosis of nucleus pulposus cells in vitro. Thus, we hypothesized that integrin α1/collagen II might play a role in exerting the anti-apoptosis effect by E2. The aim of the current study was to further investigate the anti-apoptotic effect of E2 and determine the role of integrin α1/collagen II interaction.
Material/Methods
Rat AF cells were primary cultured and used for the following experiments. AF cells were identified by immunocytochemistry of type I collagen. Cell apoptosis was detected by fluorescence-activated cell sorter (FACS) analysis. The activity of active caspase-3 was determined by use of a caspase-3 detection kit. AF cell adhesion to type I collagen was determined by cell adhesion assay. Protein level of integrin subunit α1 was quantified by Western blot and mRNA expression was determined by real-time qPCR.
Results
The immunocytochemistry of type I collagen revealed that cell purity was eligible for the following experiments with 98% of purity. FACS analysis indicated time-dependent anti-apoptosis effect of E2 at time points of 6 h, 12 h, and 24 h, which was confirmed by Caspase-3 activity. Furthermore, cell adhesion assay showed that E2 significantly increased cell binding to 95% of control, and qPCR and Western blot analysis showed that E2 effectively upregulated integrin α1. However, estrogen receptor antagonist ICI182780 prohibited the effect of E2.
Conclusions
This study shows that E2 protects against apoptosis in a time-dependent manner, and α1 integrin-mediated adhesion to collagen II is essential for estrogen-dependent anti-apoptosis in rat annulus fibrosus cells in vitro.
MeSH Keywords: Apoptosis, Collagen, Estradiol, Integrin alpha1, Intervertebral Disc Degeneration
Background
Lumbar disc herniation (LDH) is an increasingly common disease, mostly resulting from long-term disc degeneration. Degeneration of annulus fibrosus (AF) was due to repeated and long-term injuries. Cell attachment to extracellular matrix (ECM) decreases, leading to the AF becoming thinner and softer. Thus, nucleus pulposus is more likely to extrude and the spinal cord and lateral nerve roots are compressed by the prolapsed intervertebral disc, resulting in serious clinical manifestations [1].
Aberrant intervertebral disc (IVD) cell apoptosis and its acceleration of aging are regarded as the 2 major cellular processes related to disc degeneration [2–4]. Cell proliferation is often observed in nucleus pulposus (NP), leading to formation of lacunae containing multi-cell clusters [4]. Despite this, up to 50% of the cells may show signs of necrosis, while others reveal signs of apoptosis, potentially resulting in cell loss from the disc [4]. As cell activities and number of divisions are lost, the IVD is not able to maintain the large matrix macromolecules such as collagen, along with decreasing cell-to-ECM adhesion, which result in a net loss of large proteoglycans and a shift in collagen synthesis [2]. Thus, apoptosis of IVD cells plays a significant role in the pathogenesis of disc degeneration.
Previous reports have demonstrated that apoptosis in cells is under the control of environmental signals such as growth factors, glucocorticoids, radiation, cytokines, and anti-cancer drugs [5–10]. Like growth factors and hormones, extracellular matrix (ECM) molecules such as collagen, fibronectin (FN), laminin, thrombospondin, vitronectin, and proteoglycans have been shown to play important roles in hematopoiesis, proliferation, differentiation, migration, and apoptosis [11]. Many types of cells undergo apoptosis when deprived of attachment to the appropriate ECM [12]. It is well known that cell adhesion to ECM is an integrin-mediated action. Integrin, consisting of an α subunit and a β subunit, is a family of heterodimeric transmembrane proteins that participate in diverse cell-to-cell and cell-to-ECM interactions [13]. The extracellular domain can act as a receptor for ECM components, signaling protein, or adhesion molecules of other cells. In addition, ECM proteins have a modulating effect on several signalling pathways involved in the regulation of cell proliferation and apoptosis [14].
Previous studies revealed that estrogen protects cells against apoptosis and even turned over that process through upregulation of some integrin subtypes and enhancement of cell-ECM adhesion [15,16]. A recent study reported that 17β-estradiol (E2) protected NP cells against apoptosis via upregulating integrin α2β1/collagen II [1]. In addition, integrin α1β1 has been found to be the special receptor for type I collagen [17]. Thus, based on these findings, we hypothesized that integrin α1/collagen II may exert an anti-apoptosis effect via E2. E2 has been reported to protect AF cells in vitro against IL-1β-induced apoptosis in a concentration-dependent manner [18]. However, its time-response effect remains unexplored. Therefore, the aim of the present study was to further investigate the anti-apoptotic effect of E2 and to determine the role of integrin α1/collagen II interaction. We hope our study will assist development of novel therapeutic strategies for preventing and treating IVD degeneration.
Material and Methods
Reagents
We used the following reagents: DMEM/F12 (Gibco, USA), fetal bovine serum (FBS) (BI, Israel), trypsin (Sigma, USA), collagenase type II (Sigma, USA), D-Hanks and PBS (Solarbio, Beijing, China), Annexin V-FITC/PI kit (BD, USA), primary antibody of anti-α1 (Proteintech, Wuhan, China), E2 (Sigma, USA), collagen II (Sigma, USA), ICI182780 (Sigma, United Kingdom), and secondary antibody (goat anti-rabbit) (Proteintech, Wuhan, China).
Ethical statement
The protocol for animal use in these experiments was approved by the Institutional Review Board of the Affiliated Taizhou People’s Hospital of Nantong University.
Cell culture protocol
Annulus fibrosus cells were isolated from male Wistar rats (~200 g) using the culture methodology reported previously [19]. In brief, 3 male Sprague-Dawley rats were sacrificed with anesthesia overdose, the whole lumbar vertebral column was resected under aseptic conditions, and IVD were all collected. The AF was separated from the gel-like nucleus pulposus using a dissecting microscope and then put into a beaker containing 5 ml of D-Hanks solution. All AF was cut into 1-mm3 pieces and the D-Hanks solution was poured out. The AF tissue was disintegrated by 0.25% of type II collagenase for 1 h and subsequently treated with 0.2% of trypsin with EDTA for 5 min. The partially undigested tissue was removed from the rest of the medium, which included AF cells, and was then transferred into a culture flask containing DMEM and 15% FBS supplemented with 100 IU/mL penicillin and 100 ug/mL streptomycin. AF cells were cultured under a suitable environment with 5% CO2 at 37°C. AF cells proliferated attached to the bottom of a culture flask after 2–3 days. Confluent to about 80%, AF cells were subcultured in 3 culture flasks after being re-disintegrated by 0.25% trypsin solution (EDTA, 1 mmol/L).
Purification and identification of AF cells
This experiment was performed as reported previously [20]. The digested and lifted AF cells were cultured in a 50-ml dish containing DMEM/F12 without fetal bovine serum and kept static for 4 h, then AF cells were observed under an optical microscope. When AF cells were partly attached to the bottom of the dish and never suspended, we poured out DMEM/F12 with the other suspended cells. The rest of the AF cells were cultured again as above and then purified AF cells were obtained. Collagen I was identified by SP-ABC immunocytochemistry. AF cells were sequence-fixed by 4% formaldehyde for 10 min, washed 3 times with PBS for 3–5 min, kept in 0.2% Triton X-100 for 5 min at room temperature, washed in PBS 3 times, sealed off for 60 min at room temperature, washed in PBS 3 times for 3–5 min, added into rabbit anti-rat primary antibody of collagen I for 1 h at 37°C, washed in PBS 3 times for 3–5 min, then added into goat anti-rabbit secondary antibody for 30 min at 37°C, and dyed with DAB for 15 min after being washed in PBS 3 times. The cells with dyed collagen I were observed and counted under 6 random fields, and AF cellular purity was calculated.
FACS analysis
Apoptotic incidence of AF cells was detected by flow cytometry, as previously described [21]. AF cells were divided into 6 groups and cultured with a 6-well plate at the density of 2×105 cells in each well. Group A was regarded as a control group administrated with vehicle. Group B was administrated IL-1β at a concentration of 75 ng/ml. Group C was administrated IL-1β at a concentration of 75 ng/ml, with the pre-administration of E2 at a concentration of 10 μM for 6 h. Group D was administrated IL-1β at a concentration of 75 ng/ml, with the preadministration of E2 at a concentration of 10 μM for 12h. Group E was administrated IL-1β at a concentration of 75 ng/ml, with the preadministration of E2 at a concentration of 10 μM for 24 h. Group F was administrated 75 ng/ml IL-1β with the preadministration of 10 μM E2 plus 10μM ICI for 24 h. All of the groups above were cultured in DMEM/F12 medium without FBS or phenol red, for 24 h. All groups of AF cells were collected and subsequently washed twice with ice-cold PBS, and then suspended using 250 μL binding buffer (10 mm Hepes/NaOH, pH 7.4, 140 mM NaCL, 2.5 mM CaCl) to the concentration of 106 cells/ml. Finally, 100 μL of the above suspended cell mixture for each group was taken out to react with a double-staining working solution including 5 μL of Annexin V-FITC (20μg/mL) and 10 μL of propidium iodide (PI, 20 μg/mL) in the dark for 15 min at room temperature. Double staining with Annexin V and PI was considered as a positive result of early apoptotic events, which was analyzed using a FACS Calibur flow cytometer (BD Biosciences). All experiments were performed in triplicate (n=3).
Caspase-3 activity assay
Experimental groups were designed as described above. Activity of active caspase-3 was determined by a caspase-3 detection kit (Beyotime, Shanghai, China), according to the manufacturer’s instructions. Activity of caspase-3 is presented as the fold-change compared to the control group.
Cell adhesion assay
As previously reported [22], cell adhesion to collagen II was assayed. Because the dose-response effect was the focus of this study, time-dependent manner is not explored in this section. In brief, AF cells were divided into 6 groups and cultured with a 6-well plate at the density of 2×105 cells in each well. Group A was regarded as a control and was administered vehicle. Group B was administered IL-1β at a concentration of 75 ng/ml. Group C was administered IL-1β at a concentration of 75 ng/ml, with the pre-administration of E2 at a concentration of 0.1 μM for half an hour. Group D was administered IL-1β at a concentration of 75 ng/ml, with the pre-administration of E2 at a concentration of 1 μM for half an hour. Group E was administered IL-1β at a concentration of 75 ng/ml, with the pre-administration of E2 at a concentration of 10 μM for half an hour. Group F was administered 75 ng/ml IL-1β with the pre-administration of 10 μM E2 plus 10 μM ICI for half an hour. All of these groups were cultured in DMEM/F12 medium without FBS or phenol red, for 24 h. The 24-well plates were coated with type II collagen at 4°C overnight (20 μg/mL for each). To block the nonspecific binding sites, the 24-well plates were coated with 10 mg/mL of albumin for 1 h, then washed twice with PBS. A total of 3×104 cells were transplanted into each well and adhered to type II collagen at 37°C for 1 h. Finally, optical density (OD) of adherent cells was quantified at 570 nm using a Dynatech MR 5000 device (Dynatech, Germany). Values reported are the mean ±SD of 3 independent experiments (n=3).
Real-time qPCR
Experimental groups were divided as described above. Expression of mRNA encoding integrin α1 was detected by real-time qPCR as described previously [4,13]. Total RNA was extracted using TRIzol according to the instruction for use provided by the manufacturer. Total RNA was measured fluorometrically with the CyQuant-Cell Proliferation Assay Kit, produced by Molecular Probes. The RT-PCR System (Invitrogen, Carlsbad, CA) was used for cDNA synthesis. To semi-quantify the genes of interest, we used a qRT-PCR kit with 2-step method (Promega, Madison, WI) in a total volume of 20 μL. Primers of target genes were used as shown in Table 1. Standard curves were run in each optimized assay to produce a linear plot of threshold cycle (Ct) against log (dilution). The amount of the target was quantified based on the concentration of the standard curve and is shown as relative Ct value according to the 2−ΔΔCt method. The quantity of the target was normalized to the reference gene β-actin.
Table 1.
Sequences of primers used for qPCR analysis.
| No. | Gene | Primer sequence |
|---|---|---|
| 1 | α1 integrin | 5′ cttggacatcgttgtggtttat 3′(sense) |
| 5′ ttctgaagaggattaagacgga 3′(antisense) | ||
| 2 | β-actin | 5′ gcttacatgtctcgatcccacttaa 3′(sense) |
| 5′ ctcgcgctactctctctttctgg 3′(antisense) |
Western blot
Protein expression of integrin α1 was detected by Western blot analysis [23]. AF cells were washed using ice-cold PBS and then collected using 100 uL of Mammalian Protein Extraction Reagent buffer (MPER, Pierce, IL) containing protease inhibitor cocktail, NaF (5 mmol/L), and Na3VO4(200 umol/L). Lysates were centrifuged at 4°C for 3 min at 14 000rpm and resolved in 12% SDS-polyacrylamide gels. Proteins were transferred by electroblotting to a PVDF membrane, which was then blocked with 5% non-fat milk powder and incubated overnight at 4°C with respective primary antibodies. After being washed in TBST 3 times for 5 min each time, the PVDF membrane reacted with a secondary anti-IgG-HRP antibody and was incubated at room temperature for 1 h. Immunoblotting was done using the method of enhanced chemiluminescence.
Statistical analysis
Data analysis was performed using SPSS for Windows 18.0 (SPSS Inc., USA). Statistical analysis was carried out with one-way analysis of variance (ANOVA), followed by the LSD t test as pairwise comparisons. P<0.05 was regarded as significant. Data are shown as mean ±SD (standard deviation) of 3 independent experiments (n=3).
Results
Purification and identification of AF cells
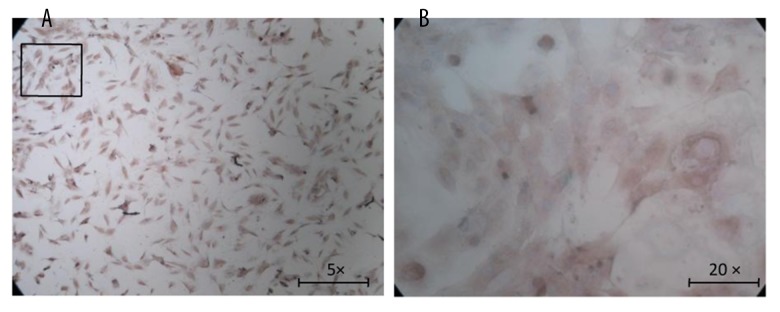
To ensure cell purity, we purified the primarily cultured cells and calculated AF cellular purity using the immunocytochemistry method of type I collagen. It is well known that collagen type I is mainly expressed in annulus fibrosus and collagen II in nucleus pulposus; therefore, as was shown in Figure 1A and 1B, collagen I was immunostained to brown in rat AF cells. The cellular purity was about 98% on average after counting 6 random views under a light microscope, indicating that AF cellular purity was sufficient for the following experiments.
Figure 1.
Immunocytochemistry of type I collagen. Collagen type I was mainly expressed in annulus fibrosus, and was immunostained to brown, as shown in the figure. (A) Original magnification ×50; (B) Original magnification×200.
FACS analysis
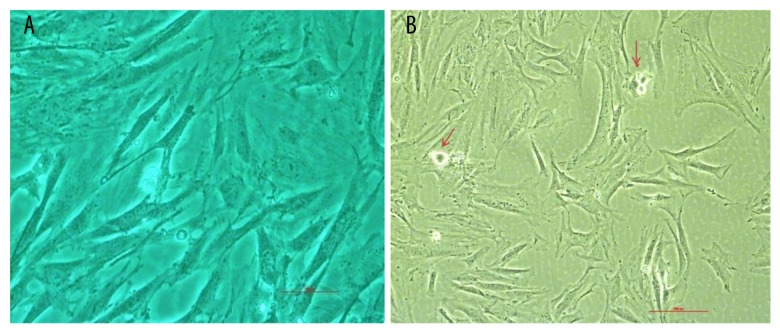
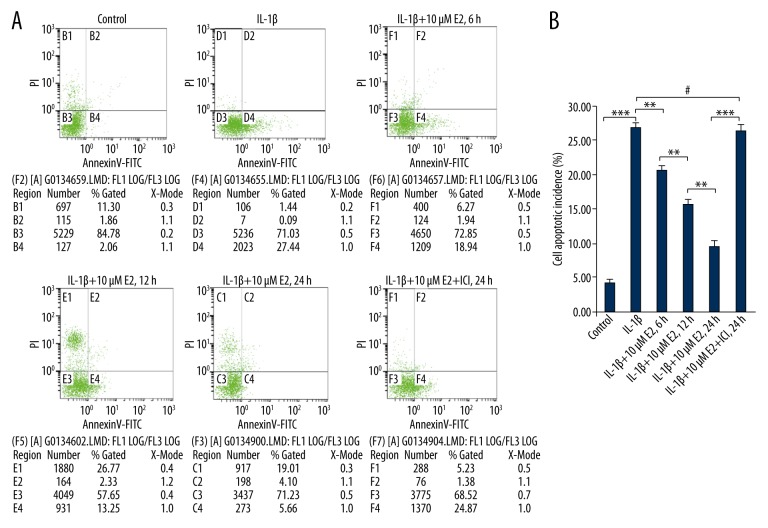
As shown in Figure 2A and 2B, apoptotic cells are marked by an arrow. As shown in Figure 3A and 3B, IL-1β led to a 27% increase in apoptotic incidence (p<0.01). However, the high-level apoptotic incidence was effectively decreased by the addition of 10 μM E2 in a time-dependent manner at time points of 6 h, 12 h, and 24 h (p<0.01).
Figure 2.
Morphologic changes in apoptotic AF cells. Using phase-contrast microscopy, apoptotic AF cells exhibited plasma membrane blebbing, as marked by an arrow (scale bar, 200 μm). AF, annulus fibrosus.
Figure 3.
FACS analysis for apoptotic incidence. (A) Apoptotic cells, stained positive for annexin V-FITC, negative for PI, or double positive, were counted. Data are represented as a percentage of the total cell count. (B) Data analysis used one-way analysis of variance (ANOVA) accompanied by pairwise comparison using the LSD t test. ** p<0.01, *** p<0.001, # p>0.05 mean ±SD (standard deviation); n=3.
Caspase-3 activity
As presented in Figure 4, IL-1β gave rise to a nearly 8-fold increase of caspase-3 activity (p<0.001), which was effectively reduced by the addition of 10 μM E2 in a time-dependent manner at time points 6 h, 12 h, and 24 h (p<0.01).
Figure 4.
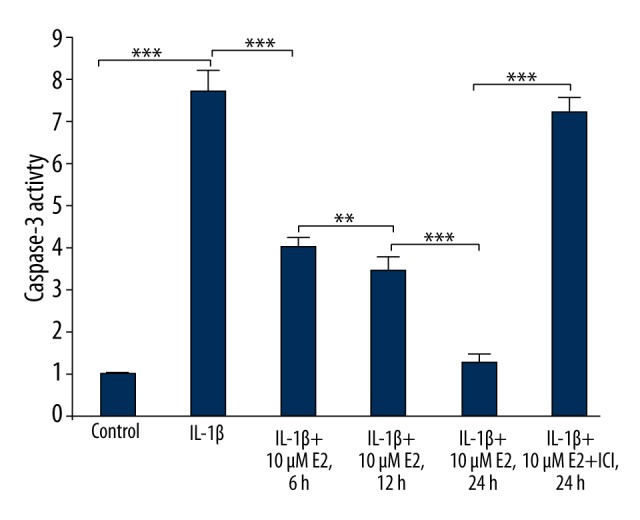
Active caspase-3 activity assay. Caspase-3 activity was determined using a caspase-3 activity kit. Caspase-3 activity is expressed as the fold-change in enzyme activity over control. ** p<0.01, *** p<0.001, by one-way analysis of variance (ANOVA) accompanied by pairwise comparison using the LSD t test. IL-1β, interleukin-1β; E2, 17β-estradiol; ICI, ICI182780; mean ±SD (standard deviation); n=3.
Cellular binding assay
As shown in Figure 5, IL-1β reduced cell binding to type I collagen by up to 40% (p<0.001). However, the trend resulting from IL-1b was effectively reversed by the addition of 0.1μM E2, 1μM E2, and 10 μM E2 in a dose- dependent manner (p<0.05).
Figure 5.
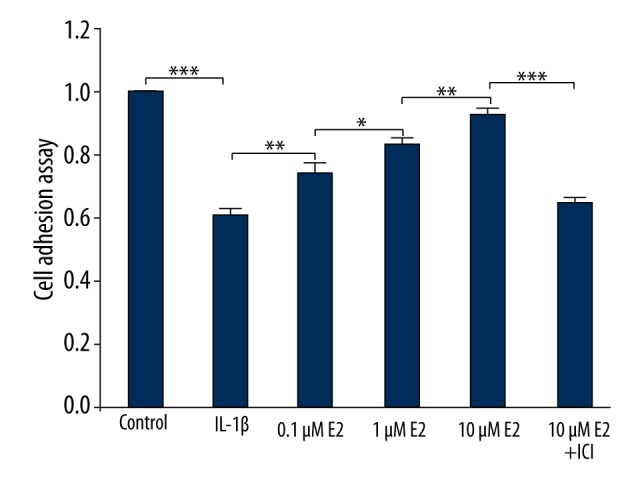
Cellular binding to type II collagen. Cellular binding is expressed as the fold-change over control. * p<0.05, ** p<0.01, *** p<0.001, by one-way analysis of variance (ANOVA) accompanied by pairwise comparison using the LSD t test. E2, 17β-estradiol; ICI, ICI182780; mean ±SD (standard deviation); n=3.
RT-qPCR
As displayed in Figure 6, IL-1β caused a significant decrease of 30% COL1α1 (the gene of type II collagen), compared with the control group (p<0.001). Notably, the trend due to IL-1β use was effectively reversed by 0.1μM E2, 1μM E2, and 10 μM E2 in a dose- dependent manner (p<0.05), further confirmed by the use of ICI (p<0.001).
Figure 6.
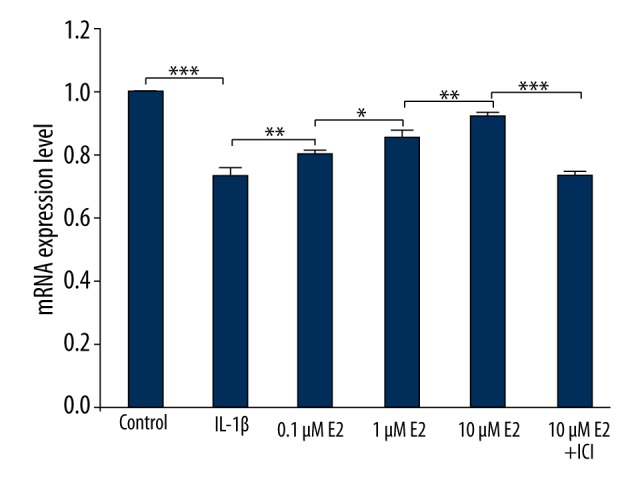
Real-time qPCR analysis. mRNA level is expressed as the fold-change over control. * p<0.05, ** p<0.01, *** p<0.001, by one-way analysis of variance (ANOVA) accompanied by pairwise comparison using the LSD t test. E2, 17β-estradiol; ICI, ICI182780; mean ±SD (standard deviation); n=3.
Western blot
As presented in Figure 7, the protein level of integrin α1 was significantly downregulated by IL-1β (p<0.001), which was partly reversed by the use of 0.1 μM E2 (p<0.001). As shown in Figure 8, E2 upregulated the protein level of integrin α1 in a dose-dependent manner by use of 0.1μM E2, 1μM E2, and 10 μM E2 (p<0.01).
Figure 7.
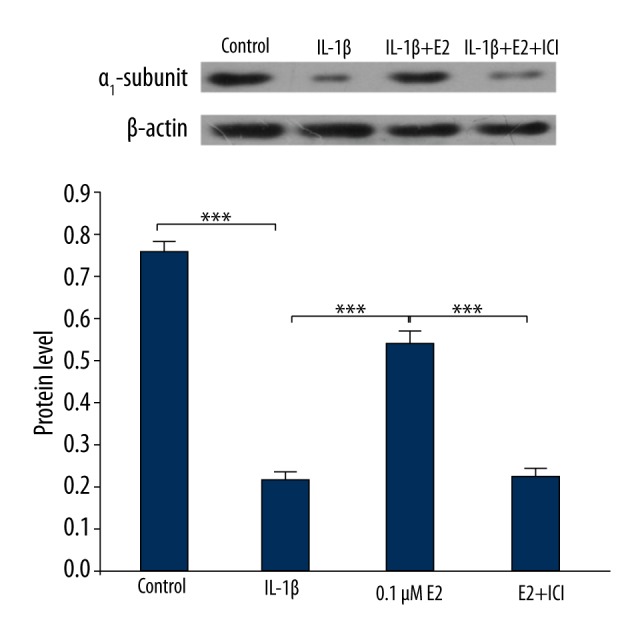
Protein level of integrin α1. *** p<0.001, by one-way analysis of variance (ANOVA) accompanied by pairwise comparison using the LSD t test. IL-1β, interleukin-1β; E2, 17β-estradiol; ICI, ICI182780; mean ±SD (standard deviation); n=3.
Figure 8.
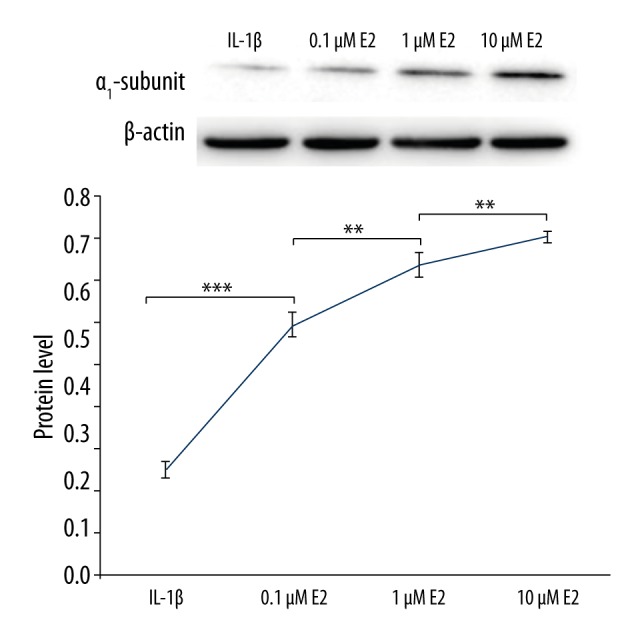
Integrin α1 was upregulated by E2 in a dose-dependent manner. ** p<0.01, *** p<0.001, by one-way analysis of variance (ANOVA) accompanied by pairwise comparison using the LSD t test. IL-1β, interleukin-1β; E2, 17β-estradiol; ICI, ICI182780; mean ±SD (standard deviation); n=3.
Discussion
It has long been known that a continuous increase in cell death occurs during IVD degeneration (IVDD) in humans. In addition, nutrient supply to the disc is reduced during IVDD in humans [24,25]. However, in this study we found that IL-1β alone was enough to cause apoptosis of AF cells with FBS deprivation. Previous studies [24,26] have reported that suppressing apoptosis can increase disc cell survival, but the cells will inevitably die if adequate nutrients are not supplied to the degenerated IVD. Hence, improving nutrient supply to the IVD is beneficial for suppressing cell apoptosis.
At present, the method of collagen immunostaining is mostly used to identify IVD cells. It is well-known that AF and NP mainly consist of type I collagen and type II collagen, respectively. Thus, immunocytochemistry of type I collagen was performed to identify AF cells and ensure cellular quality for our experiments. Since primary culture of rat AF cells may lead to a mixture of several different clusters of cells, it was necessary to repeatedly purify AF cells to acquire a high level of cellular purity. Fortunately, the current study showed that the cellular purity of rat AF cells was 98%, counted in 6 randomly selected visual fields, indicating that cultured AF cells were sufficiently pure for all experiments.
Many studies have focused on the effect of E2 on integrin. Bozzo et al. indicated that E2 can suppressβ-amyloid peptide-induced apoptosis of neuronal cells via the upregulation of integrin α1β1 expression [27]. Nilsen et al. reported that E2 protected against apoptosis via upregulating gene transcription of Bcl-2 and Bcl-XL encoding anti-apoptosis protein [28]. In prospective observational studies of estrogen replacement therapy (ERT) and Alzheimer disease (AD), Zandi and Craig et al. reported that ERT could reduce women’s risk of developing AD, perhaps by suppressing cell apoptosis [29,30]. Honda and Zhang et al. found that estrogen functioned against apoptosis by activating the protein kinase B pathway [31,32]. Nelson et al. reported that estrogen can upregulate expression level of integrin and increase cell attachment to FN-1 [13]. Data from studies of α5β1-integrin/FN interactions showed an exciting result – apoptosis can be controlled by ECM in an integrin-specific manner. Gibson reported that α5β1 integrin-mediated adhesion of Ntera2 neuronal cells to FN decreased apoptosis induced by serum withdrawal [33], and adhesion to the ECM component FN via α5β1 integrin inhibits fibroblast apoptosis [34].
Generally, previous studies focused on apoptosis induced by oxidant stress, cytokines such as TNF-α and interleukin-β, or others. However, little was done to explore the correlation among E2, integrin, and ECM in terms of AF cell apoptosis. In the present study, we explored the influence of E2 on AF cell apoptosis in vitro and detected the integrin expression level based on integrin-α1/type I collagen interaction.
Adhesion to FN can be mediated by several different integrin heterodimers, including α3β1, α4β1, α5β1, α8β1, αIIbβ3, αVβ1, αVβ3, αVβ6, and αVβ8 [35]. Among these integrin molecules, integrin α5 is distinguished from other subunits. Unlike other α subunits coupling various β subunits, the α5 subunit predominantly couples with β1 subunit and only interacts with FN [36]. In addition, integrin α6 subunit is a specific laminin receptor [37]. FBS is a mixture containing growth factors essential for survival of several types of cells in culture. Thus, some growth factors in FBS may be potent survival signals for rat AF cells. Also, the presence of phenol red may interfere with the accuracy of results, so all experiments in this study were performed without FBS and phenol red.
At present there are 2 main research fields on methods of repairing AF in intervertebral discs. One is aimed at producing healthy tissues to repair injured disc AF via gene engineering, tissue engineering, and so on. In the past several years, a series of related studies have been performed, including exploration for cell resource, suitable cell culture environment, and implantation methodology [38,39]. A recent tissue engineering study demonstrated that FN-coated scaffolds appeared to promote properly oriented AF cells and collagen type I, which should facilitate developing AF tissue that more closely mimics the native tissue [40]. The other research field in repairing AF is to directly repair AF clefts by embedding biomaterials in a surgical approach [41–43], but that still leaves some problems and complications related to the biomaterials. The present study belongs to the former type focused on the method of tissue engineering by exploring the robust state of AF cells.
Our study has some limitations. The current results obtained from this work were in vitro results. Thus, animal experiments are required to be performed to determine the in vivo situation, since results from in vitro models cannot always be applied to an in vivo situation. A new clue from the current results is that there could be an in vivo role of the investigated anti-apoptotic effects of E2 in IVDD.
Conclusions
This preliminary research found that E2 protects against apoptosis in a time-dependent manner, and α1 integrin-mediated adhesion to type II collagen is essential for estrogen-dependent anti-apoptosis in rat AF cells in vitro. This study may provide important information for developing novel therapeutic strategies for the long-term treatment of intervertebral disc degeneration.
Abbreviations
- LDH
lumbar disc herniation
- AF
annulus fibrosus
- NP
nucleus pulposus
- E2
17β-estradiol
- ICI
ICI182780
- FBS
fetal bovine serum
- ECM
extracellular matrix
- FN
fibronectin
- IVD
intervertebral disc
- IVDD
IVD degeneration
- FACS
fluorescence-activated cell sorter
- ER
estrogen receptor
- PBS
phosphate-buffered saline
- ERT
estrogen replacement therapy
- AD
Alzheimer disease
- TNF
tumor necrosis factor
- IL-1β
interleukin-1β
Footnotes
Conflict of interests
The authors indicate no potential conflicts of interest.
Source of support: Departmental sources
References
- 1.Yang SD, Ma L, Gu TX, et al. 17beta-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin alpha2beta1. Apoptosis. 2014;19:789–800. doi: 10.1007/s10495-014-0965-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhou GQ, Yang F, Leung VYL. Molecular and cellular biology of the intervertebral disc and the use of animal models. Current Orthopaedics. 2008;22:267–73. [Google Scholar]
- 3.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Majno G, Joris I. Apoptosis, oncosis, and necrosis - an overview of cell death. Am J Pathol. 1995;146:3–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Duke RC, Cohen JJ. IL-2 addition: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–99. [PubMed] [Google Scholar]
- 7.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–20. [PubMed] [Google Scholar]
- 8.Wyllie AH. Glucocorticoid-induced thymocytes apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–56. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 9.Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987;139:3199–206. [PubMed] [Google Scholar]
- 10.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–62. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 11.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–85. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson K, Helmstaedter V, Moreau C, Lage H. Estradiol, tamoxifen and ICI182,780 alter α3 and β1 integrin expression and laminin-1 adhesion in oral squamous cell carcinoma cell cultures. Oral Oncology. 2008;44:94–99. doi: 10.1016/j.oraloncology.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Jayne DG, Heath RM, Dewhurst O, et al. Extracellular matrix proteins and chemoradiotherapy: α5β1 integrin as a predictive marker in rectal cancer. Eur J Surg Oncol. 2002;28:30–36. doi: 10.1053/ejso.2001.1182. [DOI] [PubMed] [Google Scholar]
- 15.Harpel JG, Schultz-Cherry S, Murphy-Ullrich JE, Rifkin DB. Tamoxifen and estrogen effects on TGF-beta formation: role of thrombospondin-1, avβ3, and integrin-associated protein. Biochem Biophys Res Commun. 2001;284:11–14. doi: 10.1006/bbrc.2001.4922. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan KR, Blesson CS, Fatima I, et al. Expression of avβ3 integrin in rat endometrial epithelial cells and its functional role during implantation. Gen Comp Endocrinol. 2009;160:124–33. doi: 10.1016/j.ygcen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: Evidence of a differential role for alpha1beta1 and alpha2beta1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Ding W, Yang D, et al. Different concentrations of 17β-estradiol modulates apoptosis induced by interleukin-1β in rat annulus fibrosus cells. Mol Med Rep. 2014;10(5):2745–51. doi: 10.3892/mmr.2014.2514. [DOI] [PubMed] [Google Scholar]
- 19.Tim Yoon S, Su Kim K, et al. The effect of bone morphogeneric Protein-2 on rat intervertebral disc cell in vitro. Spine. 2003;28(16):1773–80. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 20.Xie QY, Ye JJ, Fu LX. In vitro culture of nucleus pulposus cells from rat and the study of its cell biology characteristics. Chinese J Trad Med Traum Orthop. 2006;14(Suppl):86–88. [Google Scholar]
- 21.Saldanha-Gama RF, Moraes JA, Mariano-Oliveira A, et al. α9β1 integrin engagement inhibits neutrophil spontaneous apoptosis: Involvement of Bcl-2 family members. Biochim Biophys Acta. 2010;1803:848–57. doi: 10.1016/j.bbamcr.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells: Regulation of integrin expression and cell survival. Spine. 2005;30:2503–9. doi: 10.1097/01.brs.0000186326.82747.13. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Zhou D, Lai Y, et al. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Lett. 2012;319(1):89–97. doi: 10.1016/j.canlet.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Yang SD, Bai ZL, Zhang F, et al. Levofloxacin increases the effect of serum deprivation on anoikis of rat nucleus pulposus cells via Bax/Bcl-2/caspase-3 pathway. Toxicol Mech Methods. 2014;24:688–96. doi: 10.3109/15376516.2014.963772. [DOI] [PubMed] [Google Scholar]
- 25.Yang SD, Yang DL, Sun YP, et al. 17beta-estradiol protects against apoptosis induced by interleukin-1beta in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis. 2015;20:348–57. doi: 10.1007/s10495-015-1086-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhao CQ, Liu D, Li H, et al. Interleukin-1beta enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis. 2007;12:2155–61. doi: 10.1007/s10495-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 27.Bozzo C, Graziola F, Chiocchetti A, Canonico PL. Estrogen and β-amyloid toxicity: Role of integrin and PI3-K. Mol Cell Neurosci. 2010;45:85–91. doi: 10.1016/j.mcn.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: Synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143(1):205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 29.Zandi PP, Carlson MC, Plassman BL, et al. Cache County Memory Study Investigators: Hormone replacement therapy and incidence of Alzheimer disease in older women: The Cache County Study. JAMA. 2002;288:2123–29. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 30.Craig MC, Maki PM, Murphy DG. The Women’s Health Initiative Memory Study: Findings and implications for treatment. Lancet Neuro. 2005;4:190–94. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- 31.Honda K, Sawada H, Kihara T, et al. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–27. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Rubinow DR, Xaing G, et al. Estrogen protects against beta-amyloid-induced neurotoxicity in rat hippocampal neurons by activation of Akt. Neuroreport. 2001;12:1919–23. doi: 10.1097/00001756-200107030-00030. [DOI] [PubMed] [Google Scholar]
- 33.Gibson RM, Craig SE, Heenan L, et al. Activation of integrin α5β1 delays apoptosis of Ntera2 neuronal cells. Mol Cell Neurosci. 2005;28:588–98. doi: 10.1016/j.mcn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on FN and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–65. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–98. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 36.Qian F, Zhang ZC, Wu XF, et al. Interaction between integrin a5 and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem Biophys Res Commun. 2005;333:1269–75. doi: 10.1016/j.bbrc.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 37.Krieger M, Scott MP, Matsudaira PT, et al. Molecular cell biology. fifth ed. New York: W.H. Freeman and Co; 2004. [Google Scholar]
- 38.Zhang Y, Anderson DG, Phillips FM, et al. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine annulus fibrosus cells: implications for anular repair. Spine. 2007;32:2515–20. doi: 10.1097/BRS.0b013e318158cc09. [DOI] [PubMed] [Google Scholar]
- 39.Chang G, Kim HJ, Kaplan D, et al. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine. 2007;16:1848–57. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attia M, Santerre JP, Kandel RA. The response of annulus fibrosus cell to fibronectin-coated nanofibrous polyurethane-anionic dihydroxyoligomer scaffolds. Biomaterials. 2011;32:450–60. doi: 10.1016/j.biomaterials.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Guo JK, Ding WY, He YL, et al. Experimental study of the duragide to annulus fibrosus closurement. Chin J Exp Surg. 2010;27:1636–38. [Google Scholar]
- 42.Taylor W. Biologic collagen PMMA injection (artifill) repairs mid-annular concentric defects in the ovine model. Spine J. 2006;6:48S–49S. [Google Scholar]
- 43.Ledet EH, Jeshuran W, Glennon JC, et al. Small intestinal submucosa for anular defect closure: long-term response in an in vivo sheep model. Spine. 2009;34:1457–63. doi: 10.1097/BRS.0b013e3181a48554. [DOI] [PubMed] [Google Scholar]