Abstract
Objective:
To identify the MRI parameters which best predict complete response (CR) to neoadjuvant chemoradiotherapy (CRT) in patients with locally advanced rectal cancer (LARC) and to assess their diagnostic performance.
Methods:
This was a prospective study of pre- and post-CRT MRI and diffusion-weighted imaging (DWI) of 64 patients with LARC who underwent neoadjuvant CRT and subsequent surgery. Histopathological tumour regression grade was the reference standard. Multivariate regression analysis was performed to identify the best MRI predictors of CR to neoadjuvant CRT, and their diagnostic performance was assessed.
Results:
The study cohort comprised 48 males and 16 females (n = 64), with mean age of 49.48 ± 14.3 years, range of 23–74 years. 11 patients had pathological complete response. The following factors predicted CR on univariate analysis: low initial (pre-CRT) tumour volume on T2 weighted high-resolution (HR) images and DWI, tumour volume-reduction rate (TVRR) of >95% on DWI and CR on post-CRT DWI (ydwiT0) as assessed by the radiologist. However, the best MRI predictors of CR on multivariate regression analysis were CR on post-CRT DWI (ydwiT0) as assessed by the radiologist and TVRR of >95% on DWI, and these parameters had an area under the curve (95% confidence interval) of 0.881 (0.74–1.0) and 0.843 (0.7–0.98), respectively. The sensitivity, specificity, positive-predictive value, negative-predictive value and accuracy of DWI in predicting CR was 81.8%, 94.3%, 75%, 96.1% and 76%; the sensitivity, specificity and accuracy of TVRR of >95% as a predictor of CR was 80%, 84.1% and 64.1%, respectively; however, this difference was not statistically significant. The interobserver agreement was substantial for ydwiT0.
Conclusion:
Visual assessment of CR on post-CRT DWI and TVRR of >95% on DWI were the best predictors of CR after neoadjuvant CRT in patients with LARC, and the former being more practical can be used in daily practice.
Advances in knowledge:
In rectal cancer, ydwiT0 as assessed by the radiologist was the best and most practical imaging predictor of CR and scores over standard T2W HR images.
INTRODUCTION
Rectal cancer is one of the most common cancers of the gastrointestinal tract, having high disease-related mortality. The incidence of rectal cancer in India is 4–5/100,000 population.1 Delay in early recognition and diagnosis causes the majority of cases to present with locally advanced disease; this scenario being true in many places with poor access to healthcare facilities.2 Neoadjuvant chemoradiotherapy (CRT) followed by surgical excision is the standard treatment in practice for locally advanced rectal cancer (LARC). However, there is a significant individual variation of response to pre-operative CRT; 9–25% of patients show complete response (CR), 54–75% of patients show tumour downstaging and others show no response.3
High-resolution (HR) MRI is routinely used for pre-operative tumour staging and for the assessment of response to therapy. The size of the tumour and qualitative assessment of change in signal intensity [tumour regression grade (TRG)] are the main criteria in use for response evalaution.4,5 Prediction of tumour response before surgery has considerable benefit in planning treatment strategy. Tumour regression and downstaging is also an important prognostic factor which affects local tumour recurrence rate and 5-year survival.3 There are, however, difficulties in the interpretation of post-treatment T2 weighted MR images owing to difficulties faced by radiologists in differentiating residual tumour, radiation-induced oedema and radiation-induced fibrosis.6 Diffusion-weighted imaging (DWI) has been found to be useful in this regard.3,4,6,7 This was based on the hypothesis that highly cellular tissues, such as tumours, restrict the diffusion of water molecules and thereby allow the identification of viable tumour from radiation-induced fibrosis and oedema.8
The goal of this study was to find the best MRI features which would predict CR to neoadjuvant chemoradiation in LARC using pathological TRG, as described by Mandard et al,9 as the reference standard.
METHODS AND MATERIALS
Study setting and study population
This Institutional Review Board-approved (IRB Min No. 7657, Christian Medical College and Hospital, Vellore, India) prospective study was undertaken by the departments of radiology, colorectal surgery and pathology in a 2800-bedded tertiary-care teaching hospital in south India. Informed consent was obtained from all patients. To estimate the difference in the MRI appearance between the responders and non-responders with 80% power and 5% significance level, a total sample size of around 80 was estimated.7 Consecutive patients who presented with LARC, i.e. clinical stage T3/T4 and any N or M stage, between April 2011 and April 2013 were included in the study if (a) they underwent CRT followed by surgery at our institution and (b) they underwent pre- and post-CRT MRI at our institution prior to surgical resection. Patients were excluded from the study if (a) there was a history of prior radiotherapy for rectal cancer or any other pelvic malignancies, (b) they could not undergo MRI owing to known contraindication to MRI or claustrophobia, (c) CRT was prematurely discontinued owing to their unwillingness to undergo further treatment, (d) surgery was delayed by more than 8 months after CRT or was cancelled owing to disease progression or inoperable malignancy and (e) post-CRT MRI was not performed.
Pre-and post-CRT MRI
All patients underwent MRI on a 3.0-T whole-body MR system (Intera 22 Achieva 3.0 T™; Philips Healthcare, Best, Netherlands) with a 16-channel phased-array coil as the receiver coil (3.0-T SENSE XL Torso MRI coil; Philips Healthcare). All patients underwent preoperative staging MRI of the abdomen and pelvis prior to initiation of treatment. Subsequently, patients underwent only MRI of the pelvis prior to surgery approximately 6–8 weeks after neoadjuvant CRT for restaging of the rectal cancer.
The MRI protocol is shown in Table 1. Standard HR T2 weighted MRI of the pelvis was performed in sagittal, oblique axial (perpendicular to the rectum) and oblique coronal (parallel to the rectum) planes. Diffusion-weighted MR images were obtained in the same oblique axial plane as T2 weighted HR images using respiratory-triggered, single-shot, echoplanar imaging with b-values of 0, 400 and 800 s mm−2, respectively. Depending on the respiratory efficiency of each patient, the acquisition time for this sequence ranged from 4 to 5 min. The apparent diffusion coefficient was calculated using a monoexponential function with b-values of 0, 400 and 800 s mm−2, respectively.
Table 1.
MRI sequences and the imaging protocol
| Sequence | TR/TE (msec) | Flip angle (degree) | Slice thickness (mm) | Slice gap (mm) | Matrix size | FOV (cm) | Acquisition time (min) |
|---|---|---|---|---|---|---|---|
| T2W axial | 941/80 | 90 | 6 | 0.6 | 288 × 189 | 375 | 2 |
| T2W SPAIR axial | 782/80 TI—220 | 90 | 6 | 0.6 | 268 × 163 | 375 | 2 |
| T1W axial | 12/2.3 | 15 | 6 | 0.6 | 256 × 153 | 380 | 1.37 |
| T2W HR coronal | 3546/80 | 90 | 3 | 0.3 | 384 × 377 | 230 | 2.43 |
| T2W HR sagittal | 3500/90 | 90 | 3 | 0.3 | 352 × 273 | 220 | 2.23 |
| T2W HR axial | 3500/90 | 90 | 3 | 0.3 | 368 × 291 | 230 | 3.19 |
| Single-shot EPI DWI transverse | 3750/75 | 90 | 4 | 0.4 | 128 × 116 | 380 | 3.15 |
DWI, diffusion-weighted imaging; EPI; echoplanar imaging; FOV, field of view; HR, high resolution; SPAIR, spectral adiabatic inversion recovery; T1W, T1 weighted; T2W, T2 weighted; TE, echo time; TI, inversion time; TR; repetition time.
Image analysis
Pre- and post-CRT MRI were reviewed on a picture archiving and communication system (GE Healthcare, Barrington, IL) workstation by two consultant gastrointestinal radiologists (AE and AC) with 10 and 5 years’ experience and one radiologist in training (KS). The radiologists were blinded to the surgical notes and pathology reports. T2W HR scans and DWI of the pre- and post-CRT MRI were assessed at different time periods.
The following observations were made in consensus on the pre- and post-CRT MRI: the location (high, mid or low); signal intensity (hypointense, isointense or hyperintense in comparison with the normal rectal wall); bulk of the tumour in terms of length and width of the tumour; T and N stage of the cancer as per the American Joint Committee on Cancer 2010 7th edition; and involvement of circumferential resection margin (CRM). Pre- and post-treatment volume of the rectal tumour was measured on both T2 weighted HR MRI and DWI. The percentage of reduction in the tumour volume compared with the pre-CRT tumour volume was termed as tumour volume-reduction rate (TVRR) and was calculated on T2 HR MRI and DWI.
Similarly, ADC values of the rectal tumour were obtained from ADC maps on pre- and post-treatment studies. The temporal changes in tumour ADC values were evaluated by calculating the absolute increase in ADC values (∆ADC) and the percentage increase in the ADC value, termed as the tumour apparent diffusion coefficient increase rate (TAIR). The ∆ADC was calculated by subtracting the pre-CRT ADC value from the post-CRT ADC value, and the TAIR was defined as the percentage increase in the ADC value.
Visual assessment of CR on DWI and T2 HR images was also documented separately for each radiologist, irrespective of the results of volume assessment on T2 HR MRI and DWI. CR on DWI (ydwiT0) was defined as the complete absence of hyperintense foci in the region of rectal tumour on B800 images. CR on T2 HR images was defined as the restoration of rectal wall thickness and morphology similar to a segment of uninvolved bowel. Marked hypointensity on T2 HR images was considered as an area of fibrosis and indeterminate for CR. The mesorectal nodes were not taken into account while assessing for CR in DWI and T2 weighted images.
Volumetric analysis
In order to standardize how the volume of the tumour will be obtained and to minimize the interobserver variability, all three observers (Observer 1—AE, Observer 2—AC and Observer 3—KS) measured the volumes of the rectal tumour in 20 consecutive patients on both T2 weighted HR MRI and DWI. After having obtained a good interobserver agreement between the observers (intraclass correlation coefficient = 0.76), Observer 3 (KS) alone assessed the volume of the tumour with the other readers contributing when necessary. Volumetric assessment took a mean time of 25 ± 7 min per MRI study.
The tumour was manually outlined on each section of the T2 HR axial images and on DWI. The volume of the tumour was calculated by multiplying the sum of areas of all sections by the sum of the interslice gap and slice thickness. For those tumours which were circumferential, the lumen of the rectum was included in the calculated volume to reduce variability in the volume assessment. While assessing post-CRT MRI, the hypointense areas on T2 weighted HR MRI were also included in the measured tumour volume. This was owing to the difficulty perceived in differentiating the tumour tissue from fibrosis. While assessing DWI, only the tissue which showed restricted diffusion, i.e. the tissue which is markedly hyperintense on B800 DWI and hypointense on ADC map, was outlined and used for calculating the tumour volume. In patients with a T2 markedly hyperintense tumour, the tumour volume was calculated on T2 weighted HR images. However, the volumetric assessment of these tumours on DWI was not accurate, since T2 markedly hyperintense rectal tumours showed T2 shine-through effect or facilitated diffusion, i.e. they appeared hyperintense on both DWI and ADC map. Thus, in these tumours, the entire hyperintense region on DWI was included for volume calculation. As it was not possible to delineate areas of true diffusion restriction in T2 markedly hyperintense tumours, a separate subgroup analysis after excluding these tumours was also carried out.
Apparent diffusion coefficient analysis using parametric maps
To obtain ADC values, regions of interest (ROIs) were drawn on an ADC map along the outline of the tumour in three sections with the maximum bulk of the tumour. The average of the three values was calculated and taken as a representative tumour ADC value. In case of studies which did not show any areas of restricted diffusion after neoadjuvant therapy (CR), the ROIs were placed in the region of the tumour in the pre-CRT MRI and region of restricted diffusion on pre-CRT DWI.
All the above-mentioned MRI findings, including the volume of the tumour in the T2 HR images and DWI, TVRR, ADC values of the tumour measured pre-CRT and post CRT and TAIR, were compared between histopathological complete responders and non-complete responders. A subgroup analysis of all the above parameters was also carried out for tumours showing restricted diffusion. The comparison between the complete and non-complete responders was made within this subgroup.
Histopathology and pathological tumour regression grade
The resected specimens of the rectal cancer were received in the laboratory and were fixed in 10% buffered formalin for approximately 24–48 h. After fixation, the entire primary tumour was sectioned at 4–5- mm intervals. The tissue was embedded in paraffin, then sliced at a thickness of 4 m and stained with haematoxylin and eosin. Four to eight sections of the tumour were examined. The pathological specimens after resection were reviewed by a single gastrointestinal pathologist with 10 years’ experience. The histopathological characteristics of the tumour and the response to pre-operative chemoradiotherapy were evaluated. The response to chemoradiotherapy was graded using a grading system adapted from Mandard et al.9 The tumour response was graded as follows: TRG 1—complete regression with the absence of residual cancer and fibrosis extending through the wall (ypT0); TRG 2—rare residual tumour cells scattered throughout the fibrosis (near CR); TRG 3—predominant fibrosis but increase in the number of cancer cells (moderate response); TRG 4—residual cancer cells outgrowing the fibrosis (minimal response); and TRG 5—absence of regressive changes (no response). Evidence of pathological complete response (pCR) was defined as the absence of viable adenocarcinoma in the surgical specimen. In the absence of tumour cells, fibrosis, haemorrhage, necrosis, inflammation and the presence of lakes of mucus without tumour cells were considered as post-treatment changes. Standard pathologic tumour staging of the resected specimen was performed in accordance with the guidelines of the American Joint Committee on Cancer. In order to compare the MRI features described above, between complete responders and non-complete responders, patients were stratified into two groups based on histopathological TRG: CR group comprised TRG 1 (ypT0) and non-complete response (non-CR) group comprised of TRG 2–5.
Statistical analysis
Statistical analysis was carried out using IBM SPSS® Analytics 16.0 software (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). The following were compared between the CR and non-CR groups using Wilcoxon signed rank test/Mann Whitney U test: the volume of the tumour on pre-CRT T2 weighted HR MRI and DWI, volume of tumour on post-CRT T2 weighted HR MRI images and DWI, percentage change in volume on T2 weighted HR MRI and DWI between pre- and post-CRT MRI, pre- and post-CRT ADC value and percentage change in ADC values between pre- and post-CRT MRI. The following were compared between the CR and non-CR groups using χ2 test: the location, signal intensity, T and N stage of the cancer, the CRM and radiologists’ independent assessment of CR on T2 HR and DWI. Univariate and multivariate analysis was performed to identify the best MR imaging predictors of CR, and their diagnostic performance was assessed using receiver-operating characteristic (ROC) curves. The best cut-off value which predicts CR for continuous variables like TVRR was obtained from ROC analysis. This cut-off value was used to convert the continuous variable into a dichotomous variable. The sensitivity, specificity, positive-predictive value (PPV), negative-predictive value (NPV) and accuracy of the best predictors of CR were calculated from χ2 test of contingency, setting as reference standard the histopathological CR. The difference in the diagnostic performance between the best predictors of CR was assessed using McNemar's test. Interobserver agreement for predicting CR on DWI (ydwiCR/ydwiT0) was determined using kappa statistics. Results of interobserver agreement were interpreted using the guidelines of Landis and Koch10 (k < 0, poor agreement; k 0–0.2, slight agreement; k 0.21–0.40, fair agreement; k 0.41–0.60, moderate agreement; k 0.61–0.80, substantial agreement; and k 0.81–1.00, almost perfect agreement). A p-value of <0.05 was considered as statistically significant.
RESULTS
Patients
The number of patients who were operable and consented for surgery was 70. Among these, six patients were excluded for the following reasons: other histological types in two patients (one patient with squamous-cell carcinoma and other patient with neuroendocrine tumour), non-availability of diffusion-weighted images in three patients and one patient was excluded because the MRI was performed after excision of a malignant polyp and there was no residual abnormality.
A total of 64 patients (48 males and 16 females) with mean age of 49.48 ± 14.3 years, range of 23–74 years, were included for final analysis. All these patients had LARC in the following stages at presentation: Stage IIA, 2 patients (3.1%); Stage IIIB, 49 (76%); Stage IIIC, 6 (9.3%); Stage IVA, 4 (6.2%); and Stage IVB, 3 (4.6%). The median time between the restaging MRI and surgery was 11.8 days. The majority of patients had low rectal cancers (n = 40, 62.5%). 19 (29.7%) patients had mid-rectal cancer and 5 (7.8%) patients had high rectal cancer. 61 (95.3%) patients underwent neoadjuvant long-course chemoradiotherapy (LCCRT) and 3 (4.7%) patients underwent neoadjuvant short-course chemoradiotherapy. 31 patients (48.4%) underwent abdominoperineal excision and 33 (51.6%) patients underwent low anterior resection. Table 2 shows the histopathological type and grade of rectal cancer along with its pathological TRG. The histopathology of 11 patients revealed ypT0 (TRG 1). The rest of the patients were non-complete responders and their TRG were as follows: TRG 2 = 12, TRG 3 = 21, TRG 4 = 17 and TRG 5 = 3 patients.
Table 2.
Histopathological type and grade of rectal cancer with pathological tumour regression grade (pTRG) of 64 patients included in the study
| Characteristics | N (%) | pTRG (n) |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Histopathological diagnosis | ||||||
| Well-differentiated adenocarcinoma | 2 (3.1%) | 1 | 1 | |||
| Moderately differentiated adenocarcinoma | 49 (76.6%) | 9 | 7 | 21 | 10 | 2 |
| Poorly differentiated adenocarcinoma (including one high-grade dysplasia with invasive adenocarcinoma) | 5 (7.8%) | 4 | 1 | |||
| Mucinous and signet-ring-cell type | 8 (12.5%) | 1 | 5 | 2 | ||
MRI features of the rectal cancer
Pre-treatment T2 weighted HR MRI revealed intermediate signal intensity tumours in 47 (73.4%) patients, hyperintense tumours in 8 (12.5%) patients and mixed intermediate and hyperintense tumours in 9 (14.1%) patients. All patients (n = 8) with T2 hyperintense rectal tumours and two patients with mixed signal intensity rectal tumours on pre-CRT MRI showed facilitated diffusion/T2 shine-through on pre-CRT DWI. The rest of the tumours showed areas of restricted diffusion. Among the 10 tumours showing facilitated diffusion, 8 tumours were mucinous/signet-ring-cell tumours, 1 tumour each was moderately differentiated adenocarcinoma and poorly differentiated adenocarcinoma, respectively.
60 (93.8%) patients had T3 disease and the rest of the patients had T4 disease. 2 (3.1%) patients had no significant mesorectal nodes, 26 (40.6%) patients had N1 disease and 36 (56.3%) patients had N2 disease in the pre-treatment staging MRI. Seven (10.9%) patients had metastatic disease which involved metastases to the liver (n = 2), lung (n = 2) and pelvic nodes (n = 3).
Among the 11 patients with pCR (TRG 1), post-CRT DWI predicted CR (ydwiT0) in 9 patients. In the other two patients with pTRG 1, yDWI predicted TRG 2 in one patient and TRG 3 in the other. On the other hand, post-CRT T2 HR MRI predicted CR (TRG 1) in only one patient; the rest were given a higher TRG on the post-CRT T2 HR MRI (TRG 2 = 6, TRG 3 = 3 and TRG 4 = 1).
Table 3 shows the median volume of tumour measured on T2 HR MRI and DWI in complete responders (CR = TRG 1) and non-responders (non-CR = TRG 2–5). The mean rate of tumour volume reduction (TVRR) as measured on DWI was 97.03% among the complete responders and 67.46% among the non-complete responders, and the difference in the TVRR among the two groups was significant, p <0.001. There was no significant difference in the mean pre-CRT ADC values between the CR (0.977 × 10−3) and non-CR group (1.013 × 10−3). Similarly, there was no difference in the ADC values of the residual tumour in the post-CRT DWI between the CR (1.46 × 10−3) and non-CR (1.41 × 10−3), p > 0.05. There was no significant difference in the TAIR (54% in CR and 45% in non-CR) and ∆ADC (0.49 × 10−3 in CR and 0.396 × 10−3 in non-CR) between the CR and non-CR group, p >0.05. Similarly, there was no significant difference in the location of the tumour, signal intensity of the tumour, T and N stage of the cancer and the CRM between the CR and non-CR groups.
Table 3.
Comparison of the median tumour volume measured on pre-chemoradiotherapy (CRT) and post-CRT MRI, tumour volume-reduction rate (TVRR) on T2 high-resolution images and diffusion-weighted imaging (DWI) between the complete responders (CR = TRG 1) and non-responders (non-CR = TRG 2–5)
| Volume mm3 | CR | Non-CR | p-value |
|---|---|---|---|
| Pre-CRT—T2W | 24.02 (±14) | 44.43 (±30) | 0.002 |
| Post-CRT—T2W | 4.84 (±1.7) | 23.81 (±26) | <0.001 |
| TVRR T2W | 71.4% (±25) | 51.47% (±31) | 0.53 |
| Pre-CRT—DWI | 21.73 (±14) | 35 (±25) | 0.025 |
| Post-CRT—DWI | 0.27 (±0.6) | 14.39 (±23) | <0.001 |
| TVRR DWI | 97.03% (±7.4) | 67.46% (±29) | <0.001 |
CR, complete response; T2W, T2 weighted; TRG, tumour regression grade.
Predictors of complete response (ypT0)
In univariate analysis, the MRI features which predicted ypT0 were low pre-CRT tumour volume on T2 weighted images (t value = −3.3, df = 31.5 and p-value = 0.002) and DWI (t value = −2.3, df = 25.7 and p-value = 0.025), high TVRR of >95% on DWI (t value = 6.4, df = 59.2, p-value <0.001) and ydwiT0 as assessed by the radiologist (Fisher’s exact test, p-value<0.001). However, the best MRI predictors of CR on multivariate regression analysis were ydwiT0 (b-value = 0.62, standard error = 0.11, p-value <0.001) as assessed by the radiologist and TVRR of >95% (b-value = 0.007, SE = 0.003 and p-value = 0.043). The agreement among the three observers in assessing ydwiT0 was substantial to excellent, with kappa values of 0.789, 0.90 and 0.792 among Observer 1 and 2, Observers 2 and 3 and Observer 1 and 3, respectively (p-value <0.001).
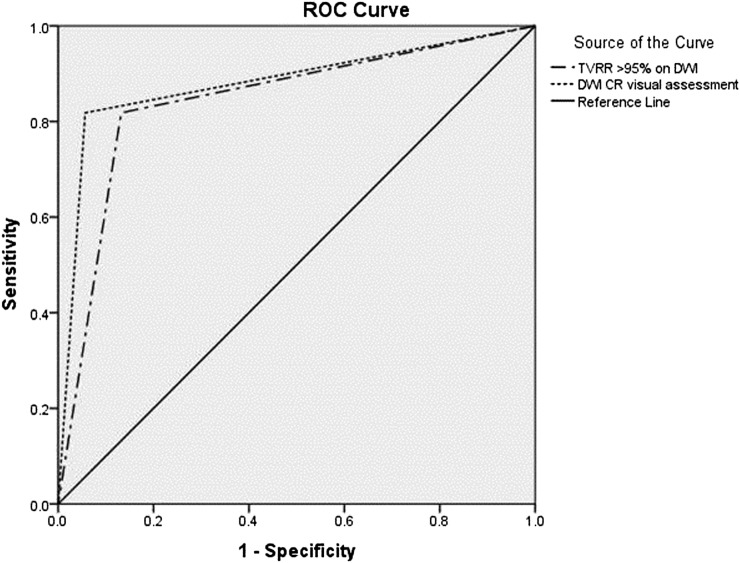
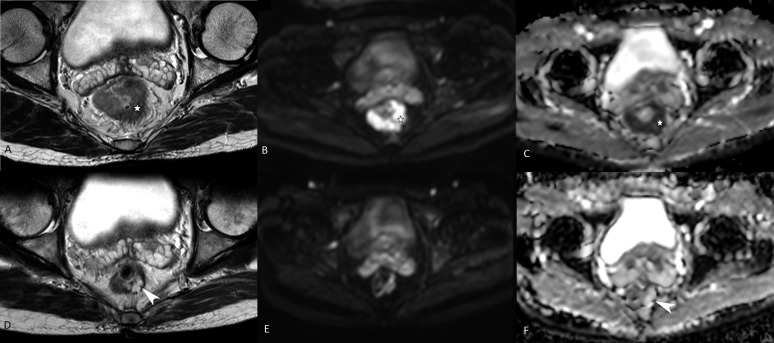
ROC analysis revealed an area under the curve (AUC) (95% confidence interval) of 0.881 (0.74–1.0) and 0.843 (0.7–0.98) for ydwiT0 and TVRR of >95% on DWI, respectively (Figure 1). The sensitivity, specificity, PPV, NPV and accuracy of visual assessment on DWI in predicting CR (ydwiT0) was 81.8%, 94.3%, 75%, 96.1% and 76%, respectively. The sensitivity, specificity and accuracy of TVRR of >95% as a predictor of CR was 80%, 84.1% and 64.1%, respectively. Figure 2 depicts a case of CR on DWI which showed residual tumour on T2W MRI.
Figure 1.
Receiver-operating characteristic (ROC) curves of tumour volume-reduction rate (TVRR) >95% [area under the curve (AUC) 0.843] and complete response (CR) on visual assessment on diffusion-weighted imaging (DWI) (AUC 0.881) as predictors of CR.
Figure 2.
Pre- and post-chemoradiotherapy T2 weighted (T2W) MRI and diffusion-weighted images in a tumour showing histopathological complete response. Upper panel (a, b and c), intermediate signal intensity tumour (asterisks) on T2W MR showing restricted diffusion on diffusion-weighted imaging and apparent diffusion coefficient. Lower panel (d, e and f), showing residual hyperintense lesion (arrowhead d) in the left posterolateral wall on T2W MRI. Facilitated diffusion is noted on diffusion-weighted imaging (arrowhead in f).
An exact McNemar's test showed that there was no statistically significant difference in the assessment of CR between ydwiT0 and TVRR of >95% on DWI (p-value = 0.125).
Analysis among the subgroup of patients showing restricted diffusion
54 patients had areas of restricted diffusion within the tumour, while 10 patients demonstrated facilitated diffusion. There was no significant improvement in the diagnostic performance of DWI in predicting complete responders when only tumours with foci of restricted diffusion on pre-CRT MRI were analysed separately. The sensitivity, specificity, PPV and NPV of DWI in predicting CR (ydwiT0) among this subgroup was 80%, 93.18%, 72.73% and 95.35%, respectively.
DISCUSSION
Surgery is the main curative option in rectal cancer. The only indication for neoadjuvant CRT, supported by randomized control trials, is locally advanced disease (T3 with threatened CRM, T4 lesions).11 These lesions are hence initially treated with neoadjuvant CRT followed by surgery. Response to neoadjuvant CRT given by pathological TRG has an important bearing on the prognosis and is obtained only after surgery. However, with increasing use of restaging MRI following LCCRT, there have been several attempts to identify MRI features that predict response to LCCRT.
Researchers initially assessed the role of T2W MR volumetry in predicting response with conflicting results.12 Following this, DWI was studied extensively as a non-invasive tool which assesses treatment response in various oncologic conditions.13
A few studies have shown DWI to have a higher sensitivity in the detection of residual tumour, clearance of mesorectal fascia and also in identifying pathological complete responders.14–20 It has been postulated that DWI can be used to identify early radiation-induced fibrosis and hence prevent overstaging of disease. Delineation of residual tumour might be better appreciated on DWI than on conventional T2W images.14,21 With this knowledge in the background, we aimed to identify the best MRI predictors of CR to neoadjuvant CRT (ypT0) in LARC.
Among the various parameters we studied, only two MRI features best predicted CR. They were CR predicted by the radiologist on B800 DWI (ydwiT0) and TVRR of >95% on DWI. None of the other MRI findings were helpful in predicting CR. The diagnostic performance of ydwiT0 as predicted by the radiologist was excellent (AUC = 0.881, PPV = 75%, NPV = 96%), with substantial-to-excellent agreement between the observers. The results are in concordance with some of the other studies.19–22 High NPV of DWI shows its usefulness in ruling out CR, as also shown by Sassen et al23 in a retrospective study. In another multicentre retrospective study of 120 patients by Lambregts et al,24 the sensitivity for the prediction of CR increased from 0–40% to 52–64% after the addition of DWI with an equally high specificity of 89–98%. Song et al, in a retrospective study on 50 patients, found that adding DWI to T2W MRI is helpful for detecting residual tumour compared with T2W or positron emission tomography/CT alone. The diagnostic accuracy increased for the two readers from 64 and 76% to 86 and 90%, while the sensitivity improved from 64–77% and 91–98%.25 Kim et al also reported similar findings. On the other hand, Wu et al26 in a meta-analysis of 14 studies involving 751 patients reported a non-significant improvement in sensitivity for the prediction of tumour response after addition of DWI to T2W MRI.
Secondly, the tumour TVRR of >95% as measured on DWI was useful in predicting CR with an AUC of 0.843. Multivariate analysis showed that individual volume measurements obtained on T2 HR images and DWI obtained on pre- and post-CRT imaging were not useful in predicting CR. These results are in concordance with most studies that have shown benefit in performing TVRR for assessing disease response.27,28 However, most studies have evaluated TVRR based on T2W volumetry. Their results indicate that a TVRR of >70–80% had a significant association with CR.27–29 In a prospective study of 34 patients, Kim et al12 found no difference in the TVRR on T2 HR MRI among the responders (TRG 1 and 2) and non-responders (TRG 3–5).
Studies on DWI revealed that volume analysis on post-CRT DWI was more accurate than on T2W images for the prediction of CR. In a retrospective study of 35 patients, Jung et al30 observed a significant difference in the post-CRT volumes measured on DWI among the responders (2.1 cm3) and non-responders (6 cm3). Curvo-Semedo et al,7 in another retrospective study of 50 patients, observed similar findings, median values measuring 0.03 and 1.5 cm3 among the 2 groups, and they found that TVRR measured on T2 weighted images and DWI were equally accurate in assessing response. However, in our series, we found that neither post-CRT volumes measured on T2 HR images or DWI nor the TVRR measured on T2 HR MRI was useful in predicting CR. The only volume parameter which was useful in predicting response was TVRR >95% measured on DWI. While some studies have shown ADC values to be a useful predictor of response, others have shown no such use. High post-CRT ADC and low pre-CRT ADC values were found to be helpful in predicting CR,3,19,31 and in post-CRT MRI, the viable tumour had lower ADC value (0.93 × 10−3 mm2 s−1) than non-viable tumour (1.55 × 10−3 mm2 s−1).25 According to Sun et al,3 early increase in ADC along with a low pre-treatment ADC value correlated with a good response to chemoradiation. However, we did not find any significant difference in the ADC values, TAIR and ∆ADC values between the complete responders and non-responders. There are other studies which have shown similar results.7,14 This discrepancy may be related to the fact that a significant component of our study population comprised of the mucinous and signet-ring-cell type of adenocarcinoma (approximately 13%). These tumours demonstrate a T2 shine-through effect on DWI, and in view of this, the ADC values were high on both pre- and post-CRT ADC maps. These tumours are also known to be resilient to CRT.
A subgroup analysis was performed among the patients showing rectal cancer with restricted diffusion (n = 54). This was performed because in the tumours showing facilitated diffusion (T2 shine-through effect), the prediction of CR on DWI alone is not possible. It was thought that this might influence the results. However, there was no significant improvement in the diagnostic performance of DWI in predicting complete responders when only tumours with foci of restricted diffusion on pre-CRT MRI were analysed separately.
Overall, our results are in concordance with most studies which have shown promise in the use of DWI in radiated tissues. Although the usefulness of all the described parameters could not be confirmed, we observed that visual assessment of DWI alone and tumour volume reduction of >95% on DWI were the most useful MRI predictors of pCR. The clinical relevance would be that these parameters can be used for the selection of patients with CR, thereby aiding in prognostication and also allowing an option for conservative management. As there was no statistically significant difference in the diagnostic performance of these two parameters and volume measurements being more cumbersome, visual assessment alone can provide the same information with a high diagnostic accuracy.
Our study has a few limitations. Firstly, we assessed the volume of the tumour on DWI by manually tracing the areas of restricted diffusion, and the volume measured may not be accurate. Use of the parametric response map may have increased the accuracy. Secondly, we assessed the mean ADC value of the tumour in the same locations of the tumour pre-CRT and post CRT. This method does not take into account the heterogeneity in the region assessed. This may have led to no significant change in ADC between the pre-CRT and post CRT. A histogram analysis of the tumour may have given a more accurate picture of post-treatment change in ADC. But, this was beyond the scope of this study. Thirdly, it must be admitted that the conclusions were based on 11 patients with pCR among the 64 recruited patients with LARC. Thus, findings of this study may need to be tested in larger number of patients. Lastly, this study assessed the primary tumour only. The nodal stage was not assessed separately to identify the tumour response. However, this was not clinically relevant, as surgery was not withheld in any of our patients owing to progression of nodal involvement.
In conclusion, the visual assessment of CR on DWI (ydwiT0) is an excellent predictor of pCR with accuracy comparable with volumetric assessment of the tumour volume reduction. Quantitative methods, although promising, are cumbersome and are not standardized among various studies. CR predicted by the visual assessment of B800 DWI can be used as a more practical method of predicting complete responders in day-to-day practice.
Contributor Information
Kirthi Sathyakumar, Email: kirthi86s@yahoo.com, kirthi86sk@gmail.com.
Anuradha Chandramohan, Email: anuradhachandramohan@gmail.com.
Dipti Masih, Email: dlondz@yahoo.com.
Mark Ranjan Jesudasan, Email: ranjanbernice@cmcvellore.ac.in.
Anna Pulimood, Email: annapulimood@cmcvellore.ac.in.
Anu Eapen, Email: anuepn@yahoo.com.
REFERENCES
- 1.Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging–detected tumour response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011; 29: 3753–60. doi: 10.1200/JCO.2011.34.9068 [DOI] [PubMed] [Google Scholar]
- 2.Deo S, Kumar S, Shukla NK, Kar M, Mohanti BK, Sharma A, et al. Patient profile and treatment outcome of rectal cancer patients treated with multimodality therapy at a regional cancer center. Indian J Cancer 2004; 41: 120–4. [PubMed] [Google Scholar]
- 3.Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumour histopathologic downstaging1. Radiology 2010; 254: 170–8. doi: 10.1148/radiol.2541082230 [DOI] [PubMed] [Google Scholar]
- 4.Sun YS, Cui Y, Tang L, Qi LP, Wang N, Zhang XY, et al. Early evaluation of cancer response by a new functional biomarker: apparent diffusion coefficient. AJR Am J Roentgenol 2011; 197: W23–29. doi: 10.2214/AJR.10.4912 [DOI] [PubMed] [Google Scholar]
- 5.Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology 2013; 268: 330–44. doi: 10.1148/radiol.13121361 [DOI] [PubMed] [Google Scholar]
- 6.Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumour clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 2011; 260: 771–80. doi: 10.1148/radiol.11102135 [DOI] [PubMed] [Google Scholar]
- 7.Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 2011; 260: 734–43. doi: 10.1148/radiol.11102467 [DOI] [PubMed] [Google Scholar]
- 8.Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics 2009; 29: 1797–810. doi: 10.1148/rg.296095521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumour regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–6. doi: [DOI] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 11.Hyams DM, Mamounas EP, Petrelli N, Rockette H, Jones J, Wieand HS, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum 1997; 40: 131–9. doi: 10.1007/BF02054976 [DOI] [PubMed] [Google Scholar]
- 12.Kim YC, Lim JS, Keum KC, Kim KA, Myoung S, Shin SJ, et al. Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging 2011; 34: 570–6. doi: 10.1002/jmri.22696 [DOI] [PubMed] [Google Scholar]
- 13.Mannelli L, Kim S, Hajdu CH, Babb JS, Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: prediction and assessment of response to transarterial chemoembolization. Preliminary experience. Eur J Radiol 2013; 82: 577–82. [DOI] [PubMed]
- 14.Kim YC, Lim JS, Keum KC, Kim KA, Myoung S, Shin SJ, et al. Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging 2011; 34: 570–6. doi: 10.1002/jmri.22696 [DOI] [PubMed] [Google Scholar]
- 15.Carbone SF, Pirtoli L, Ricci V, Venezia D, Carfagno T, Lazzi S, et al. Assessment of response to chemoradiation therapy in rectal cancer using MR volumetry based on diffusion-weighted data sets: a preliminary report. Radiol Med 2012; 117: 1112–24. doi: 10.1007/s11547-012-0829-3 [DOI] [PubMed] [Google Scholar]
- 16.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002; 360: 307–8. doi: 10.1016/S0140-6736(02)09520-X [DOI] [PubMed] [Google Scholar]
- 17.Carbone SF, Pirtoli L, Ricci V, Carfagno T, Tini P, Lazzi S, et al. Diffusion-weighted MR volumetry for assessing the response of rectal cancer to combined radiation therapy with chemotherapy. Radiology 2012; 263: 311. doi: 10.1148/radiol.12112454 [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumour response to neoadjuvant chemo- and radiation therapy. Radiology 2009; 253: 116–25. doi: 10.1148/radiol.2532090027 [DOI] [PubMed] [Google Scholar]
- 19.Ha HI, Kim AY, Yu CS, Park SH, Ha HK. Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol 2013; 23: 3345–53. doi: 10.1007/s00330-013-2936-5 [DOI] [PubMed] [Google Scholar]
- 20.Monguzzi L, Ippolito D, Bernasconi DP, Trattenero C, Galimberti S, Sironi S. Locally advanced rectal cancer: value of ADC mapping in prediction of tumour response to radiochemotherapy. Eur J Radiol 2013; 82: 234–40. doi: 10.1016/j.ejrad.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 21.MERCURY Study Group. Extramural depth of tumour invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 2007; 243: 132–9. [DOI] [PubMed] [Google Scholar]
- 22.Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 2011; 18: 2224–31. doi: 10.1245/s10434-011-1607-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sassen S, de Booij M, Sosef M, Berendsen R, Lammering G, Clarijs R, et al. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol 2013; 23: 3440–9. doi: 10.1007/s00330-013-2956-1 [DOI] [PubMed] [Google Scholar]
- 24.Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 2011; 18: 2224–31. doi: 10.1245/s10434-011-1607-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song I, Kim SH, Lee SJ, Choi JY, Kim MJ, Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol 2012; 85: 577–86. doi: 10.1259/bjr/68424021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu LM, Zhu J, Hu J, Yin Y, Gu HY, Hua J, et al. Is there a benefit in using magnetic resonance imaging in the prediction of preoperative neoadjuvant therapy response in locally advanced rectal cancer? Int J Colorectal Dis 2013; 28: 1225–38. doi: 10.1007/s00384-013-1676-y [DOI] [PubMed] [Google Scholar]
- 27.Yeo SG, Kim DY, Park JW, Oh JH, Kim SY, Chang HJ, et al. Tumour volume reduction rate after preoperative chemoradiotherapy as a prognostic factor in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2012; 82: e193–9. doi: 10.1016/j.ijrobp.2011.03.022 [DOI] [PubMed] [Google Scholar]
- 28.Kang JH, Kim YC, Kim H, Kim YW, Hur H, Kim JS, et al. Tumour volume changes assessed by three-dimensional magnetic resonance volumetry in rectal cancer patients after preoperative chemoradiation: the impact of the volume reduction ratio on the prediction of pathologic complete response. Int J Radiat Oncol Biol Phys 2010; 76: 1018–25. doi: 10.1016/j.ijrobp.2009.03.066 [DOI] [PubMed] [Google Scholar]
- 29.Nougaret S, Rouanet P, Molinari N, Pierredon MA, Bibeau F, Azria D, et al. MR volumetric measurement of low rectal cancer helps predict tumour response and outcome after combined chemotherapy and radiation therapy. Radiology 2012; 263: 409–18. doi: 10.1148/radiol.12111263 [DOI] [PubMed] [Google Scholar]
- 30.Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, et al. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging 2012; 35: 110–6. doi: 10.1002/jmri.22749 [DOI] [PubMed] [Google Scholar]
- 31.Ha HI, Kim AY, Yu CS, Park SH, Ha HK. Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol 2013; 23: 3345–53. doi: 10.1007/s00330-013-2936-5 [DOI] [PubMed] [Google Scholar]