Abstract
Objective:
To investigate the correlation between apparent diffusion coefficient (ADC) values and prognostic factors in patients with invasive ductal carcinoma (IDC).
Methods:
48 lesions belonging to 47 patients with histopathologically proven IDC were examined using conventional MR and diffusion-weighted imaging at a 3.0-T system. All of the patients had modified radical mastectomies or breast-sparing surgery plus axillary lymph node dissection. The ADC values acquired from the ADC maps consisted of six different b-values (0, 50, 100, 500, 1000 and 1500 s mm−2) and were compared with the patients' ages, tumour size, histological grade of the lesions, tumour localization, lesions' distance to skin surface and nipples, the existence of axillary lymph node involvement, the number of involved axillary lymph nodes, oestrogen/progesterone receptor status, peritumoral lymphovascular invasion status and the existence of human epidermal growth factor 2 (c-erbB-2) overexpression.
Results:
A statistically significant relationship was found regarding axillary lymph node involvement (p = 0.027), and oestrogen/progesterone receptor status (p = 0.013). No significant relationship was detected regarding other prognostic factors (p > 0.05).
Conclusion:
Among various prognostic factors, ADC values were significantly correlated with only axillary lymph node positivity and oestrogen/progesterone receptor status.
Advances in knowledge:
In the present study, the relationship between ADC values of IDC lesions that are acquired at a high magnetic field (3.0 T) system by using multiple b-values and some specific prognostic factors that were not evaluated before in the medical literature was investigated.
INTRODUCTION
Axillary lymph node status, younger age (<35 years), tumour size, histological grade, existence of peritumoral vascular invasion and overexpression of human epidermal growth factor 2 (HER2 or c-erbB-2) can account for the major prognostic factors in patients with breast cancer.1 Additionally, some of these prognostic factors, such as axillary lymph node status, and hormone receptor and c-erbB-2 expression in lesions affect operation procedures (sentinel lymph node biopsy or axillary dissection) and treatment alternatives.2
Ultrasonography and mammography are the primary imaging modalities for evaluating breasts that have good specificity but low sensitivity.3–5 However, in the past decade, dynamic contrast-enhanced breast MRI, including diffusion-weighted imaging (DWI), has been routinely and effectively used to evaluate breasts with mass lesions and/or dense structures, or other factors (i.e. asymmetric density and architectural distortion). DWI is an MRI technique that can provide unique information about the microenvironment and biophysical properties of tissue as well detect molecular diffusion (i.e. the Brownian motion of water molecules in biological tissues). DWI measures the apparent diffusion coefficient (ADC) of water in tissue, which is sensitive to certain parameters, such as cell organization, cell density, microstructure and microcirculation.6,7 The usefulness of DWI and the ADC value for the evaluation of primary breast lesions and axillary lymph node involvement have been researched in many studies.7–13 Those reports demonstrated that DWI may be a valuable technique for identifying and characterizing breast lesions and axillary lymph nodes, as well as for monitoring treatment response.13–16 However, reports documenting the relationship between the ADC values of primary breast lesions and the prognostic factors are lacking.
This article aimed to study the possible relationship between the ADC values of 48 invasive ductal carcinoma (IDC) lesions and the prognostic factors acquired from a 3.0-T system. To increase the accuracy and the homogeneity of the ADC measurements, six different b-values were used in the DWI protocol, namely 0, 50, 100, 500, 1000 and 1500 s mm−2.
METHODS AND MATERIALS
Patient selection
This retrospective study was approved by the institutional review board of Gulhane Military Medical School. Radiological and pathological data related to the consecutive patients with IDC who underwent surgical treatment plus axillary dissection between April 2011 and April 2015 in the university hospital were reviewed. Patients who had complete breast MRI, including DWI acquired from a 3.0-T scanner, were evaluated. Among this group, patients were excluded from the study if any of the following criteria applied: more than one malignant primary lesion in one breast; a DWI examination judged inadequate because of the motion or susceptibility artefacts during the scan; the presence of recurrent breast cancer or neoadjuvant chemotherapy history; primary lesions <1 cm due to difficulty in drawing regions of interests (ROIs); and ductal carcinoma in situ lesions. Finally, 47 females (48 breasts and 48 axillae) with histopathologically proven IDC who met the criteria were enrolled in the study. In all of the cases, the final histopathological diagnosis was made by modified radical mastectomy or breast-sparing surgery plus axillary lymph node dissection. Patients with one lymph node at the same axilla involved were accepted as lymph node positive.
MRI protocol
All of the MR examinations were performed with a 3.0-T system (Achieva® 3.0 T X-series, Philips Medical Systems, Best, The Netherlands) with a dedicated 16-channel phased-array breast coil in the prone position. DWI MR images were acquired in the axial plane by using an echo-planar imaging sequence, parallel imaging with fat suppression (spectral attenuated inversion recovery). Scanning parameters: time to repetition/echo time = 8.430/62 ms, field of view = 340 × 340 mm (right to left × anterior to posterior), matrix = 136 × 133, flip angle 90°, slice thickness = 4 mm, b-values: 0, 50, 100, 500, 1000 and 1500 s mm−2, acquisition time: 8 min 51 s.
MRI analysis
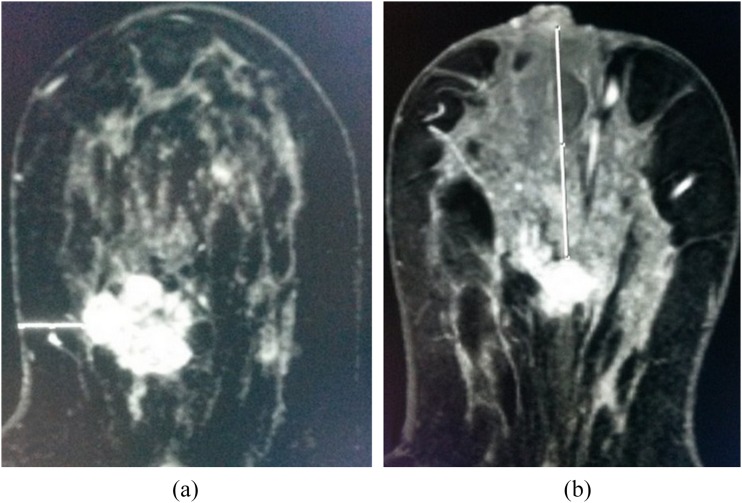
The size of the lesions and the distance from the most superficial aspect of the primary lesion to the nearest skin surface and nipple were respectively evaluated from the dynamic images obtained from the hospital's picture archiving and communication system by two radiologists (6 and 2 years' of experience in interpreting breast images, respectively) in consensus (Figure 1).
Figure 1.
(a, b) Axial dynamic early enhanced MR images of two different patients. (a) The distance between the mass and the nearest skin surface in a 42-year-old female with invasive ductal carcinoma (caliper = 13.52 mm). (b) The distance to the nipple of an invasive ductal carcinoma lesion in a 45-year-old female (caliper = 66.07 mm).
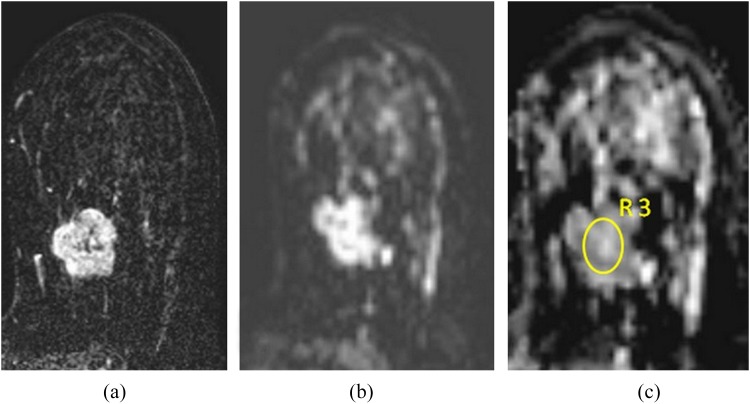
A combined ADC map was formed for each patient automatically using six different b-values from a specific software (DynaCAD revised v. 2.1.1, 2009; Invivo Corporation, Orlando, FL). Then, with the aid of the software, the ADC values of the primary masses were recorded in consensus, resulting from the average of three measurements obtained at slightly different positions of manually drawn ROIs by one of the two radiologists. Circular ROIs were placed to encompass as large an area as possible within the confines of the lesions (mean size of ROIs = 20 mm2) (Figure 2). With reference to T2 weighted images, cystic, necrotic and haemorrhagic parts of the tumour were not included.
Figure 2.
(a–c) A 41-year-old female with invasive ductal carcinoma in her right breast. The histological grade was 3, and one axillary lymph node was involved. The lesion was positive for oestrogen/progesterone receptors and for peritumoral lymphovascular invasion and was negative for human epidermal growth factor 2 overexpression. A dynamic delayed enhanced image (a) showing a 3.5-cm heterogeneous mass lesion with a lobulated and irregular margin, prominent enhancement is readily seen. Diffusion-weighted image (b) demonstrates high signal intensity and corresponding apparent diffusion coefficient (ADC) map (c) reveals diffusion restriction inside the lesion (ADC value = 0.75 s mm−2). R3, third region of interest used for measuring the ADC value.
Histopathological analysis
The pathological data of the patients with IDC who met the criteria were evaluated consecutively. The gross tumour size, histological grade, receptor status (oestrogen/progesterone), the existence of axillary lymph node involvement, peritumoral lymphovascular invasion, the number of involved axillary lymph nodes and HER2 (c-erbB-2) status that were determined by the breast pathologists in our tertiary centre were recorded.
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY. The variables were investigated using visual (histograms, probability plots) and analytical methods (Shapiro–Wilk's test) to determine whether or not they were normally distributed. Descriptive analyses were presented using tables of frequencies for the ordinal variables, medians and minimum–maximum values for the non-normally distributed and means and standard deviations for normally distributed variables. Since the ADC measurements were not normally distributed; non-parametric tests (Mann–Whitney U test, Kruskal–Wallis test) were conducted to compare ADC between the groups. Although investigating the associations between ADC and continuous variables, correlation coefficients and their significance were calculated using Spearman test. A p-value of <0.05 was considered to show a statistically significant result.
RESULTS
The mean age of our case group was 48.1 ± 8.7 years (range: 31–68 years). The pathological and radiological data of 48 lesions belonging to 47 females were evaluated. In one patient, two lesions in total were detected in two breasts.
While the median length of the lesions was 21.50 mm (range: 10.00–69.00 mm), this value was found to be 21.50 mm (range: 10.00–70.00 mm) in the pathological specimens.
Although the median distance between the lesions and the nearest skin surface was 14 mm (range: 0–36 mm), the median distance to nipple was found to be 44 mm (range: 0–126 mm).
Among 26 cases with axillary metastases, the median number of the involved axillary lymph nodes was 1.5 (range: 1–16).
The median ADC value of 48 IDC lesions was found to be 0.70 × 10−3 s mm−2 (range: 0.50–1.15 × 10−3 s mm−2). The recorded ADC values were statistically compared with the patients' ages, tumour size, tumour localization, lesions' distance to skin surface and nipple, the existence of axillary lymph node involvement, the number of involved axillary lymph nodes, histological grade of the lesions, oestrogen/progesterone receptor status, peritumoral lymphovascular invasion status and the existence of HER2 (c-erbB-2) overexpression. Among those factors, only axillary lymph node positivity and the hormone receptor status have shown a statistically significant correlation with ADC values. The relationships between the ADC values and examined prognostic factors are shown in Table 1.
Table 1.
Correlation between apparent diffusion coefficient (ADC) values and various prognostic factors of breast cancer in our case group
| Prognostic factors | Number of lesions (n) | Median ADC valuea | Correlation coefficient (rho) | p-value |
|---|---|---|---|---|
| Age | −0.085 | 0.567 | ||
| Localization | ||||
| UOQ | 25 (52.1%) | 0.70 | 0.733 | |
| UIQ | 6 (12.5%) | 0.66 | ||
| LOQ | 7 (14.6%) | 0.74 | ||
| LIQ | 5 (10.4%) | 0.685 | ||
| Periareolar | 5 (10.4%) | 0.72 | ||
| Size | 48 | 0.034 | 0.820 | |
| Histological grade | ||||
| Grade 1 | 5 (10.4%) | 0.68 | 0.582 | |
| Grade 2 | 19 (39.6%) | 0.70 | ||
| Grade 3 | 24 (50%) | 0.75 | ||
| Distance to skin surface and nipple | ||||
| Skin surface | 48 | 0.054 | 0.717 | |
| Nipple | 48 | 0.144 | 0.327 | |
| Axillary lymph node involvement | ||||
| Positive | 26 (54.2%) | 0.68 | 0.027 | |
| Negative | 22 (45.8%) | 0.78 | ||
| Number of involved lymph nodes | −0.209 | 0.306 | ||
| E/P receptors | ||||
| Positive | 38 (79.2%) | 0.69 | 0.013 | |
| Negative | 10 (20.8%) | 0.835 | ||
| PLVI | ||||
| Positive | 12 (25%) | 0.755 | 0.520 | |
| Negative | 36 (75%) | 0.69 | ||
| HER2 (c-erbB-2) status | ||||
| Positive | 25 (52.1%) | 0.71 | 0.975 | |
| Negative | 23 (47.9%) | 0.70 | ||
E/P, oestrogen/progesterone; HER2, human epidermal growth factor 2; LIQ, lower inner quadrant; LOQ, lower outer quadrant; PLVI, peritumoral lymphovascular invasion; UIQ, upper inner quadrant; UOQ, upper outer quadrant.
Statistically significant p-values are in bold.
ADC values are written as ×10−3 s mm−2.
DISCUSSION
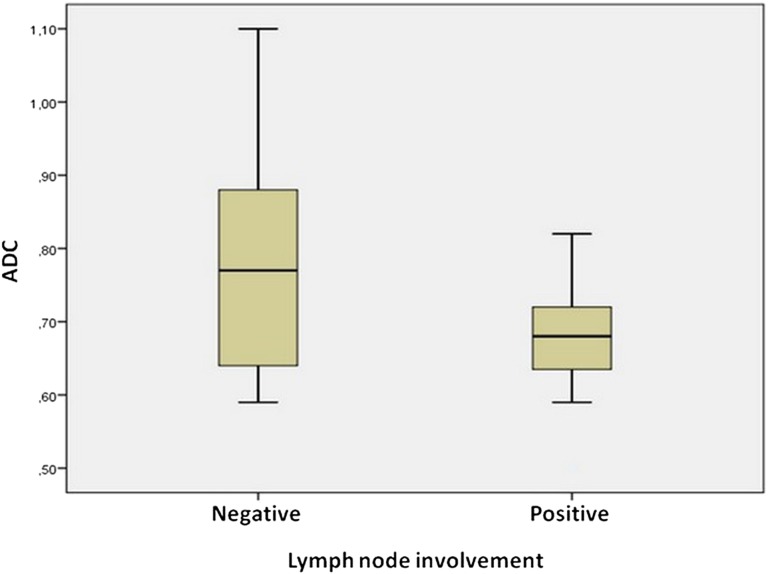
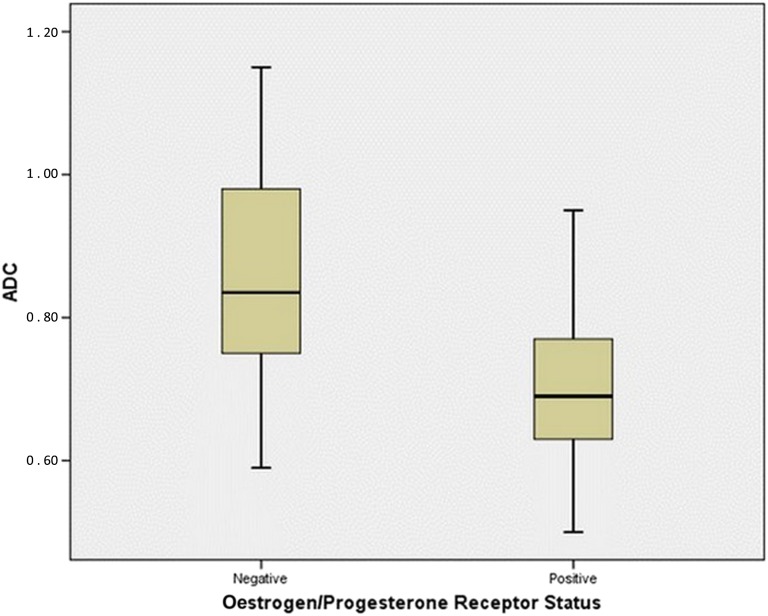
In this study's patient group, the ADC values of the IDC lesions with axillary lymph node involvement were significantly lower than for cancers without axillary involvement (Figure 3). Additionally, there was a statistically significant relationship between the ADC values and the oestrogen/progesterone receptor status of the lesions (Figure 4). However, in terms of other prognostic factors that were studied, no statistically significant relationship was found with the ADC values.
Figure 3.
Box plot showing the distribution of apparent diffusion coefficient (ADC) values of invasive ductal carcinoma lesions according to axillary lymph node involvement. As shown, the ADC value of the lesions with axillary lymph node involvement is significantly lower than that of the lesions without lymph node involvement.
Figure 4.
Box plot showing the distribution of apparent diffusion coefficient (ADC) values of invasive ductal carcinoma lesions according to oestrogen/progesterone receptor status. As shown, the ADC value of the lesions with oestrogen/progesterone receptor positivity is significantly lower than that of the lesions without those receptors.
The median ADC value of IDC lesions was 0.70 × 10−3 s mm−2 in the present study, which is lower than previous reports that were performed with 1.5-T systems (ADC range: 0.89 to 1.22 ×10−3 s mm−2).10,17–21 In their study performed with a 3.0-T scanner, Park et al22 reported that the mean ADC value of IDC lesions was 0.88 ± 0.15 × 10−3 s mm−2 (b-values: 0 and 1000 s mm−2), which is higher than the results of the present study. The existence of a negative correlation between the maximum b factor15 and the ADC values was previously reported and that small b-values produce larger ADC values.17,21,23,24 In the present study, the median ADC value was lower than the results of the previous reports mentioned above. This lower ADC value may be because of higher spatial resolution of the 3.0-T system and lower partial volume artefacts compared with the 1.5-T system and the exclusion of <1 cm lesions from the study group. Additionally, by using multiple b-values, we believe that more homogeneous ADC measurements were obtained, which may be the main reason why this study's ADC measurements are different (lower) from previous studies in the literature.
The existence of axillary lymph node metastasis is one of the major prognostic factors in breast cancer.25 Kim et al26 and Park et al22 found no statistically significant correlation between the existence of axillary lymph node involvement and the ADC values of the primary masses in their case groups. By contrast, Kamitani et al2 found that axillary node positivity was positively correlated with the ADC values. Conversely, Razek et al27 reported an opposite result that lower ADC values of IDC lesions were correlated with axillary lymph node positivity in their 59-patient case study performed with a 1.5-T system. Similar to Razek et al, an inverse correlation between those variables was also found in the present study. In this study, the median ADC value of the lesions with axillary lymph node involvement was significantly lower than that of the lesions without lymph node involvement. This result is opposed to the results of the previous studies whose measurements mostly show a positive correlation. This difference may arise from the higher spatial resolution of the 3.0-T system and more homogeneous and correct ADC measurements with the help of the higher resolution, lower partial volume artefacts and multiple b-values that were used. Among those studies mentioned above, only Park et al used a 3.0-T scanner, a study which also included patients with <1 cm lesions. Although both studies were performed with a 3.0-T system, the differences in ADC values between the present study and that of Park et al may arise from the minor case number, that is excluding patients with <1 cm lesions, and/or the multiple b-values used in this research.
Oestrogen/progesterone receptor status is another major factor that affects the operation procedures and treatment alternatives.2 Kim et al26 found no significant relationship between the ADC values and the hormone receptor status. However, Jatoi et al25 reported that oestrogen/progesterone receptor expression has a weak but significant negative correlation with ADC values. Additionally, Kamitani et al2 reported significantly lower ADC values in oestrogen- and progesterone-positive breast cancer masses. However, Park et al22 found no relationship between oestrogen or progesterone receptor status and the ADC values in their case group. In the present study, we found statistically significant lower ADC values in the receptor-positive group.
A younger age at diagnosis (<35 years) is another major prognostic factor in breast cancer,1 and it has been correlated with axillary lymph node status.28,29 A probable relationship between this variable and the ADC values was researched in this study's case group. However, no statistically significant relationship was detected between those variables. To the best of the researchers' knowledge, no previous report exists in the literature regarding the relationship between patient age and the ADC values of IDC lesions.
Tumour localization is also a prognostic factor for breast cancer. 25 out of the 48 IDC lesions (52.1%) were at the upper outer quadrant in this study's case group, whereas the other localizations were nearly at the same rate. A probable correlation between tumour localizations and the ADC values of the same lesions was evaluated. However, no statistically significant correlation between those variables was detected in this study's group. To the best of the researchers' knowledge, no previous report exists in the literature regarding the relationship between the ADC values and tumour localization.
In the current study, the size of the tumors and their relationship with the ADC values were also evaluated. Tumour size is a very important prognostic factor for patients with breast cancer, and it is directly associated with overall survival.30 Razek et al27 reported a positive correlation between tumour size and the ADC values in their 59-patient case study. Park et al22 divided their 110 patients into 3 subgroups (<2, 2–5 and >5 cm) and made multiple comparisons with their ADC values. They found a statistically significant relationship between the three groups in multiple comparisons. On the other hand, Kim et al26 did not find a significant correlation between those two variables. A statistically significant relationship between the ADC value and radiological tumour size was also not detected in this study.
Cellularity is a crucial index for tumour grade.18 Now, it is known that tumour cellularity is inversely correlated with the histological grade, and high cellularity is related to markedly decreased ADC values.8,9 Razek et al27 reported that the ADC values of grade 3 IDC lesions were lower than those of grades 1 and 2. By contrast, Yoshikawa et al18 and Kamitani et al2 found no correlation between those two variables. In the current study, although ROIs were manually drawn, the cystic, necrotic and haemorrhagic parts of the lesions were excluded using the reference of T2 weighted images. ROIs were drawn as large as possible for better reflecting the diffusion characteristics of the entire lesion as performed in similar studies in the medical literature.2,7,22,31–34 Similar to the last two reports above, no significant relationship was found between the ADC values and the histological grades of the tumours in this study's group.
It is reasonable that tumours closer to the rich plexus of lymphatics in the dermal and subareolar locations have greater access to the lymphatic networks than tumours in other locations, therefore resulting in a greater risk of lymphatic dissemination and finally a higher risk of axillary lymph node metastases.35 Based on this idea the probable relationship between the ADC values and the distance to skin surface and nipple of primary lesions was evaluated in the present study. But no statistically significant relationship was detected between the ADC values and the contiguity of the lesions to the skin surface and nipple. To the best of the researchers' knowledge, there is no previous report regarding this subject in the medical literature except the present study.
Assessing the number of the involved axillary lymph nodes in patients with breast cancer was another main subject of our study. We found 26 positive axillae out of the 48 and the median number of the involved axillary lymph node was 1.5. We also did not detect statistically significant relationship between the ADC values of the IDC lesions and the number of the involved axillary lymph nodes on the same side. To the best of the researchers' knowledge, no previous report exists in the literature regarding the relationship between those two variables.
Lymphovascular invasion is defined as tumour emboli present within a definite endothelial-lined space in the breast-surrounding invasive carcinoma.36 The presence of this finding may help to identify who is at high risk for axillary lymph node involvement and distant metastasis,37,38 and it has been accepted as a major prognostic factor in patients with lymph node-negative invasive breast cancer.39 The reports regarding peritumoral lymphovascular invasion and its relationship to ADC values are very limited in the medical literature. Kamitani et al2 did not find any significant relationship between peritumoral vascular invasion and the ADC values in their 81-patient case study. Similarly, no significant correlation between those two variables was found in the present study.
Overexpression of HER2 (c-erbB-2) is seen in 20–25% of the patients with IDC.40 HER2-positive cells have more malignant phenotypes than HER2-negative cells, which is associated with a higher rate of cell proliferation, invasion and metastasis. ADC values of HER2-positive IDC are assumed to be lower than those of HER2-negative IDC owing to increased cellularity.23 However, Park et al22 reported an opposite result and showed significantly higher ADC values in their study involving 110 IDC cases. On the other hand, Kamitani et al2 found no correlation between HER2 overexpression and the ADC values of IDC lesions. The current study also did not detect any relationship between these two variables.
In recent years, 3.0-T scanners that provide great spatial resolution and signal-to-noise ratios are widely used in many institutions worldwide. High-resolution DWI studies performed better, in terms of diagnostic accuracy, than studies performed with a lower spatial resolution, which is probably due to reduced partial volume effects.8,21,41 In addition, Matsuoka et al42 reported that small cancers were more clearly visible on DWI at 3.0 T than at 1.5 T. On the other hand, higher magnetic fields increase susceptibility artefacts and sensitivity to motion that distorts the images. The current study examined a possible relationship between the ADC values of IDC lesions and the prognostic factors of breast cancer, assisted by the added advantages of a 3.0-T system. The aim was to evaluate many of the prognostic factors researched in earlier work that were also found in this study's group, as well as add some factors that were not evaluated before in the medical literature. Although some reports about this subject performed with 1.5-T scanners exist in the literature,2,13,14 the number of studies performed with a 3.0-T system, as used in the present study, are limited.
The ADC in each voxel is a sum of true ADCs and noise. The noise must be minimized to obtain the true ADC value. This is especially important for small lesions, where the mean ADC relies on only a few voxels. Also, the assessment of tumour borders improves with higher precision.31 Although using higher b-values may distort the image, in this way, the ADC difference between benign and malign breast lesions is bigger as well.10,17,43 It is also known that with high b-values, the signal-to-noise ratio decreases.44 The b-value is generally selected to be <1000 s mm−2 in routine breast DWI practice, although the optimum b-value for tumour detection has not yet been agreed upon.32 However, there have been many recent studies regarding the use of the b-values >1000 s mm−2 in breast DWI.23,31,33,44–47 Tamura et al32 reported that increased contrast between the normal breast tissue and malignant tumour with the b-value from b = 0 to around 1500 s mm−2 and decreased contrast with b > 1500 s mm−2 in almost every case of their study. They concluded that the maximum contrast b-values were of high frequency at approximately b = 1500 s mm−2. However, they did not suggest the use of high b-values >1000 s mm−2 only and recommended performing multiple b-value combinations (b = 750–850 and 1400–1500 s mm−2). Takanaga et al48 also reported that b = 1500 s mm−2 provided the maximum contrast between normal breast tissue and malignant breast tumour. In the same study, which was performed with a 3.0-T scanner, they concluded that the highest contrast b-value for 3.0 T might be higher than that of 1.5 T. In another study, Ochi et al33 reported that ADC values obtained from b = 1500 s mm−2 were useful to improve the diagnostic accuracy for malignant tumours and benign lesions. The same study also showed that b-values of 0 and 1500 s mm−2 provided superior detection capability for malignant breast lesions. In the current study, by adding b = 1500 s mm−2 as one of the b-values, we aimed to (1) obtain more accurate ADC maps and measurements, (2) increase the normal breast-to-malignant tumour contrast and (3) provide a superior detection capability for the malignant breast lesions with 3.0-T system.
In the present study, a significant relationship was found between axillary lymph node involvement and the ADC values of IDC lesions, which is in contrast to most of the previous reports in the literature. If the vital role of axillary lymph node positivity in the survival and the treatment plan in patients with breast cancer is considered, the ability to know the axillary lymph node status without invasive procedures provides enormous benefits. Hence, these research findings suggest that the ADC values of primary lesions may provide this type of information in the future with the help of further studies involving 3.0-T scanners, larger case groups and a prospective model.
The present study has some limitations. Although we included all consecutive patients with IDC who were operated in our tertiary breast centre to the present study, the number of the cases was limited owing to the lack of MR examinations performed with the 3.0-T scanner in most of them. Second, because other types of breast cancers can have higher ADC values8 that can affect our results (for example, mucinous carcinoma), these carcinomas were not included in this study, except IDC. Third, the six acquired b-values increased the total DWI scan time (8 min 51 s) compared with scans with two b-values. This could cause more susceptibility and movement artefacts. However, despite this time increase, the image quality was very satisfactory. Fourth, the limited number of oestrogen/progesterone receptor-negative lesions (n = 10) and lesions with positive peritumoral lymphovascular invasion (n = 12) may have affected related statistical results. Finally, not including the <1 cm lesions in the study due to probable partial volume artefacts may be viewed a limitation.
CONCLUSION
The relationship between the ADC values of IDC lesions and well-known prognostic factors of breast cancer were evaluated in the present study. Additionally, the probable correlation between ADC values and some unusual factors, such as patients' age, tumour localization, distance to skin surface and nipple, and the number of involved axillary lymph nodes, were also examined, which were not previously reported in the medical literature. Among all of these factors, a statistically significant correlation was only found regarding axillary lymph node positivity and the hormone receptor status of IDC lesions.
Contributor Information
Inanc Guvenc, Email: inancguvenc@yahoo.com.
Sinan Akay, Email: akaysinan04@yahoo.com.
Selami Ince, Email: turkselo@yahoo.com.
Ramazan Yildiz, Email: ryildiz@gata.edu.tr.
Zafer Kilbas, Email: zkilbas@gata.edu.tr.
Fahrettin G Oysul, Email: guven@oysul.com.
Mustafa Tasar, Email: mtasar@gata.edu.tr.
REFERENCES
- 1.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005; 16: 1569–83. doi: 10.1093/annonc/mdi326 [DOI] [PubMed] [Google Scholar]
- 2.Kamitani T, Matsuo Y, Yabuuchi H, Fujita N, Nagao M, Jinnouchi M, et al. Correlations between apparent diffusion coefficient values and prognostic factors of breast cancer. Magn Reson Med Sci 2013; 12: 193–9. doi: 10.2463/mrms.2012-0095 [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology 2007; 244: 672–91. doi: 10.1148/radiol.2443051661 [DOI] [PubMed] [Google Scholar]
- 4.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med 2008; 148: 671–9. doi: 10.7326/0003-4819-148-9-200805060-00007 [DOI] [PubMed] [Google Scholar]
- 5.Riedl CC, Ponhold L, Flöry D, Weber M, Kroiss R, Wagner T, et al. Magnetic resonance imaging of the breast improves detection of invasive cancer, preinvasive cancer, and premalignant lesions during surveillance of women at high risk for breast cancer. Clin Cancer Res 2007; 13: 6144–52. doi: 10.1158/1078-0432.CCR-07-1270 [DOI] [PubMed] [Google Scholar]
- 6.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986; 161: 401–7. doi: 10.1148/radiology.161.2.3763909 [DOI] [PubMed] [Google Scholar]
- 7.Luo N, Su D, Jin G, Liu L, Zhu X, Xie D, et al. Apparent diffusion coefficient ratio between axillary lymph node with primary tumor to detect nodal metastasis in breast cancer patients. J Magn Reson Imaging 2013; 38: 824–8. doi: 10.1002/jmri.24031 [DOI] [PubMed] [Google Scholar]
- 8.Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 2008; 7: 23–9. doi: 10.2463/mrms.7.23 [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002; 16: 172–8. doi: 10.1002/jmri.10140 [DOI] [PubMed] [Google Scholar]
- 10.Woodhams R, Matsunaga K, Iwabuchi K, Kan S, Hata H, Kuranami M, et al. Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 2005; 29: 644–9. doi: 10.1097/01.rct.0000171913.74086.1b [DOI] [PubMed] [Google Scholar]
- 11.Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 2005; 4: 35–42. doi: 10.2463/mrms.4.35 [DOI] [PubMed] [Google Scholar]
- 12.Yabuuchi H, Matsuo Y, Okafuji T, Kamitani T, Soeda H, Setoguchi T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging 2008; 28: 1157–65. doi: 10.1002/jmri.21570 [DOI] [PubMed] [Google Scholar]
- 13.Kamitani T, Hatakenaka M, Yabuuchi H, Matsuo Y, Fujita N, Jinnouchi M, et al. Detection of axillary node metastasis using diffusion-weighted MRI in breast cancer. Clin Imaging 2013; 37: 56–61. doi: 10.1016/j.clinimag.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Youk JH, Kim JA, Gweon HM, Kim EK, Ryu YH, et al. Role of diffusion-weighted MRI: predicting axillary lymph node metastases in breast cancer. Acta Radiol 2014; 55: 909–16. doi: 10.1177/0284185113509094 [DOI] [PubMed] [Google Scholar]
- 15.Tsushima Y, Takahashi-Taketomi A, Endo K. Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging 2009; 30: 249–55. doi: 10.1002/jmri.21854 [DOI] [PubMed] [Google Scholar]
- 16.Vandecaveye V, De Keyzer F, Nuyts S, Deraedt K, Dirix P, Hamaekers P, et al. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys 2007; 67: 960–71. doi: 10.1016/j.ijrobp.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita T, Yashiro N, Ihara N, Funatu H, Fukuma E, Narita M. Diffusion-weighted half-Fourier single-shot turbo spin echo imaging in breast tumors: differentiation of invasive ductal carcinoma from fibroadenoma. J Comput Assist Tomogr 2002; 26: 1042–6. doi: 10.1097/00004728-200211000-00033 [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Mochizuki T, et al. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med 2008; 26: 222–6. doi: 10.1007/s11604-007-0218-3 [DOI] [PubMed] [Google Scholar]
- 19.Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol 2007; 17: 2646–55. doi: 10.1007/s00330-007-0621-2 [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Kikuchi K, et al. Comparison of breast cancer detection by diffusion-weighted magnetic resonance imaging and mammography. Radiat Med 2007; 25: 218–23. doi: 10.1007/s11604-007-0128-4 [DOI] [PubMed] [Google Scholar]
- 21.Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol 2007; 8: 390–6. doi: 10.3348/kjr.2007.8.5.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging 2015; 41: 175–82. doi: 10.1002/jmri.24519 [DOI] [PubMed] [Google Scholar]
- 23.Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW. In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 2002; 15: 693–704. doi: 10.1002/jmri.10116 [DOI] [PubMed] [Google Scholar]
- 24.Buadu LD, Murakami J, Murayama S, Hashiguchi N, Sakai S, Masuda K, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology 1996; 200: 639–49. doi: 10.1148/radiology.200.3.8756909 [DOI] [PubMed] [Google Scholar]
- 25.Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol 1999; 17: 2334–40. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 2009; 30: 615–20. doi: 10.1002/jmri.21884 [DOI] [PubMed] [Google Scholar]
- 27.Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed 2010; 23: 619–23. doi: 10.1002/nbm.1503 [DOI] [PubMed] [Google Scholar]
- 28.Anan K, Mitsuyama S, Tamae K, Nishihara K, Iwashita T, Abe Y, et al. Axillary lymph node metastases in patients with small carcinomas of the breast: is accurate prediction possible? Eur J Surg 2000; 166: 610–5. [DOI] [PubMed] [Google Scholar]
- 29.Olivotto IA, Jackson JS, Mates D, Andersen S, Davidson W, Bryce CJ, et al. Prediction of axillary lymph node involvement of women with invasive breast carcinoma: a multivariate analysis. Cancer 1998; 83: 948–55. doi: [DOI] [PubMed] [Google Scholar]
- 30.Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit Rev Oncol Hematol 1999; 31: 209–23. doi: 10.1016/S1040-8428(99)00034-7 [DOI] [PubMed] [Google Scholar]
- 31.Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology 2009; 253: 341–51. doi: 10.1148/radiol.2532081718 [DOI] [PubMed] [Google Scholar]
- 32.Tamura T, Murakami S, Naito K, Yamada T, Fujimoto T, Kikkawa T. Investigation of the optimal b-value to detect breast tumors with diffusion weighted imaging by 1.5-T MRI. Cancer Imaging 2014; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochi M, Kuroiwa T, Sunami S, Murakami J, Miyahara S, Nagaie T, et al. Diffusion-weighted imaging (b value = 1500 s/mm(2)) is useful to decrease false-positive breast cancer cases due to fibrocystic changes. Breast Cancer 2013; 20: 137–44. doi: 10.1007/s12282-011-0319-9 [DOI] [PubMed] [Google Scholar]
- 34.Chen X, He XJ, Jin R, Guo YM, Zhao X, Kang HF, et al. Conspicuity of breast lesions at different b values on diffusion-weighted imaging. BMC Cancer 2012; 12: 334. doi: 10.1186/1471-2407-12-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suami H, Pan WR, Taylor GI. Historical review of breast lymphatic studies. Clin Anat 2009; 22: 531–6. doi: 10.1002/ca.20812 [DOI] [PubMed] [Google Scholar]
- 36.de Mascarel I, MacGrogan G, Debled M, Sierankowski G, Brouste V, Mathoulin-Pélissier S, et al. D2-40 in breast cancer: should we detect more vascular emboli? Mod Pathol 2009; 22: 216–22. doi: 10.1038/modpathol.2008.151 [DOI] [PubMed] [Google Scholar]
- 37.Woo CS, Silberman H, Nakamura SK, Ye W, Sposto R, Colburn W, et al. Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg 2002; 184: 337–40. doi: 10.1016/S0002-9610(02)00950-9 [DOI] [PubMed] [Google Scholar]
- 38.Davis BW, Gelber R, Goldhirsch A, Hartmann WH, Hollaway L, Russell I, et al. Prognostic significance of peritumoral vessel invasion in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Hum Pathol 1985; 16: 1212–8. doi: 10.1016/S0046-8177(85)80033-2 [DOI] [PubMed] [Google Scholar]
- 39.de Mascarel I, Bonichon F, Durand M, Mauriac L, MacGrogan G, Soubeyran I, et al. Obvious peritumoral emboli: an elusive prognostic factor reappraised. Multivariate analysis of 1320 node-negative breast cancers. Eur J Cancer 1998; 34: 58–65. doi: 10.1016/S0959-8049(97)00344-4 [DOI] [PubMed] [Google Scholar]
- 40.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 2003; 95: 142–53. doi: 10.1093/jnci/95.2.142 [DOI] [PubMed] [Google Scholar]
- 41.Wenkel E, Geppert C, Schulz-Wendtland R, Uder M, Kiefer B, Bautz W, et al. Diffusion weighted imaging in breast MRI: comparison of two different pulse sequences. Acad Radiol 2007; 14: 1077–83. doi: 10.1016/j.acra.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka A, Minato M, Harada M, Kubo H, Bandou Y, Tangoku A, et al. Comparison of 3.0-and 1.5-tesla diffusion-weighted imaging in the visibility of breast cancer. Radiat Med 2008; 26: 15–20. doi: 10.1007/s11604-007-0187-6 [DOI] [PubMed] [Google Scholar]
- 43.Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol 2003; 45: 169–84. doi: 10.1016/S0720-048X(02)00303-0 [DOI] [PubMed] [Google Scholar]
- 44.Sinha S, Sinha U. Functional magnetic resonance of human breast tumors: diffusion and perfusion imaging. Ann N Y Acad Sci 2002; 980: 95–115. doi: 10.1111/j.1749-6632.2002.tb04891.x [DOI] [PubMed] [Google Scholar]
- 45.Woodhams R, Kakita S, Hata H, Iwabuchi K, Umeoka S, Mountford CE, et al. Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol 2009; 193: 260–6. doi: 10.2214/AJR.08.1670 [DOI] [PubMed] [Google Scholar]
- 46.Tamura T, Usui S, Murakami S, Arihiro K, Akiyama Y, Naito K, et al. Biexponential signal attenuation analysis of diffusion-weighted imaging of breast. Magn Reson Med Sci 2010; 9: 195–207. doi: 10.2463/mrms.9.195 [DOI] [PubMed] [Google Scholar]
- 47.Tamura T, Usui S, Murakami S, Arihiro K, Fujimoto T, Yamada T, et al. Comparisons of multi b-Value DWI signal analysis with pathological specimen of breast cancer. Magn Reson Med 2012; 68: 890–7. doi: 10.1002/mrm.23277 [DOI] [PubMed] [Google Scholar]
- 48.Takanaga M, Hayashi N, Miyachi T, Kawashima H, Hamaguchi T, Ohno N, et al. Influence of b value on the measurement of contrast and apparent diffusion coefficient in 3.0 Tesla breast magnetic resonance imaging. [In Japanese.] Jpn J RadioTech 2012; 68: 201–8. doi: 10.6009/jjrt.2012_JSRT_68.3.201 [DOI] [PubMed] [Google Scholar]