Abstract
Objective:
This study evaluated the potential benefit of a split-parotid delineation approach on the parotid gland in the treatment planning of patients with nasopharyngeal carcinoma (NPC).
Methods:
50 patients with NPC with parapharyngeal space (PPS) and/or level IIa cervical node involvements were divided into three groups: PPS only, level IIa cervical node only and both. Two volumetric-modulated arc therapy plans were computed. The first plan (control) was generated based on the routine treatment-planning protocol, while the second plan (test) was computed with the split-parotid delineation approach, in which a line through the anterolateral margin of the retromandibular vein was created that divided the parotid gland into anterolateral and posteromedial subsegments. For the test plan, the anterolateral subsegment was prescribed, with a dose constraint of 25 Gy in the plan optimization. Dosimetric data of the parotid gland, target volumes and selected organs at risk (OARs) were compared between the control and test plans.
Results:
The mean dose to the anterolateral subsegment of the parotid gland in all three groups was kept below 25 Gy. The test plan demonstrated significantly lower mean parotid dose than the control plan in the entire gland and the anterolateral subsegment in all three groups. The difference was the greatest in Group 3.
Conclusion:
The split-parotid delineation approach significantly lowered the mean dose to the anterolateral subsegment and overall gland without greatly compromising the doses to target volumes and other OARs. The effect was more obvious for both PPS and level IIa cervical node involvements than for either of them alone.
Advances in knowledge:
It is the first article based on the assumption that parotid gland stem cells are situated at the anterolateral segment of the gland, and applied the split-parotid delineation approach to the parotid gland in the treatment planning of patients with NPC with PPS and level IIa cervical node involvements, so that the function of the post-radiotherapy parotid gland might be better preserved.
INTRODUCTION
In external beam radiotherapy of patients with nasopharyngeal carcinoma (NPC), the parotid gland often receives a high radiation dose owing to its relatively close proximity to the tumour, especially for patients with parapharygneal space (PPS) or upper cervical node involvements. Because of this, long-term complications such as xerostomia, sore throat, altered taste, dental decay, changes in voice quality, impaired chewing and swallowing have been reported.1–4 With the introduction of more advanced radiotherapy techniques such as intensity-modulated radiotherapy and volumetric arc therapy in the past two decades, the dose to the parotid glands can be reduced compared with conventional techniques, resulting in a lower incidence of severe xerostomia and better post-treatment life quality.5–7 However, total sparing of the parotid gland is still not possible even with these techniques; about 40% of patients with NPC were still reported to have moderate or severe xerostomia after treatment.8
Most patients with NPC present with moderate-to-advanced stage disease at initial diagnosis, with the tumour usually extending outside the nasopharyngeal region. Over 60% of them involve the PPS9 and/or level IIa cervical lymph nodes,10 which are in close proximity to the deep lobe of the parotid gland. Therefore, it is likely that relatively high doses would be delivered to the parotid in radiotherapy. Deasy et al11 reported that severe radiation-induced xerostomia could be avoided if both entire parotid glands were kept to a mean dose of below 25 Gy, which poses a challenge to the dosimetrists for computing treatment plans for these patients.
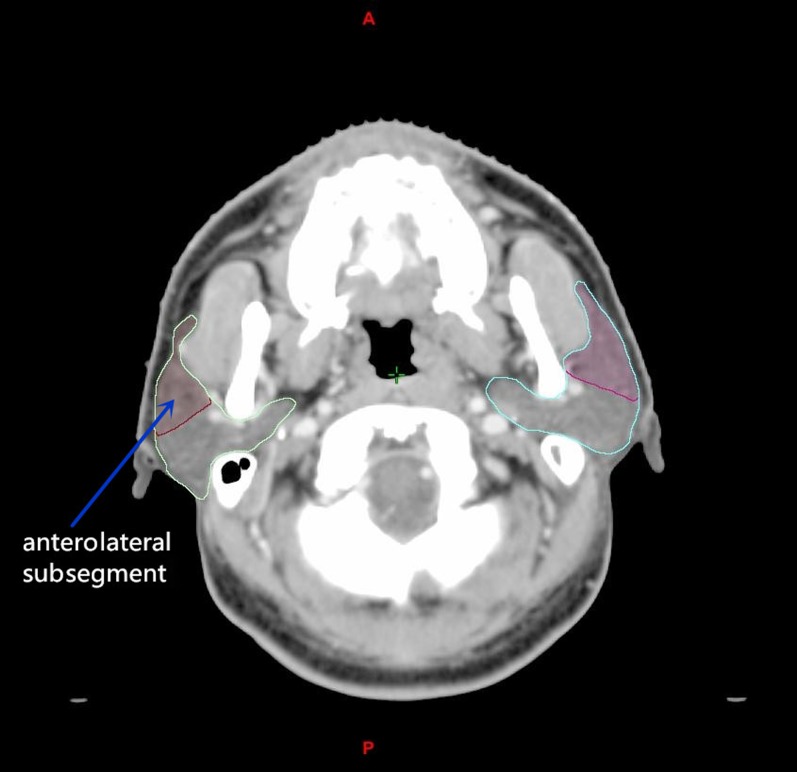
Recently, it has been reported that the recovery of a salivary gland injury after radiation therapy was dependent on the radiation dose and amount of residual dynamic stem cell in the salivary gland pre-clinically.12 Therefore, the reduction of dose in parotid gland stem cells might promote its recovery in patients. Pre-clinical studies on mice revealed that restricting the dose to this region of the gland produced more rapid recovery of gland function after irradiation.13,14 Since with reference to the mice model, the stem cells of the salivary gland were detected at the main excretory ducts,15,16 which are mainly located at the anterolateral subsegment of the parotid gland,17 in order to better protect the stem cells in the parotid gland during radiotherapy, a “split-parotid delineation” approach would be useful in which an imaginary line is drawn through the anterolateral margin of the retromandibular vein, which divides the parotid gland into the anterolateral and posteromedial subsegments (Figure 1). By applying a more stringent dose constraint to the anterolateral subsegment, which is where the stem cells are mainly located, there might be a better chance to preserve the function of the parotid gland.
Figure 1.
A transverse CT image showing the parotid gland contours being split into anterolateral and posteromedial subsegments. It is expected that most stem cells will be located at the anterolateral subsegment of the parotid gland.
The aim of this study was to evaluate the dosimetric impact of applying the split-parotid delineation method in volumetric-modulated radiotherapy (VMAT) of patients with NPC with PPS and/or level IIa cervical node involvements and how it might reduce the risk of xerostomia in patients by better sparing the putative stem cell niche in the parotid gland.
METHODS AND MATERIALS
50 newly diagnosed patients with NPC treated with VMAT between February 2012 and June 2014 were recruited from the Cancer Hospital, Shantou University Medical College. The patient characteristics are shown in Table 1. They were divided into three groups according to the location of the tumour extension. Group 1 consisted of patients with level IIa cervical lymph node metastases only (n = 15); Group 2 was patients with unilateral PPS invasion only (n = 15); and Group 3 consisted of patients who had both unilateral PPS and level IIa cervical lymph node involvements (n = 20).
Table 1.
Clinical characteristic of the patients with nasopharyngeal carcinoma in different patient groups
| Characteristic | Group 1 (n =15) | Group 2 (n = 15) | Group 3 (n = 20) |
|---|---|---|---|
| Sex | |||
| Male | 11 (73.33%) | 9 (60.00%) | 12 (60.00%) |
| Female | 4 (26.67%) | 6 (40.00%) | 8 (40.00%) |
| Age (years) | |||
| <60 | 10 (66.67%) | 11 (73.33%) | 14 (70.00%) |
| ≥60 | 5 (33.33%) | 4 (26.67%) | 6 (30.00%) |
| T stage | |||
| T1 | 5 (33.33%) | 0 (0%) | 0 (0%) |
| T2 | 8 (53.33%) | 4 (26.67%) | 5 (25.00%) |
| T3 | 2 (13.34%) | 7 (46.66%) | 10 (50.00%) |
| T4 | 0 (0%) | 4 (26.67%) | 5 (25.00%) |
| N stage | |||
| N0 | 0 (0%) | 1 (6.67%) | 0 (0%) |
| N1 | 5 (33.33%) | 6 (40.00%) | 1 (5.00%) |
| N2 | 10 (66.67%) | 8 (53.33%) | 14 (70.00%) |
| N3 | 0 (0%) | 0 (0%) | 5 (25.00%) |
| Clinical stage | |||
| I | 0 (0%) | 0 (0%) | 0 (0%) |
| II | 4 (26.67%) | 2 (13.33%) | 0 (0%) |
| III | 11 (73.33%) | 9 (60.00%) | 10 (50.00%) |
| IVa | 0 (0%) | 4 (26.67%) | 5 (25.00%) |
| IVb | 0 (0%) | 0 (0%) | 5 (25.00%) |
All patients underwent planning CT scan in a supine treatment position and were immobilized with custom thermoplastic immobilization devices. The scan covered from the vertex to the upper mediastinum with a slice thickness of 3 mm. A VMAT plan was computed for each patient with the Eclipse treatment-planning system (Varian® Medical Systems, Palo Alto, CA) with 6 MV using a TrueBeam linear accelerator (Varian Medical Systems). The target volumes were delineated according to the International Commission on Radiation Units and Measurements reports 50 and 62 guidelines.18,19 Image registration with MRI of the patient was performed to provide a delineation reference for the targets. Gross tumour volume was defined as the gross extent of the tumour shown by CT/MRI images, and this included the gross tumour at nasopharynx (GTVnx) and regional lymph nodes (GTVnd). Planning target volume at nasopharynx (PTVnx) and lymph node (PTVnd) were generated by adding 5–10 mm to the GTVnx and GTVnd, respectively. In addition, the high-risk regions outside the PTVnx and PTVnd that included the entire nasopharyngeal mucosa plus 5 mm into the submucosal, and the high-risk lymphatic drainage area was delineated as clinical target volume 1 (CTV1). By adding a 5-mm margin to the CTV1, the planning target volume 1 (PTV1) was created. In this study, only the parotid gland that was situated at the same side (ipsilateral side) of the PPS and/or level II nodes were evaluated in detail. The organs at risk (OARs), apart from the ipsilateral parotid gland, including the contralateral parotid gland, spinal cord, brain stem, ipsilateral temporomandibular joint, oral cavity, thyroid and larynx, were also contoured. The prescriptions for all patients were to give 70 Gy to PTVnx; 66 Gy to PTVnd; and 60 Gy to PTV1 in 30 fractions using a simultaneous integrated boost. For each VMAT plan, the collimator rotation was set at 30° and two coplanar arcs were delivered in opposite gantry rotation directions (clockwise and anticlockwise). All patients were treated with a 6-MV photon using TrueBeam linear accelerator (Varian Medical System). The planning objectives for the target volumes and OARs were set with reference to Radiation Therapy Oncology Group 0615 recommendations (Table 2). The treatment plan generated was labelled as the “control plan”, in which the parotid gland was contoured as a single OAR to which the routine dose constraint was applied. A second plan, named the “test plan” in this study, was then computed for each patient using the same set of CT data and contoured structures. In this plan, a line through the anterolateral margin of the retromandibular vein was created that divided the parotid gland into the anterolateral and posteromedial subsegments (Figure 1). A mean dose constraint of 25 Gy was applied to the anterolateral subsegment before the optimization, with the same dose requirements for the target volumes and other OARs as the control plan.
Table 2.
The optimization objectives of the control and test plans for patients with nasopharyngeal carcinoma patients
| Structures | Optimization objectives |
|---|---|
| PTVnx | V100 ≥ 95%, D1 ≤ 77 Gy |
| PTVnd | V100 ≥ 95% |
| PTV1 | V100 ≥ 95% |
| Brain stem | D1 < 60 Gy |
| Spinal cord | D1 < 45 Gy |
| Optic nerve | D1 < 50 Gy |
| Optic chiasm | Dmax < 54 Gy |
| Lens | D1 < 10 Gy |
| Pituitary | Dmean < 50 Gy |
| Larynx | Dmean < 40 Gy |
| TM joint | Dmean < 50 Gy |
| Oral cavity | Dmean < 40 Gy |
| Parotid gland | Dmean < 35 Gy |
| Thyroid gland | Dmean < 50 Gy |
D1, dose received by 1% volume; Dmax, maximum dose; Dmean, mean dose; PTV1, planning target volume 1; PTVnd, planning target volume of lymph nodes; PTVnx, planning target volume at nasopharynx; TM, temporomandibular; V100, percentage of the volume that received 100% of the prescribed dose.
The dosimetric data of each treatment plan were collected and recorded through the generation of dose–volume histograms. The evaluated dose parameters for the parotid gland were V15, V20, V25, V30 and V35 (volume of organ receiving 15, 20, 25, 30 and 35 Gy, respectively) and Dmean (mean dose). For the PTV1, the Dmax (maximum dose), Dmean, conformity index (CI) and homogeneity index (HI) were included as the assessment parameters. CI was calculated using the equation: CI = (PTVref ÷ VPTV) × (PTVref ÷ Vref),20 where PTVref represents the volume receiving the prescribed dose in the target volume; VPTV was the volume of the planning target volume (PTV) and Vref was the volume that received the prescribed dose. HI was calculated as the difference between D1 and D99 divided by the prescribed dose,21,22 where D99 and D1 represent the dose received by 99% and 1% of the volume, respectively. For the OARs, D1 (Dose received by 1% volume of the organ) was used to evaluate the doses to the brain stem, spinal cord, optic nerves and lens, and Dmean was used to evaluate the doses to the pituitary, ipsilateral temporomandibular joints, contralateral parotid glands, oral cavity, thyroid and larynx. Dmax was used to evaluate the doses to the optic chiasm. A summary of all the dose parameters for the targets, parotid gland and the various OARs are listed in Supplementary Table A. For each of the three patient groups, the mean of each dose parameter was compared between the control plan and test plan. SPSS® v. 20.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was applied for statistical analysis. Paired t-test or Wilcoxon test was conducted depending on the normality of the data to evaluate the differences between the two types of plans for each patient group.
RESULTS
Dose to the ipsilateral parotid gland
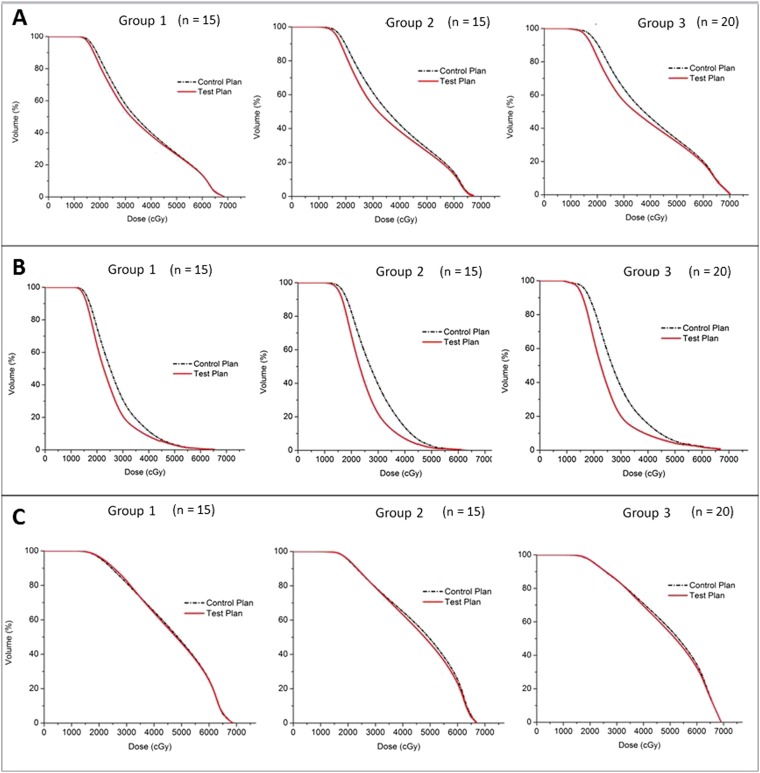
With regard to the entire parotid gland, the mean doses in all treatment plans were above 36 Gy and they increased from Group 1 to Group 3. The differences of their means were significant (analysis of variance, p < 0.001), in which Group 2 was significantly greater than Group 1 (Tukey's test, p = 0.004), and Group 3 was significantly greater than Group 2 (Tukey test, p = 0.006). All the dose parameters of the test plans were significantly lower than that of the control plans in all the three patient groups (Table 3, Figure 2). The mean doses of the anterolateral subsegment in all the three groups were all less than 25 Gy, and they also increased from Group 1 to Group 3. Besides, all the dose parameters of the test plans were significantly lower than that of the control plans. For the posteromedial subsegment, its mean doses were in general higher than those in the anterolateral subsegment. Most of the dose parameters between the control and test plan did not show significant difference, except for the Dmean of Groups 2 and 3 and V25 of Group 1.
Table 3.
Comparisons of doses to the ipsilateral parotid gland between the control and test plans in the three different patient groups
| Dose–volume parameters | Group 1 (n = 15) |
Group 2 (n = 15) |
Group 3 (n = 20) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | |
| Whole gland | |||||||||
| Dmean (Gy) | 37.50 ± 2.30 | 36.42 ± 2.04 | 0.001 | 38.45 ± 4.00 | 36.44 ± 3.45 | 0.000 | 40.55 ± 2.85 | 38.45 ± 2.77 | 0.000 |
| V15 (%) | 98.53 ± 1.66 | 97.29 ± 2.49 | 0.001 | 98.93 ± 1.84 | 97.74 ± 2.56 | 0.001 | 98.56 ± 4.56 | 96.80 ± 4.94 | 0.000 |
| V20 (%) | 86.33 ± 7.10 | 81.55 ± 5.98 | 0.000 | 89.23 ± 6.99 | 83.08 ± 6.59 | 0.000 | 90.78 ± 7.95 | 82.69 ± 7.69 | 0.000 |
| V25 (%) | 71.08 ± 7.06 | 66.20 ± 5.01 | 0.000 | 74.00 ± 9.95 | 66.18 ± 8.52 | 0.000 | 76.39 ± 8.55 | 67.39 ± 5.84 | 0.000 |
| V30 (%) | 57.81 ± 6.36 | 53.94 ± 4.90 | 0.000 | 61.35 ± 11.25 | 53.65 ± 8.92 | 0.001 | 63.93 ± 7.90 | 56.35 ± 6.22 | 0.000 |
| V35 (%) | 48.09 ± 6.23 | 45.44 ± 4.95 | 0.001 | 50.98 ± 10.83 | 45.26 ± 8.88 | 0.001 | 54.20 ± 7.91 | 49.10 ± 6.93 | 0.000 |
| Anterolateral subsegment | |||||||||
| Dmean (Gy) | 27.30 ± 1.58 | 24.98 ± 0.73 | 0.001 | 28.82 ± 2.74 | 25.07 ± 0.78 | 0.001 | 29.26 ± 1.65 | 25.15 ± 0.41 | 0.000 |
| V15 (%) | 97.45 ± 3.03 | 94.79 ± 4.89 | 0.001 | 98.28 ± 3.07 | 95.72 ± 5.05 | 0.001 | 97.40 ± 8.14 | 93.50 ± 9.17 | 0.000 |
| V20 (%) | 75.32 ± 11.60 | 64.17 ± 10.00 | 0.000 | 82.47 ± 9.31 | 68.46 ± 9.22 | 0.000 | 83.40 ± 13.09 | 65.10 ± 12.85 | 0.000 |
| V25 (%) | 50.42 ± 10.64 | 38.35 ± 6.32 | 0.001 | 58.32 ± 12.44 | 40.25 ± 5.95 | 0.001 | 57.98 ± 12.07 | 37.67 ± 5.46 | 0.000 |
| V30 (%) | 30.83 ± 8.64 | 20.72 ± 5.05 | 0.001 | 39.23 ± 13.68 | 21.85 ± 3.10 | 0.001 | 37.47 ± 8.93 | 20.13 ± 5.36 | 0.000 |
| V35 (%) | 19.20 ± 6.62 | 13.30 ± 4.19 | 0.000 | 24.60 ± 12.07 | 12.55 ± 3.76 | 0.001 | 23.92 ± 7.35 | 13.07 ± 5.04 | 0.000 |
| Posteromedial subsegment | |||||||||
| Dmean (Gy) | 46.33 ± 2.86 | 46.24 ± 2.76 | 0.597 | 46.15 ± 5.21 | 45.51 ± 5.00 | 0.042 | 49.44 ± 4.13 | 48.94 ± 4.11 | 0.005 |
| V15 (%) | 99.60 ± 0.90 | 99.59 ± 0.90 | 0.600 | 99.54 ± 1.09 | 99.54 ± 1.22 | 0.917 | 99.64 ± 1.32 | 99.69 ± 1.15 | 0.612 |
| V20 (%) | 96.12 ± 4.30 | 96.41 ± 4.23 | 0.140 | 95.10 ± 6.40 | 95.32 ± 5.96 | 0.496 | 96.85 ± 3.97 | 96.85 ± 3.71 | 0.570 |
| V25 (%) | 89.29 ± 6.27 | 90.09 ± 6.06 | 0.022 | 87.02 ± 10.40 | 87.20 ± 10.43 | 0.910 | 90.92 ± 7.63 | 91.01 ± 7.36 | 0.723 |
| V30 (%) | 81.35 ± 7.21 | 82.40 ± 6.57 | 0.091 | 79.21 ± 12.76 | 79.02 ± 12.78 | 0.722 | 85.01 ± 10.04 | 85.06 ± 9.45 | 1.000 |
| V35 (%) | 73.14 ± 7.04 | 72.99 ± 5.89 | 0.838 | 71.92 ± 13.50 | 71.09 ± 13.62 | 0.092 | 78.12 ± 10.55 | 77.51 ± 9.87 | 0.108 |
Dmean, mean dose; SD, standard deviation.
Figure 2.
Comparison of dose–volume histograms between the control plan and test plan in the three different patient groups (1–3) for the (a) whole ipsilateral parotid gland, (b) anterolateral subsegment and (c) posteromedial subsegment of the ipsilateral parotid gland.
Dose to targets and organs at risk
All the targets met the dose requirements set for plan optimization. For the doses to the PTVs, the dosimetric differences between the control and test plans were small (<1%), with only a few differences in Group 1 and 3 reaching statistical significance (Table 4). For the doses to the OARs, all of them received doses within their dose limits. Apart from the contralateral parotid gland and oral cavity, there was no significant difference between the control and test plans for the rest of the OARs in all the three groups (Table 5).
Table 4.
Comparisons of doses to the target between the control and test plans in different patient groups
| Dose–volume parameters | Group 1 (n = 15) |
Group 2 (n = 15) |
Group 3 (n = 20) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | |
| PTVnx | |||||||||
| V100 (%) |
96.47 ± 1.79 |
95.91 ± 1.73 |
0.024 |
95.89 ± 1.51 |
95.70 ± 1.56 |
0.267 |
95.71 ± 1.28 |
95.45 ± 1.14 |
0.825 |
| V93 (%) |
100.00 ± 0.00 |
100.00 ± 0.00 |
1.000 |
99.95 ± 0.13 |
99.96 ± 0.14 |
0.655 |
99.92 ± 0.23 |
99.93 ± 0.20 |
0.109 |
| Dmean (Gy) |
71.71 ± 0.54 |
71.66 ± 0.61 |
0.609 |
71.82 ± 0.51 |
71.85 ± 0.68 |
0.910 |
72.04 ± 0.48 |
72.11 ± 0.42 |
0.232 |
| PTVnd | |||||||||
| V100 (%) |
98.15 ± 1.75 |
98.04 ± 1.95 |
0.666 |
97.45 ± 2.07 |
97.32 ± 1.73 |
0.690 |
97.21 ± 2.02 |
97.29 ± 2.22 |
0.825 |
| V93 (%) |
100.00 ± 0.00 |
100.00 ± 0.00 |
1.000 |
99.81 ± 0.60 |
99.80 ± 0.63 |
1.000 |
99.87 ± 0.27 |
99.85 ± 0.30 |
0.024 |
| Dmean (Gy) |
68.61 ± 1.34 |
68.66 ± 1.40 |
0.784 |
68.41 ± 1.07 |
68.58 ± 1.56 |
0.790 |
69.08 ± 1.19 |
69.29 ± 1.31 |
0.027 |
| PTV1 | |||||||||
| V100 (%) |
95.06 ± 1.02 |
95.62 ± 0.84 |
0.016 |
95.48 ± 1.07 |
95.71 ± 0.87 |
0.184 |
94.81 ± 1.25 |
95.23 ± 1.36 |
0.015 |
| V93 (%) |
99.90 ± 0.21 |
99.92 ± 0.18 |
0.192 |
99.84 ± 0.29 |
99.82 ± 0.32 |
0.859 |
99.52 ± 0.55 |
99.50 ± 0.57 |
0.568 |
| Dmean (Gy) |
64.60 ± 1.04 |
64.66 ± 1.11 |
0.427 |
65.08 ± 0.91 |
65.10 ± 1.01 |
0.865 |
65.99 ± 0.93 |
66.12 ± 0.94 |
0.030 |
|
Dmax (Gy) |
74.54 ± 0.91 |
74.46 ± 1.01 |
0.766 |
74.54 ± 0.80 |
74.61 ± 1.14 |
0.688 |
75.09 ± 0.55 |
75.33 ± 0.61 |
0.029 |
| CI |
0.87 ± 0.03 |
0.87 ± 0.03 |
1.000 |
0.86 ± 0.04 |
0.86 ± 0.04 |
1.000 |
0.87 ± 0.02 |
0.87 ± 0.02 |
1.000 |
| HI | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.880 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.609 | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.017 |
CI, conformity index; Dmax, maximum dose; Dmean, mean dose; HI, homogeneity index; PTV1, planning target volume 1; PTVnd, planning target volume of lymph nodes; PTVnx, planning target volume at nasopharynx; SD, standard deviation.
Table 5.
Comparisons of doses to the organs at risk between the control and test plans in different patient groups
| Dose parameters | Group 1 (n = 15) |
Group 2 (n = 15) |
Group 3 (n = 20) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | Control plan (mean ± SD) | Test plan (mean ± SD) | p-value | |
| Contra parotid gland Dmean (Gy) |
36.65 ± 2.32 |
35.85 ± 2.07 |
0.040 |
38.18 ± 3.00 |
36.49 ± 2.70 |
0.010 |
39.15 ± 2.07 |
37.70 ± 2.31 |
0.010 |
| Brain stem D1 (Gy) |
45.10 ± 3.02 |
45.06 ± 3.04 |
0.765 |
47.16 ± 3.91 |
47.07 ± 4.00 |
0.458 |
48.66 ± 5.13 |
48.35 ± 5.49 |
0.344 |
| Spinal cord D1 (Gy) |
39.21 ± 1.46 |
39.27 ± 1.45 |
0.865 |
38.77 ± 1.48 |
38.94 ± 1.50 |
0.190 |
39.98 ± 1.87 |
40.22 ± 1.84 |
0.141 |
| Ipsi TM joint Dmean (Gy) |
33.35 ± 4.45 |
33.46 ± 4.25 |
0.663 |
35.27 ± 6.22 |
34.60 ± 6.20 |
0.090 |
38.06 ± 9.67 |
37.92 ± 9.91 |
0.550 |
| Oral cavity Dmean (Gy) |
35.91 ± 2.07 |
35.89 ± 3.21 |
0.012 |
37.24 ± 2.62 |
37.83 ± 2.65 |
0.001 |
36.50 ± 2.98 |
37.24 ± 3.28 |
0.001 |
| Larynx Dmean (Gy) |
38.60 ± 2.36 |
38.75 ± 2.25 |
0.271 |
37.77 ± 3.30 |
37.56 ± 3.29 |
0.075 |
38.76 ± 2.02 |
38.59 ± 2.28 |
0.341 |
| Thyroid Dmean (Gy) | 42.09 ± 7.83 | 42.13 ± 7.89 | 0.754 | 38.73 ± 10.18 | 38.82 ± 10.17 | 0.201 | 45.43 ± 4.76 | 45.48 ± 4.82 | 0.615 |
Contra, contralateral; D1, dose received by 1% volume of the organ; Dmean, mean dose; ipsi, ipsilateral; SD, standard deviation; TM, temporomandibular.
DISCUSSION
The application of the split-organ delineation approach to the parotid gland in treatment planning had been used in some previous studies. Chau et al23 had reported splitting the organs into target-overlapping and non-target-overlapping subsegments in contouring the parotid gland, while Zhang et al24 reported splitting of the parotid glands into superficial and deep lobes. Shao et al17 studied the influence of a similar parotid-split delineation approach on patients with NPC with bilateral cervical nodes. Our study employed the split-parotid delineation method in the VMAT planning of patients with NPC to spare the stem cells of the parotid gland, which are thought to be concentrated at the anterolateral segment of the gland. The uniqueness of this study was that it focused on patients with NPC with PPS invasion and level IIa cervical lymph node metastases, which were anatomically close to the parotid gland.
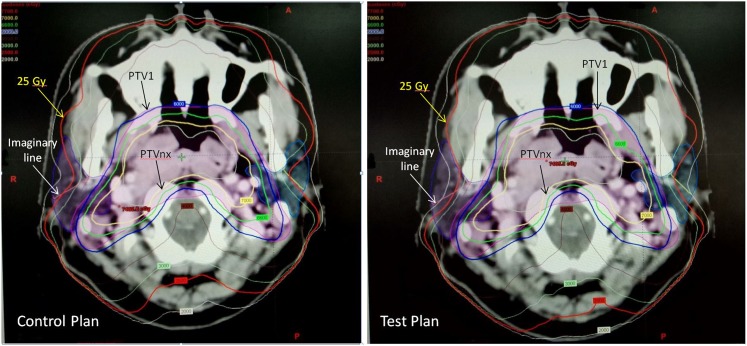
By applying the split-parotid delineation approach and with a more stringent dose constraint to the anterolateral subsegment, our study demonstrated that this could lower the mean dose of the parotid gland and limit the mean dose of the anterolateral subsegment below 25 Gy without greatly compromising the doses to the target volumes and OARs. The dosimetric effect is illustrated in Figure 3, in which the 25 Gy isodose curve was pushed away from the anterolateral subsegment of the parotid gland in the test plan. Comparatively, the impact on PTV dose was the greatest in Group 3 than in the other two groups, where the Dmean, Dmax and HI of this group showed significant differences. The explanation for this was because the PTVs in Group 3 with both PPS and level IIa nodal involvements were expected to be larger and closer to the parotid gland. The magnitude of dose reduction in the PTV would then be relatively more obvious when the dose to the anterolateral subsegment is reduced by applying the dose constraint. For the OARs, the doses to the oral cavity showed difference between the control and test plans. This could be owing to the fact that the oral cavity was the OAR that was located close to the parotid gland, and it was more affected by the change in the parotid dose. Nevertheless, the absolute differences in these parameters in both PTV and OARs were small; it was expected that they would not have significant clinical impact on the patients. For the contralateral parotid gland, since similar dose constraint as for the ipsilateral gland was applied in the test plan, its mean dose was lower than that of the control plan, although the differences were not as great as the ipsilateral side.
Figure 3.
Comparison of the isodose distribution of a volumetric-modulated radiotherapy plan between the control plan and test plan for a patient with nasopharyngeal carcinoma in Group 3.
In addition, the mean dose of the anterolateral subsegment of the parotid gland demonstrated a significant increase from Group 1 to Group 3. This indicated that the influence of PPS invasion was greater than that of the level IIa cervical node involvement, whereas when both PPS invasion and level IIa cervical node involvement existed together, the dose impact to the anterolateral subsegment was the greatest.
At present, there is little knowledge about the dose limit of the stem cell in the parotid gland; it was logical to take the mean dose of 25 Gy suggested by Deasy et al11 as the reference in this study because it has been the most conservative dose limit for the parotid gland of all previous related studies. The success in keeping the anterolateral subsegment below this dose level would be expected to help preserve the parotid gland function and reduce the risk of xerostomia, because this is the location where most of the parotid gland stem cells are thought to be located. This effect was relatively more obvious in Group 3 than in Group 1, indicating that this split-parotid delineation approach offered greater benefit for patients with both PPS and level IIa cervical node involvements. It was because for patients in Group 3, the PTVs were more close to the parotid gland that caused a relatively higher mean dose to the anterolateral region of the gland in the control plan. Therefore, a greater dose reduction would be observed for this group when the dose of this subsegment is pushed below 25 Gy in the test plan. On the other hand, it was logical to see such dose difference effect did not exist in the posteromedial subsegment for all the three groups because no stringent dose constraint was applied to this region. With the positive results obtained from this study, it was expected that the application of this split-parotid delineation approach could be extended to other head and neck cases with the irradiation fields involving the parotid gland, so that the function of the gland could be better preserved after radiotherapy. However, clinical validations of this planning approach followed by larger scale randomized clinical studies are required to evaluate the clinical outcomes.
CONCLUSION
The split-parotid delineation approach significantly lowered the mean dose to the anterolateral subsegment of the parotid gland and the overall gland in the VMAT of patients with NPC with PPS and/or level IIa cervical node involvements, without greatly compromising the doses to the target volumes and OARs. The effect was more obvious for both PPS and level IIa cervical node involvements (Group 3) than for either of them alone (groups 1 and 2). It was expected that such an approach could be extended to other head and neck cancers when irradiation fields involve the parotid gland so as to achieve better preservation of the gland function after radiotherapy.
Contributor Information
Wei Xiao, Email: xw631314@126.com.
Zhixiong Lin, Email: zxlin5@qq.com.
Wuzhe Zhang, Email: 14032426@qq.com.
Mei Li, Email: limei00182@139.com.
Vincent WC Wu, Email: htvinwu@polyu.edu.hk.
REFERENCES
- 1.Abendstein H, Nordgren M, Boysen M, Jannert M, Silander E, Ahlner-Elmqvist M, et al. Quality of life and head and neck cancer: a 5 year prospective study. Laryngoscope 2005; 115: 2183–92. doi: 10.1097/01.MLG.0000181507.69620.14 [DOI] [PubMed] [Google Scholar]
- 2.Hammerlid E, Taft C. Health-related quality of life in long-term head and neck cancer survivors: a comparison with general population norms. Br J Cancer 2001; 84: 149–56. doi: 10.1054/bjoc.2000.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammerlid E, Silander E, Hörnestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer—a longitudinal study. Head Neck 2001; 23: 113–25. doi: [DOI] [PubMed] [Google Scholar]
- 4.Bjordal K, Ahlner–Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biorklund A, et al. A prospective study of quality of life in head and neck cancer patients. Part II: longitudinal data. Laryngoscope 2001; 111: 1440–52. doi: 10.1097/00005537-200108000-00022 [DOI] [PubMed] [Google Scholar]
- 5.Bian X, Song T, Wu S. Outcomes of xerostomia-related quality of life for nasopharyngeal carcinoma treated by IMRT. Expert Rev Anticancer Ther 2015; 15: 109–19. doi: 10.1586/14737140.2015.961427 [DOI] [PubMed] [Google Scholar]
- 6.Marucci L, Marzi S, Sperduti I, Giovinazzo G, Pinnarò P, Benassi M, et al. Influence of intensity-modulated radiation therapy technique on xerostomia and related quality of life in patients treated with intensity-modulated radiation therapy for nasopharyngeal cancer. Head Neck 2012; 34: 328–35. doi: 10.1002/hed.21736 [DOI] [PubMed] [Google Scholar]
- 7.Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stagenasopharyngeal carcinoma patients. J Clin Oncol 2007; 25: 4873–9. [DOI] [PubMed] [Google Scholar]
- 8.Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Siotman BJ, Langendijk JA. Intensity modulated radiotherapy reduces radiation-induced morbidity and improves health related quality of life results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 2009; 74: 1–8. doi: 10.1016/j.ijrobp.2008.07.059 [DOI] [PubMed] [Google Scholar]
- 9.Yu ZH, Xu GZ, Huang YR, Hu YH, Su XG, Gu XZ. Value of computed tomography in staging the primary lesion (T-staging) of nasopharyngeal carcinoma (NPC): an analysis of 54 patients with special reference to the parapharyngeal space. Int J Radiat Oncol Biol Phys 1985; 11: 2143–7. doi: 10.1016/0360-3016(85)90095-1 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Li L, Hu C, Zhou Z, Ying H, Ding J, et al. Patterns of level II node metastasis in nasopharyngeal carcinoma. Radiother Oncol 2008; 89: 28–32. doi: 10.1016/j.radonc.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 11.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 2010; 76: 58–63. doi: 10.1016/j.ijrobp.2009.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008; 26: 2595–601. doi: 10.1634/stemcells.2007-1034 [DOI] [PubMed] [Google Scholar]
- 13.Konings AW, Cotteleer F, Faber H, van Luijk P, Meertens H, Coppes RP. Volume effects and region-dependent radiosensitivity of the parotid gland. Int J Radiat Oncol Biol Phys 2005; 62: 1090–5. doi: 10.1016/j.ijrobp.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 14.Konings AW, Faber H, Cotteleer F. Secondary radiation damage as the main cause for unexpected volume effects: a histopathologic study of the parotid gland. Int J Radiat Oncol Biol Phys 2006; 64: 98–105. doi: 10.1016/j.ijrobp.2005.06.042 [DOI] [PubMed] [Google Scholar]
- 15.Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis 2011; 17: 143–53. doi: 10.1111/j.1601-0825.2010.01723.x [DOI] [PubMed] [Google Scholar]
- 16.Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 2009; 92: 466–71. doi: 10.1016/j.radonc.2009.06.023 [DOI] [PubMed] [Google Scholar]
- 17.Shao MH, Ke WT, Jia HJ. A split-parotid delineation approach for dose optimisation in intensity-modulated radiotherapy for nasopharyngeal carcinoma with bilateral neck lymph node metastasis in level II. J Chin Oncol 2014; 20: 40–6. [Google Scholar]
- 18.A. International Commission on Radiation Units and Measurements. ICRU report 50. Prescribing, recording, and reporting photon beam therapy. Bethesda, MD; ICRU; 1993. [Google Scholar]
- 19.B. International Commission on Radiation Units and Measurements. ICRU report 62. Prescribing, recording, and reporting photon beam therapy. Bethesda, MD; ICRU; 1999. [Google Scholar]
- 20.van't Riet A, Mak AC, Moerland MA. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 1997; 37: 731–6. [DOI] [PubMed] [Google Scholar]
- 21.Clivio A, Fogliata A, Franzetti-Pellanda A. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 118–24. doi: 10.1016/j.radonc.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Sun T, Wang C, Yin Y, Liu T, Cehn J. Dosimetric comparison of fixed field intensity modulated radiation therapy and RapidArc volumetric modulated arc therapy in treatment of multiple intracranial metastases. Chin J Radiother Med Protect 2010; 30: 585–9. [Google Scholar]
- 23.Chau RM, Leung SF, Kam MK, Cheung KY, Kwan WH, Yu KH, et al. A split organ delineation approach for dose optimization for intensity modulated radiotherapy for advanced T stage nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol 2008; 20: 134–41. doi: 10.1016/j.clon.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 24.Zhang HB, Lu X, Huang SM, Wang L, Zhao C, Xia WX, et al. Superficial parotid lobe–sparing delineation approach: a better method of dose optimization to protect the parotid gland in intensity-modulated radiotherapy for nasopharyngeal carcinoma. Curr Oncol 2013; 20: 577–84. doi: 10.3747/co.20.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]