Abstract
Objective:
The purpose of this study was to evaluate whether high mammographic density can be used as one of the selection criteria for MRI in invasive lobular breast cancer (ILC).
Methods:
In our institute, high breast density has been used as one of the indications for performing MRI scan in patients with ILC. We divided the patients in two groups, one with MRI performed pre-operatively and other without MRI. We compared their surgical procedures and analyzed whether surgical plan was altered after MRI. In case of alteration of plan, we analyzed whether the change was adequate by comparing post-operative histological findings.
Results:
Between 2011 and 2015, there were a total of 1601 breast cancers with 97 lobular cancers, out of which 36 had pre-operative MRI and 61 had no MRI scan. 12 (33.3%) had mastectomy following MRI, out of which 9 (25%) had change in surgical plan from conservation to mastectomy following MRI. There were no unnecessary mastectomies in the MRI group. However, utilization of MRI in this cohort of patients did not reduce reoperation rate (19.3%). Lobular carcinoma in situ (LCIS) was identified in 60% of reoperations on post-surgical histology. Patients in the “No MRI” group had higher mastectomy rate 26 (42.6%), which was again appropriate.
Conclusion:
High mammographic density is a useful risk stratification criterion for selective MRI in ILC within a multidisciplinary team meeting setting. Provided additional lesions identified on MRI are confirmed with biopsy, pre-operative MRI does not cause unnecessary mastectomies. Used in this selective manner, reoperation rates were not eliminated, albeit reduced when compared to literature.
Advances in knowledge:
High mammographic breast density can be used as one of the selection criteria for pre-operative MRI in ILC without an increase in inappropriate mastectomies with potential time and cost savings. In this cohort, re-excisions were not reduced markedly with pre-operative MRI.
INTRODUCTION
Invasive lobular cancer (ILC) is known for incomplete surgical excision compared with other histological types of breast cancer. Re-excision rates in ILC after breast conservation have been reported to vary from 29 to 67%.1–3 Conversion to mastectomy after breast-conserving surgery (BCS) has been reported in 16–48%.1,3–5
Mammography, ultrasound and MRI are all used in assessing the extent of ILC. Whilst mammography has primarily served as a screening test, it has limited potential in young patients with dense breasts.6 Reports indicate sensitivity of mammography in dense breasts to be as low as 30–48%.6,7 Furthermore, the potential of mammography is even more limited in ILC owing to its intraductal, permeative pattern of spread within the breast tissue. Both tumour size and multifocality are underestimated with mammography.8
Ultrasound is useful for detecting mammographically occult cancer but has limited value in detecting multifocality, unsuspected bilateral cancer and often underestimates tumour size.7,9–11
Contrast-enhanced MRI of breast is considered most accurate of the imaging modalities, capable of detecting multifocal/multicentric or contralateral cancers. It has the potential to change therapeutic plans.12 MRI is strongly recommended routinely in pre-operative planning for all ILC.13 However, some authors contend that MRI may overestimate disease, causing wider excision than needed or conversion to mastectomy.14,15
In the Breast Centre, all patients with ILC are discussed in multidisciplinary team meeting (MDTM) as part of their pre-operative planning. If they are deemed to have dense breasts on mammograms and if MRI could potentially change management, we proceed with MRI. In this way, two cohorts of patients were created with ILC, one cohort of patients who received pre-operative MRI (MRI+) and another cohort of patients who did not receive MRI (MRI−). The aim of this study was to assess the utility of performing MRI in selected group of lobular cancers and also to compare surgical procedures between the two groups and analyze whether surgical procedure was altered with or without MRI. In case of alteration, we analyzed whether the change was adequate by comparing post-operative histological findings.
METHODS AND MATERIALS
Patients
Database of the University Hospital was searched for all ILC diagnosed by biopsy and proven by final pathology of the surgical specimens between January 2011 and March 2015. Patients included both screening and symptomatic population groups and had undergone curative surgery/management at the centre. There were a total of 1601 breast cancers with 101 lobular breast cancers. This study was registered as a service evaluation project in the institute and was performed according to good clinical practice. Local ethics committee approval or informed consent of the patients was not deemed necessary owing to the retrospective nature of the study. Pre-operative mammograms and ultrasound were performed in all patients, and MRI was performed in a subgroup of patients with high mammographic density. If pre-operative imaging or surgical pathology results were not available, they were excluded from the analysis. After exclusions (4), there were 97 patients, with 36 in the MRI group (MRI+) and 61 in the “No MRI” group (MRI−).
Breast density
Breast density was classified into one of the four categories as defined by the American college of radiology breast imaging reporting and data system (BI-RADS) (5th edition) categories:16 Category A: the breasts are almost entirely fatty; Category B: there are scattered areas of fibroglandular density; Category C: the breasts are heterogeneously dense, which may obscure small masses; and Category D: the breasts are extremely dense, which lowers the sensitivity of mammography.
Assigning patients to these density subgroups was performed by one of the two radiologists (GJB) with 5 years' experience in breast imaging. This was performed retrospectively blinded to the knowledge of MRI+/MRI− grouping.
MRI
MRI was performed on a 1.5-T magnetic resonance scanner (GE Signa HDxt 1.5 T; GE Healthcare, Tokyo, Japan), using an eight-channel phased-array breast coil. Imaging was performed with the patient lying in the prone position. Following a three-plane localizer and axial three-dimensional (3D) T1 (high-resolution) sequences, multiphase volume imaging breast assessment dynamic post-contrast axial 3D T1 fat-suppressed high-resolution images were obtained. Axial images were obtained using a spin echo sequence (repetition time/echo time 500/7.6 ms), with a 2-mm slice thickness, matrix 512 × 256, field of view 34 cm and number of excitations 1. A dynamic study of both breasts was obtained after intravenous injection of gadopentetate dimeglumine (0.1 mmol kg−1 body weight), and fat-suppressed subtracted images were obtained in axial plane approximately every minute for 8 min. A 3D spoiled gradient-recalled acquisition in the steady state sequence (repetition time/echo time/TI 6/2.5/18 ms) with flip angle 10°, field of view 34 cm, section thickness 2 mm, matrix 512 × 256 and acquisition time of 1 min 5 s was used for post-contrast images. The images were interpreted by the breast radiologist (GJB), reporting >120 breast MRIs per year.
Following definitions were applied for additional findings on MRI:
Clinically relevant increase in size of index lesion: increment of >5 mm in largest diameter for index lesions >20 mm. This was because increase in size on MRI for lesions <20 mm will not alter the clinical management, which are generally managed by BCS/lumpectomy.
New malignant lesions detected: additional lesions which are highly suspicious for malignancy, usually with similar morphological and kinetic appearance as index lesions, proven post MRI on second-look ultrasound and biopsy.
Pre-operative surgical plan
All patients with ILC were discussed in MDTM, comprising surgeons, radiologists, oncologists and pathologists, as part of normal departmental protocol. Triple assessments including clinical, radiological and histological examination are the “gold standard” for the evaluation of all patients with breast cancer. The decision to perform MRI was usually taken in the MDTM by consensus at this stage, taking into consideration the breast mammographic density, patient's choice and tentative surgical plan. Following MRI, patients were discussed in the MDTM again, when treatment plans are confirmed or changed or modified. The departmental policy was that all new MRI findings required pathological proof of malignancy, usually by “second-look” ultrasound and biopsy. Pathological proof was not deemed necessary if only small extension of the local excision was required following MRI.
For BCS, surgeons tried to secure 10-mm margin from the tumour boundary during first lumpectomy. The need for re-excision or secondary surgery depended on histopathological opinion of the surgical specimens. Histological size (invasive and in situ component separately), status of resection margins and lymph-node status were reported for each surgical specimen during MDTM. Maximum tumour size was used for comparing histological size with imaging tumour size.
Statistical analyses
The primary end point of this study was to compare the two groups (MRI+) and (MRI−) with respect to re-excision rate, initial mastectomy rate and conversion of BCS to mastectomy. The tumour sizes were compared between different imaging modalities. All mean values were expressed as mean ± 2 standard deviation. Binomial comparisons were performed with χ2 test or Fisher exact test to check for statistical significance. Continuous variables were tested with Student paired/unpaired t test. Statistical analysis was performed using SPSS® v. 21.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). p-value <0.05 was taken as statistically significant.
RESULTS
Patients, imaging and tumour characteristics
In total, 97 patients met the inclusion criteria, out of which 36 were in the MRI+ group and 61 were in the MRI− group. There was no statistical difference between the two groups (with or without MRI) with respect to T or N stage, histological grade or hormone receptor status.
The mean age of patients in the MRI+ group was 59.4 years compared with 71.8 years in the MRI− group (p = 0.004) (Table 1). There was statistically significant difference in the breast density distribution between the two groups, with 86.1% in the MRI+ group with breast density BI-RADS categories C and D compared with 22.9% in the MRI− group (p = 0.003).
Table 1.
Comparison of age, mammographic density, histological and imaging sizes between two groups (MRI+ and MRI−)
| MRI (n = 36) | No MRI (n = 61) | p-value | |
|---|---|---|---|
| Age (years) | 59.4 | 71.8 | 0.004 |
| Density categories (A and B) | 5 | 47 | 0.009 |
| Density categories (C and D) | 31 | 14 | 0.003 |
| Mean histological size, mm (±2SD) | 41.9 (±32.5) | 43.6 (±30.2) | 0.750 |
| Mean mammographic size, mm (±2SD) | 17.3 (±12.6) | 32.7 (±20.2) | 0.004 |
| Mean ultrasound size, mm (±2SD) | 16.9 (±9.9) | 23.2 (±10.8) | 0.008 |
| Mean MRI size, mm (±2SD) | 30 (±17.5) | – | – |
SD, standard deviation.
The ultrasound and mammographic sizes of tumours showed statistical difference between the two groups, with larger sizes seen in the “No MRI” (MRI−) group (p = 0.004) (Table 1). Average tumour size was underestimated by all imaging modalities, when compared with histological size (Table 2). MRI underestimated the mean tumour size least (when compared with other imaging modalities) by 7 mm and showed maximum correlation with final histology (paired t sample correlation 0.834). The histopathological size of tumours in both groups did not show any statistical size difference (p = 0.750).
Table 2.
Mean difference between imaging and histological sizes between different imaging modalities
| Mean imaging size and histological size difference, mm (±2SD) | Correlation with histological size (paired t sample correlation coefficient) | |
|---|---|---|
| Mammogram | 16.9 (±22.2) | 0.577 |
| Ultrasound | 22.5 (±22.4) | 0.496 |
| MRI | 7 (±12.3) | 0.834 |
SD, standard deviation.
MRI findings
Out of the total 36 patients who had MRI as part of pre-operative planning, new malignant lesions were detected in 5 patients (13.8%), clinically relevant increase in size of index lesion were detected in 12 patients (33.3%) and both new lesions and increase in size were detected in another 5 patients (13.8%). In total, MRI picked up clinically relevant findings in 22 patients (61%).
Surgical outcomes
Comparison was made between primary surgery rates between the two groups (MRI+ and MRI−). Primary mastectomy rate in the MRI+ group was 12/36 (33%) vs 26/61 (42.6%) in the MRI− group (Table 3). There were proportionately greater numbers of BCS in the MRI+ group at 61.1% (p = 0.001) vs 13 (21%) in the MRI− group. Rate of resection margin positivity between the MRI− and MRI+ group was compared. There were five patients with re-excision of margins and two patients needed conversion to mastectomy after BCS in the MRI+ group. This compared with only one conversion to mastectomy and no re-excisions for margin involvement in the MRI− group.
Table 3.
Comparison of surgical procedures between the two groups (MRI+ and MRI−)
| MRI, n (%) | No MRI, n (%) | p-value | |
|---|---|---|---|
| Wide local excision | 22 (61.1) | 13 (21.3) | 0.001 |
| Mastectomy | 12 (33.3) | 26 (42.6) | 0.003 |
| WLE-mastectomy | 2 (5.5) | 1 (1.6) | – |
| No surgery | 21 (34.4) | – | |
| Reoperation of margins | 5 (13.8) | 0 | – |
| ANC | 10 (27.7) | 14 (22.9) | 0.658 |
| ANC following radiologically normal axilla | 5 (13.8) | 8 (13.11) | 0.793 |
ANC, axillary node clearance; WLE, wide local excision.
Out of the 12 patients who had mastectomy following MRI, all had post-surgical size of tumour >25 mm (25–72 mm). Six patients had post-surgical size >70 mm, one had >50 mm, one had >40 mm, two had >30 mm and two had post-surgical size of 25 mm. MRI changed management to mastectomy in 9/12 patients (25%), by either picking new malignant lesions in one patient, clinically relevant increase in size of the index lesion in 5 patients or both in 3 patients. In the other three patients, MRI did not change the size of index lesion nor found new lesions. Size on MRI of these patients varied between 24 and 26 mm, and the MDTM decision to perform mastectomy was based on the relative size of lesion to breast size. After surgery, one had post-surgical histological size of 30 mm and other two had 25 mm of tumour. Attempted BCS would have resulted in positive margins and subsequent re-excision/mastectomy in all these patients. In the MRI− group, 26/61 patients (42.6%) had mastectomy with a histological size >25 mm in all except in 4 patients, where it was a patient's choice.
Two patients in the MRI+ group were converted after wide local excision to mastectomy, out of whom one was on pre-operative hormone therapy and was found to have 62 mm of invasive cancer on the second post-operative histology. The other had lobular carcinoma in situ (LCIS) measuring 57 mm in the post-surgical specimen. The full extent of lesions was difficult to delineate on all pre-operative imaging modalities including MRI in both these cases (Figures 1 and 2). Both patients had positive margins for invasive disease. The second patient had MRI size of 21 mm and was found to have 31 mm of invasive cancer and 57 mm of LCIS, and margins were involved with both invasive cancer and LCIS. With respect to re-excisions in the MRI+ group, two of five had non-invasive cancer on the second post-surgical specimens, with measurement varying from 5 to 55 mm. Invasive cancer varied from 4 to 2 mm on the second post-surgical specimens.
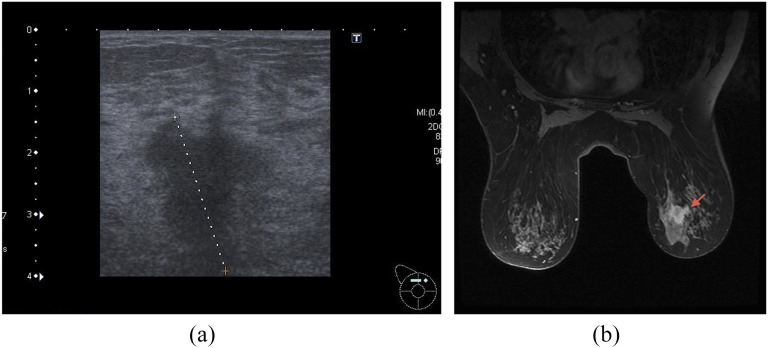
Figure 1.
(a) Ultrasound of the patient which was converted from breast-conserving surgery to mastectomy owing to lobular carcinoma in situ (LCIS); pre-operative ultrasound size 27 mm, MRI size 28 mm; histology showed 31 mm of invasive cancer and 57 mm of LCIS. (b) MRI of the above patient. Dotted line (a) and arrow (b) indicate ultrasound and MRI abnormality, respectively.
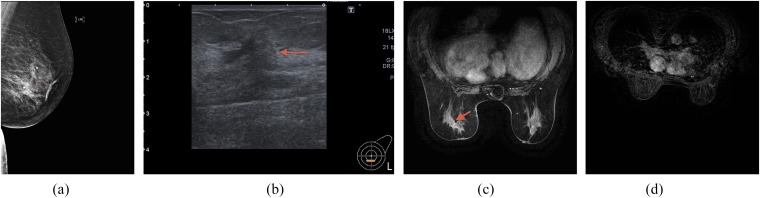
Figure 2.
(a) Patient on hormonal treatment pre-operatively and at the time of imaging; mammogram showing subtle asymmetric density within lower half of the left breast. (b) Ultrasound of the above patient showing subtle attenuation. (c) MRI of the above patient demonstrating subtle enhancement within the lower half of the left breast. (d) MRI of the axillary regions of the above patient: normal axilla on imaging (post-operatively, there was 62 mm of invasive cancer with seven positive axillary nodes leading to mastectomy and axillary node clearance). Arrows indicate imaging abnormality.
DISCUSSION
This is the first study of its kind, which questions the role of MRI performed routinely in all ILC. By selecting a subgroup of patients with high mammographic density for MRI staging and avoiding MRI in others, potential cost saving and delay in treatment can be avoided. Additional lesions detected by MRI often lead to additional work-up, which may not prove to be malignant, thus delaying definitive treatment.
MRI as an accurate predictor of disease
This study demonstrates that MRI is most accurate for estimating the full extent of ILC, when compared with ultrasound or mammography. There was an underestimation of size by all imaging modalities but least with MRI, which correlated best with histopathological size. Similar experience was reported in other studies.17–19 MRI did not reduce the reoperation rate (7/36; 19.4%) markedly in our study, when compared with literature 29–67%.1–3 Three of seven patients with reoperation had non-invasive cancer (LCIS) and one patient was on hormonal treatment pre-operatively. Moreover, as mean ultrasound and mammographic size was smaller in the MRI+ group than in the “No MRI” group, with no significant difference in the histological size, it can be speculated that without MRI, there would have been many more reoperations in the MRI group. The discrepancy between mammographic/ultrasound size and histological size was bigger in the MRI group, underscoring the importance of MRI in this group. Shin et al20 determined the accuracy of MRI in non-invasive cancer (including both lobular and ductal components) and found that MRI correlated better with pure non-invasive disease size than ultrasound.
Appropriate change in surgical therapy after supplementary pre-operative MRI
Of the patients studied, none were harmed owing to overtreatment in the MRI+ group. 25% of patients experienced an appropriate change in surgical therapy owinb to MRI. Patient selection for MRI based on mammographic density seemed appropriate. None of the patients in the “No MRI” group experienced re-excisions of margins, with only one conversion to mastectomy. We have assumed complete pathologically confirmed surgical eradication of tumour as a surrogate for local recurrence rates and possibly overall survival in ILC. However, small overlooked ipsilateral or contralateral focus of breast cancer may not be the leading prognostic indicator and only large randomized clinical trials can address the full significance of MRI-detected lesions.
Our findings are in keeping with other studies.19,21,22 MRI is known to change the therapeutic plans in approximately one-third of patients with ILC.19,21,22 In a systemic review by Mann et al,22 a change in surgical therapy based on MRI findings occurred in 28% of patients, as has been observed in this study. There was an overtreatment rate of 2% (95% confidence interval 0–4%) in their study comprising a review of six studies with number of patients varying from 20 to 51. Similar results were reported by Heil et al,23 comprising 92 patients, where change in surgical therapy due to MRI was seen in 25% with overtreatment in 3% (95% confidence interval 0–6%). We had no overtreatment within both groups of patients, apart from 4/26 within the “No MRI” group, where it was a patient's choice for mastectomy.
New suspicious findings on MRI
In literature, different definitions of new suspicious findings on MRI have been applied, and this varies between study protocols. The review by Mann et al,22 summarizing all definitions stated a 32% increase in additional lesions detected on MRI in patients with ILC. Similar results were seen by Heil et al.23 In our study, there was a clinically relevant additional finding on MRI in 61% of patients, although change in surgical therapy was in 25% of patients. Better sensitivity of MRI facilitates complete removal of tumour24 and therefore less local recurrences. However, this was refuted by the comparative effectiveness of MRI in breast cancer (COMICE) trial25 for the ILC subgroup.25 It is debatable if additional lesion detected on MRI has any relevance in the era of multimodal therapy with radiotherapy and chemotherapy.
Mastectomy and re-excision rates
In this study, mastectomy rate within the MRI group was 33.3% and 42.6% in the “No MRI” group (p = 0.003). This is in contrast to some studies. Katipamula et al26 reported a higher mastectomy rate in patients undergoing MRI before surgery (54% vs 36%). Similar findings were reported by Bleicher et al.14 However, none of these studies evaluated ILC separately and in this way differed from the present study, in which pre-operative MRI was performed in patients with dense breasts with ILC. This discrepancy may also be because, in our study, all suspicious lesions were reviewed with ultrasound, and malignant and benign lesions were distinguished through biopsy. However, this difference could also be related to selection bias in our cohort of patients owing to younger patients in the MRI group and smaller imaging (mammogram and ultrasound) sizes leading to an inclination towards BCS. We did not notice a marked reduction of reoperation rates after pre-operative MRI as a result of surgical margin involvement, similar to some studies.25,27 In COMICE trial,25 one of the largest randomized controlled trial on this subject randomized 1623 patients with breast cancer to pre-operative breast MRI and found no difference in the reoperation rates of 19%. MR mammography of non-palpable breast tumours (MONET) trial27 found an increased rate of re-excision in the MRI group (34% vs 12% in the control). Mann et al28 found otherwise in invasive lobular cancer, with reduced re-excision rates without increase in the rate of mastectomy. Their patients comprised historical patients over long time period, leading to scans being performed by varied MRI protocols and field strengths. In a recent meta-analysis of all studies with a control group (a total of 766 patients with ILC),29 there were increased mastectomies with routine pre-operative MRI and weak evidence in favour of reduced re-excisions rates in ILC subgroup. Overall, there was unfavourable harm–benefit ratio for routine use of pre-operative MRI in breast cancer. In another recent population-based study from Canada, there was correlation between routine use of pre-operative MRI and increasing mastectomies from 2003 to 2010.30 Using a population-based cancer registry, the authors found that pre-operative MRI was performed in 15% of all patients with breast cancers. They concluded that patients who had pre-operative MRI had longer wait times between diagnosis and surgery, additional biopsies prior to surgery and higher rate of mastectomies. Unfortunately, none of the authors of the above-mentioned studies controlled for breast density. In the COMICE randomized trial,25 breast density was used as a stratification factor but was not found to alter the rate of re-excision.
This study has certain strengths compared with other studies in the literature. Firstly, this is one of the first studies, which utilizes breast mammographic density as a selection criterion for MRI in the pre-operative planning for ILC. This has the potential to reduce overutilization of MRI for all ILC in the clinical setting. Moreover, by selecting a subgroup of patients with ILC out of all breast cancers, it was possible to control for the histological subtype, which is found wanting in many previous studies.14,26 Secondly, all images, pathological data and surgical techniques were maintained by “in-house” radiologists, pathologists and surgeons, who were specialists in breast cancer in a university hospital. This led to tight control of the environment in which the patients were treated. It has been shown in previous studies that patients seen at teaching hospital are more likely to undergo breast MRI.30 Therefore, our results are applicable to a teaching hospital setting with high-volume work. Thirdly, as all patients were discussed in an MDTM, and decisions were taken by consensus by all members of MDTM, surgeon's experience, volume load and temporal effects on mastectomy rates, as a confounding factor has been somewhat accounted for. Fourthly, this is one of the few studies with a comparator group,29 which allows for more meaningful analysis of short-term surgical outcomes than studies in which all subjects received MRI.
There are few limitations in this study. Firstly, this is a retrospective non-randomized single-centre study. Secondly, the selection of patients for MRI may have a selection bias owing to younger age and inclination towards BCS in those patients. However, we feel that these are the sort of patients which are usually selected for MRI in the clinical setting, and if breast density can be used as one of the criterion, cost savings can be made by avoiding MRI in some patients with ILC, without any change in the clinical outcome. Thirdly, study patients were not followed up on a long-term basis. However, this was not the objective of this study, and complete removal of tumour tissue was taken as a surrogate for local recurrence rates. Fourthly, mammographic assessment of density was performed subjectively, which is prone to both interobserver and intraobserver variations. Owing to lack of robust and non-expensive quantitative method for the assessment of breast density, most clinical departments rely on subjective measurements only. Lastly, this study did not account for the effect of age, as patients who received MRI were of younger age. This could be better delineated in future studies using individual patient data meta-analysis, in the absence of which this study provides the best available evidence on how to utilize pre-operative MRI selectively.
In summary, high mammographic density is a useful risk stratification tool for selective MRI in ILC within a MDTM setting. Provided additional lesions identified on MRI are confirmed with biopsy, pre-operative MRI does not cause unnecessary mastectomies. Used in this selective manner, reoperation rates were not eliminated, albeit reduced when compared to literature. Further large randomized clinical trials can address the full significance of MRI-detected lesions in terms of local recurrence rates in an era of increasing use of local and systemic therapy.
Contributor Information
Gaurav J Bansal, Email: gjbansal@gmail.com.
Divya Santosh, Email: divyasantosh@wales.nhs.uk.
Eleri L Davies, Email: eleridavies@wales.nhs.uk.
REFERENCES
- 1.Keskek M, Kothari M, Ardehali B, Betambeau N, Nasiri N, Gui GP. Factors predisposing to cavity margin positivity following conservation surgery for breast cancer. Eur J Surg Oncol 2004; 30: 1058–64. doi: 10.1016/j.ejso.2004.07.019 [DOI] [PubMed] [Google Scholar]
- 2.Van den Broek N, Van den Sangen MJ, Van de Poll-Franse LV, Van Beek MW, Nieuwenhuijzen GA, Voogd AC. Margin status and the risk of local recurrence after breast conserving treatment of lobular breast cancer. Breast Cancer Res Treat 2007; 105: 63–8. doi: 10.1007/s10549-006-9431-5 [DOI] [PubMed] [Google Scholar]
- 3.Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast conserving surgery for cancer. Ann Surg Oncol 2008; 15: 1297–303. doi: 10.1245/s10434-007-9777-x [DOI] [PubMed] [Google Scholar]
- 4.Hussien M, Lioe TF, Finnegamn J, Spence RA. Surgical treatment of invasive lobular carcinoma of the breast. Breast 2003; 12: 23–35. doi: 10.1016/S0960-9776(02)00182-0 [DOI] [PubMed] [Google Scholar]
- 5.Morrow M, Keeney K, Scholtens D, Wei J, Steel J, Khan SA. Selecting patients for breast-conserving therapy: the importance of lobular histology. Cancer 2006; 12: 2563–68. doi: 10.1002/cncr.21921 [DOI] [PubMed] [Google Scholar]
- 6.Mandelson M, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval and screen detected cancers. J Natl Cancer Inst 2000; 92: 1081–7. doi: 10.1093/jnci/92.13.1081 [DOI] [PubMed] [Google Scholar]
- 7.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002; 225: 165–75. doi: 10.1148/radiol.2251011667 [DOI] [PubMed] [Google Scholar]
- 8.Pain JA, Ebbs SR, Hern RP, Lowe S, Bradbeer JW. Assessment of breast cancer size: a comparison of methods. Eur J Surg Oncol 1992; 18: 44–8. [PubMed] [Google Scholar]
- 9.Nothacker M, Duda V, Hahn M, Warm M, Degenhardt F, Madjiar H, et al. Early detection of breast cancer: benefits and risks of supplemental breast US in asymptomatic women with mammographically dense breast tissue. A systemic review. BMC Cancer 2009; 9: 335. doi: 10.1186/1471-2407-9-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schelfout K, Van Goethem M, Kersschot E, Colpaert C, Schelfout AM, Leyman P, et al. Contrast enhanced MR imaging of breast lesions and effect on treatment. Eur J Surg Oncol 2004; 30: 501–7. doi: 10.1016/j.ejso.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 11.Hwang KT, Kim H, Chung JK. A comparative study between the preoperative diagnostic tumour size and the postoperative pathologic tumour size in patients with breast tumours. J Breast Cancer 2010; 13: 187–97. doi: 10.4048/jbc.2010.13.2.187 [DOI] [Google Scholar]
- 12.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of pre-operative contrat enhanced MRI on the therapeutic approach. Radiology 1999; 213: 881–8. doi: 10.1148/radiology.213.3.r99dc01881 [DOI] [PubMed] [Google Scholar]
- 13.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from European society of breast imaging. Eur Radiol 2008; 18: 1307–18. doi: 10.1007/s00330-008-0863-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleicher RJ, Ciocca RM, Egleston BL, Sesa L, Evers K, Sigurdson ER, et al. Association of routine pretreatment MRI with time to surgery, mastectomy rate and margins status. J Am Coll Surg 2009; 209: 180–7. doi: 10.1016/j.jamcollsurg.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biglia N, Bounous VE, Martincich L, Panuccio E, Liberale V, Ottino L, et al. Role of MRI versus conventional imaging for breast cancer pre-surgical staging in young women or with dense breasts. Eur J Surg Oncol 2011; 37: 199–204. doi: 10.1016/j.ejso.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 16.American College of Radiology. American college of radiology breast imaging reporting and data system (BI-RADS). 5th edn. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 17.Caramella T, Chapellier C, Ettore F, Raoust I, Chamorey E, Balu-Maestro C. value of MRI in the surgical planning of invasive lobular breast carcinoma: a prospective and retrospective study of 57cases: comparison with physical examination, conventional imaging and histology. Clin Imaging 2007; 31: 155–61. doi: 10.1016/j.clinimag.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Mann RM, Veltman J, Barentsz JO, Wobbes T, Blickman JG, Boetes C. The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol 2008; 34: 135–42. doi: 10.1016/j.ejso.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 19.Quan ML, Sclafani L, Heerdt AS, Fey JV, Morris EA, Borgen PI. Magnetic resonance imaging detects unsuspected disease in patients with invasive lobular cancer. Ann Surg Oncol 2003; 10: 1048–53. doi: 10.1245/ASO.2003.03.016 [DOI] [PubMed] [Google Scholar]
- 20.Shin HC, Wonshik H, Moon HG. Limited value and utility of breast MRI in patients undergoing Breast-conserving cancer surgery. Ann Surg Oncol 2012; 19: 2572–79. doi: 10.1245/s10434-012-2289-3 [DOI] [PubMed] [Google Scholar]
- 21.Bedrosian I, Mick R, Orel SG, Schnall M, Reynolds C, Spitz FR, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer 2003; 98: 468–73. doi: 10.1002/cncr.11490 [DOI] [PubMed] [Google Scholar]
- 22.Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat 2008; 107: 1–14. doi: 10.1007/s10549-007-9528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heil J, Buehler A, Golatta M, Rom J, Schipp A, Harcos A, et al. Do patients with invasive lobular breast cancer benefit in terms of adequate change in surgical therapy from a supplementary preoperative breast MRI? Ann Oncol 2011; 23: 98–104. doi: 10.1093/annonc/mdr064 [DOI] [PubMed] [Google Scholar]
- 24.Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast conservation treatment with radiation for women with early stage inavasive breast cancer or ductal carcinoma in situ. J Clin Oncol 2008; 26: 386–91. doi: 10.1200/JCO.2006.09.5448 [DOI] [PubMed] [Google Scholar]
- 25.Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised control trial. Lancet 2010; 375: 563–71. doi: 10.1016/S0140-6736(09)62070-5 [DOI] [PubMed] [Google Scholar]
- 26.Katipamula R, Degnim AC, Hoskin T, Boughey JC, Loprinzi C, Grant CS, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009; 27: 4082–8. doi: 10.1200/JCO.2008.19.4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, et al. Preoperative MRI and surgical management in patients with non-palpable breast cancer: the MONET-randomised controlled trial. Eur J Cancer 2011; 47: 879–86. doi: 10.1016/j.ejca.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 28.Mann RM, Loo CE, Wobbes T, Bult P, Barentsz JO, Gilhuijs KG, et al. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat 2010; 119: 415–22. doi: 10.1007/s10549-009-0616-6 [DOI] [PubMed] [Google Scholar]
- 29.Houssami N, Turner R, Morrow M. Pre-operative magnetic resonanace imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013; 257: 249–55. doi: 10.1097/SLA.0b013e31827a8d17 [DOI] [PubMed] [Google Scholar]
- 30.Arnaout A, Catley C, Booth C, Mcinnes M, Graham I, Kumar V, et al. Use of pre-operative magnetic resonance imaging for breast cancer—a Canadian population-based study. JAMA Oncol 2015; 1: 1238–50. doi: 10.1001/jamaoncol.2015.3018 [DOI] [PubMed] [Google Scholar]