Abstract
Objective:
To study the CT and MR imaging features of carcinoma showing thymus-like differentiation (CASTLE) and to raise awareness of this rare thyroid tumour.
Methods:
The imaging appearances of 10 CASTLE tumours confirmed by surgical pathology were retrospectively reviewed and correlated with clinical and histological findings.
Results:
Seven patients with newly diagnosed and three patients with recurrent tumours were identified (six males and four females). CASTLE tumours were commonly located in the lower neck between the inferior pole of the thyroid and the upper mediastinum. The average tumour size was 4.2 cm (range: 2.5–6 cm). On plain CT scans, most tumours were ill-defined nodular masses of uniform density. After enhancement, most cases showed mild enhancement, while heterogeneous enhancement could be seen in more than half the cases. On the MR images, tumours presented with homogeneous isointensity on T1 weighted images and they appeared to be slightly hyperintense on T2 weighted images. On post-contrast images, marked enhancement was seen in two patients, and heterogeneous enhancement was seen in three cases. Aggressive local infringements mainly included the ipsilateral strap muscle, tracheoesophageal groove area and tracheal wall. The specimens stained positively for CD5 and CD117, indicating thymic differentiation.
Conclusion:
CASTLE has no characteristic imaging features when compared with other thyroid nodules, except for its unique location in the lower neck between the inferior pole of the thyroid and the upper mediastinum. CD5- and CD117-specific immunoreactivity is useful for diagnosis.
Advances in knowledge:
We reported 10 cases of CT and MR images illustrating the features of CASTLE, and we raised the level of awareness of this rare malignant thyroid tumour.
INTRODUCTION
Carcinoma showing thymus-like differentiation (CASTLE) is a rare occurrence and represents a special type of thyroid cancer; it originates in the ectopic thymus tissue within the thyroid gland or rudimentary branchial pouches along the thymic line. This neoplasm was first described by Miyauchi et al1 in 1985 as “intrathyroidal epithelial thymoma”. The clinical and pathological features of the tumour were morphologically clarified by Chan and Rosai,2 and it was not until 2004 that this disease was designated as an independent clinicopathologic type of thyroid tumours in the World Health Organization classification of tumours of endocrine organs.3 Histologically, with the exception of thymic carcinoma, CASTLE also resembles squamous-cell carcinoma and anaplastic carcinoma of the thyroid, but it features an indolent clinical course and favourable prognosis;2,4,5 so, it is crucial to distinguish between CASTLE and other aggressive thyroid tumours to avoid overtreatment. Until now, <100 cases have been reported in the English literature, and the radiological imaging findings are solely limited to individual case reports.6–8 Given the rarity of CASTLE, most radiologists are not familiar with it. Thus, we intend to report the CT and MR imaging appearances of 10 cases of CASTLE in this study along with a review of the relevant literature and also wish to raise awareness of this uncommon thyroid malignancy.
METHODS AND MATERIALS
Patients
Between January 2000 and September 2013, an electronic cross-search of our radiological (picture archiving and communication system) and pathological database was performed in our cancer centre; then, 10 CASTLE cases were collected, including six male and four female patients. The patient median age upon admission was 46 years (range: 37–56 years). The institutional ethics board of our cancer centre approved this retrospective study and they waived the requirement for informed consent. Pre-operative laboratory findings, ultrasonographic and radiological imaging features and fine-needle aspiration biopsy (FNAB) specimens were evaluated. All newly diagnosed patients were treated with curative surgery; others with recurrent disease were treated with salvage surgery. Post-operative radiotherapy (three patients) or chemotherapy (one patient) was also implemented in four primary cases.
IMAGING PROTOCOL
CT technique
Nine patients underwent plain and contrast-enhanced neck CT scans in the axial plane. The images were obtained using consecutive 5-mm-thick scans with a 40 (n = 3) or 64 (n = 6) multislice helical CT system (SOMATOM®; Siemens Medical, Erlangen, Germany). The scan range was from the mandibular angle to the carina, with a maximized 45 × 45-cm field of view and a 512 × 512-voxel matrix. The imaging parameters were as follows: voltage, 120 kV; current, 100 mAs; pitch, 0.9; slice thickness, 5 mm; and layer space, 5 mm. The acquisition time ranged from 15 to 20 s. Enhancement was administered intravenously using a power injector with a non-ionic contrast agent (100 ml) (Bracco Sine Pharmaceutical Co, Shanghai, China) with an injection rate of 1.5 ml s−1, followed by a 10-ml flush of saline solution.
MRI technique
Among the 10 patients, 4 patients underwent MR scans performed with a 16-channel head and neck joint coil on a 1.5-T MR system (Signal Infinity TwinSpeed™ Excite; GE Healthcare, Milwaukee, WI) in the axial and coronal planes, including 3 patients with simultaneous CT examinations. The MRI protocol was as follows: (1) axial T1 weighted fast spin-echo (FSE) images were obtained from the hyoid bone to the aortic arch level [repetition time/echo time (TR/TE) range: 520/9.8 ms; slice thickness: 5 mm; interslice gap: 1.5 mm; field of view: 24 cm; number of acquisitions: 2; and matrix: 320 × 224]. (2) Transverse and coronal T2 weighted fast spin-echo inversion-recovery images with fat suppression were obtained (TR/TE range: 3700/88 ms; echo train length: 15; slice thickness: 5 mm; interslice gap: 1.5 mm; field of view: 24 cm; number of acquisitions: 2; and matrix: 320 × 160). The axial and coronal fast spoiled gradient-echo T1 images with frequency-selective fat suppression were obtained immediately after a bolus infusion of 0.1 mmol kg−1 of gadolinium–diethylene triamine pentaacetic acid (Gd-DTPA) (Magnevist®, Schering, Berlin, Germany) (TR/TE range: 190/1.9 ms; slice thickness: 5 mm; interslice gap: 1.5 mm; field of view: 24 cm; number of acquisitions: 2; and matrix: 256 × 160). The injection rate was 2 ml s−1 for a total of 16 ml. The standard protocol for the dual-phase contrast-enhanced dynamic imaging of head and neck neoplasms was performed by obtaining early-phase images (30–60 s after starting the injection), followed by delayed-phase images (120–180 s after starting the injection).
Imaging analysis
All of the CT and image findings were reviewed by two experienced clinical radiologists (Dr B Wu and Dr YJ Gu, who have engaged in head and neck cancer diagnostic imaging for 10 years and 20 years, respectively) in consensus. The CT and MR imaging characteristics were analyzed, and particular attention was paid to the tumour location, shape, size, margin, radiographic manifestations (including the enhancement pattern and degree), local aggression and metastatic lymph node involvement. The shape of the tumour was classified as round, oval or irregular. The margin of the tumour was classified as well defined or ill defined. The size of each tumour was measured at the maximal diameter of the tumour. Compared with the adjacent normal muscle tissue of the neck, each neoplasm's imaging appearance was determined by the attenuation on plain CT scans and the signal intensity on T1 and T2 weighted MR images, while the presence of characteristics like calcification or cystic necrosis within the tumour were specially marked. On post-contrast CT and MR images, the degree of enhancement was also subjectively assessed as mild (enhancement less than or equal to that of the ipsilateral sternocleidomastoid muscle) or marked (enhancement greater than that of the ipsilateral sternocleidomastoid muscle).
Pathological examination
Slides were retrospectively reviewed by a single professional pathologist (Dr R Bi) for each case, with emphasis placed on the tumour cellularity, architecture, calcification and cystic necrosis. The histopathological analysis included both standard haematoxylin and eosin (HE) staining and immunohistochemical evaluation; the latter was performed on 4-mm-thick sections cut from a representative block using the standard EnVision method. CD5 (clone CD5/54/F6, 1 : 100; Dako Denmark A/S) and CD117 (polyclonal, 1 : 600; Dako Denmark A/S) antibodies were used to evaluate the tumours for the presence of thymic differentiation. Thyroglobulin (clone DAK-Tg6, 1 : 600; Dako Denmark A/S), thyroid transcription factor-1 (TTF-1) (clone 8G7G3/1, 1 : 200; Dako Denmark A/S) and calcitonin (polyclonal, 1 : 200; Dako Denmark A/S) served as markers for thyroid-derived tumours. Other antibodies that were also used included cytokeratin (clone AE1/AE3, 1 : 60; Dako Denmark A/S), which usually produces positive results for thymic carcinoma.
RESULTS
Clinical data
Seven cases of primary tumours and three recurrent tumours were included; the male-to-female ratio was 1.5 : 1. Clinical manifestations included slowly growing neck masses with no tenderness (as detected by physical examination or ultrasound screening) (n = 6), hoarse voice due to ipsilateral recurrent laryngeal nerve infringement (n = 2) and progressive dysphagia (n = 2). Primary tumours showed unilateral lesions; recurrent tumours were noted during the original part of the surgery, following partial or total thyroid gland resection. The time span for recurrence ranged from 2 weeks to 12 years. Nine cases underwent pre-operative fine-needle aspiration biopsy (FNAB) guided by ultrasonography, and it turned out that two cases were diagnosed as papillary thyroid carcinoma, one as Hashimoto's thyroiditis and one as metastatic squamous-cell carcinoma, and the remaining five cases were assessed as poorly differentiated carcinomas of the thyroid.
Imaging findings
Cervical ultrasound was performed in all patients and commonly revealed a solid, hypoechoic mass without calcification. The CT and MR imaging features of the ten patients with CASTLE are summarized in Table 1. The imaging results showed that all cases were single lesions that were commonly located in the lower neck between the inferior pole of the thyroid and the upper mediastinum; there were eight tumours in the left neck and two in the right neck. The average tumour size was 4.2 cm (range: 2.5–6 cm). All primary tumours had unclear boundaries at the inferior portion of the ipsilateral thyroid. For the three recurrent tumours, all were located behind the strap muscles, close to the trachea at the site of the suprasternal fossa. All primary lesions were irregularly shaped, and this was evident in two recurrent tumours as well. On pre-contrast CT scans, most tumours were ill-defined nodular masses of uniform density (Figure 1a), except for one tumour that featured internal necrotic cystic areas at the lower part. The attenuation of the tumour was similar to that of the adjacent muscle, but it was significantly lower than that of the normal thyroid tissue. Calcification was absent in all cases. Eight CT cases showed mild enhancement, while one demonstrated marked enhancement; meanwhile, heterogeneous enhancement could be seen in more than half the cases (n = 5) (Figure 1b). On the MR images, all four tumours presented with homogeneous isointensity on the T1 weighted images (Figure 2a), while slight hyperintensity was observed on the T2 weighted images (Figure 2b) compared with the intensity of the muscle. Signal intensity was significantly different between the tumour and the normal thyroid tissue on T2 weighted imaging. Following contrast, two patients showed marked enhancement (Figure 2c), while heterogeneous enhancement could be seen in three cases on post-contrast T1 weighted images.
Table 1.
CT and MR features of carcinoma showing thymus-like differentiation
| Patient number/age (years)/gender | CT/MRI | Location | Size (cm) | Shape/margin | CT |
MRI |
||
|---|---|---|---|---|---|---|---|---|
| Attenuationa | Enhancementb | T1 weighted imagea | T2 weighted imagea | |||||
| 1/55/M | CT/MR | L/SF | 3.5 | IR/ID | Isodense | Mi/Hetero | Isointense | Hyperintense |
| 2/50/M | CT/MR | L/IPT | 5 | IR/ID | Isodense | Mi/Homo | Isointense | Hyperintense |
| 3/37/M | CT | L/SF | 6 | IR/ID | Isodense | Mi/Homo | ||
| 4/52/F | CT | R/IPT | 4 | IR/ID | Isodense | Mi/Hetero | ||
| 5/56/F | CT | L/IPT | 4 | IR/ID | Isodense | Mi/Homo | ||
| 6/53/M | CT | R/SF | 2.5 | RO/ID | Isodense | Mi/Homo | ||
| 7/40/F | MR | L/IPT | 4 | IR/ID | Isointense | Hyperintense | ||
| 8/39/M | CT/MR | L/IPT | 4 | IR/ID | Isodense | Ma/Hetero | Isointense | Hyperintense |
| 9/42/F | CT | L/IPT | 4 | IR/ID | Isodense | Mi/Hetero | ||
| 10/40/M | CT | R/IPT | 4.9 | IR/ID | Isodense | Mi/Hetero | ||
F, female; Hetero, heterogeneous; Homo, homogenous; ID, ill-defined; IPT, inferior portion of thyroid; IR, irregular; L, left neck; M, male; Ma, marked enhancement; Mi, mild enhancement; R, right neck; RO, round; SF, suprasternal fossa.
Compared with the attenuation of the ipsilateral sternocleidomastoid muscle.
Compared with the enhancement of the ipsilateral sternocleidomastoid muscle.
Figure 1.
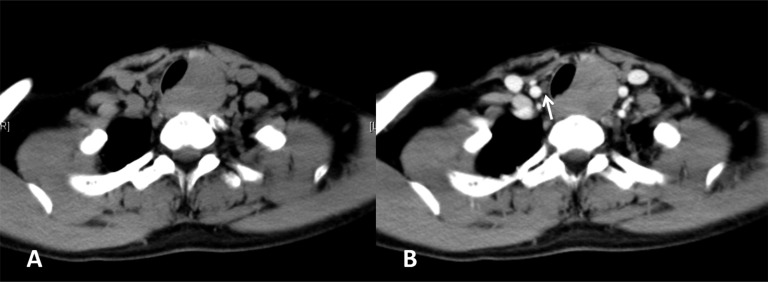
(a, b). CT imaging of the carcinoma showing thymus-like differentiation. (a) Plain CT scan showing an irregular nodular mass in the left pole of the thyroid with uniform attenuation. (b) Mild and slightly heterogeneous enhancement of the mass is displayed after contrast administration. Metastatic paratracheal lymph node (Level VI) in the contralateral site could be found (white arrow).
Figure 2.
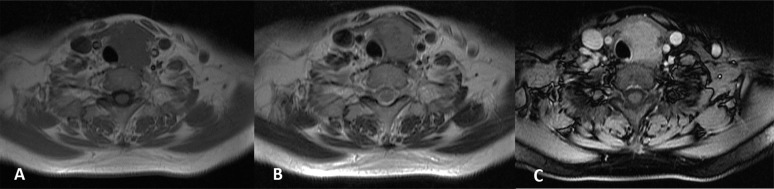
(a–c). MRI of the carcinoma showing thymus-like differentiation: the tumour presents homogeneously isointense on axial T1 weighted image (a) and slightly hyperintense on axial T2 weighted imaging (b). Marked heterogeneous enhancement can be seen in axial contrast-enhanced T1 weighted imaging (c).
Aggressive local infringements were found, including in the ipsilateral strap muscle, tracheoesophageal groove area and tracheal wall. On the image, strap muscle involvement usually displayed as an unclear boundary between the organ and tumours with tissue plane obliteration (Figure 3); tumour involvement in the tracheoesophageal groove presented as a mass that extended into that area. In that case, ipsilateral recurrent laryngeal nerve paralysis can be identified by the changes of the larynx, such as ipsilateral fixation of the vocal cords, dilation and relaxation of the pyriform sinus. For violation of the tracheal airway, intraluminal extension mass was found specifically surrounding the tracheal wall or membrane (Figure 4). Vessel displacement could be seen in three primary tumours owing to mass effect, with no obvious sign of invasion. Metastatic lymphadenopathy was not uncommon and was found in four cases at Regions IV and VI (Figure 5), according to the imaging classification of the American Joint Cancer Committee (AJCC),9 two cases for each neck. The appearance of metastatic lymph nodes correlated with the same enhancement pattern in primary tumours. Primary tumours were initially diagnosed as thyroid malignant tumour (n = 4), cervical mass with unknown cause (n = 2) and tracheal tumour (n = 1).
Figure 3.
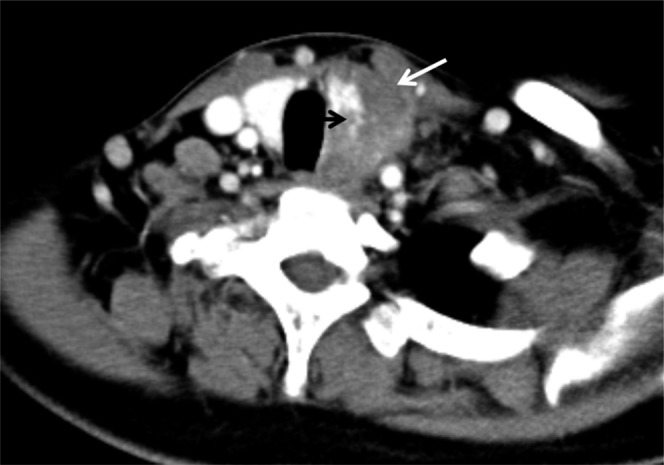
Tumour has unclear boundaries with the inferior portion of ipsilateral thyroid (black short arrow). Aggressive surrounding infringements with strap muscle showing the absence of fat gap (white long arrow).
Figure 4.
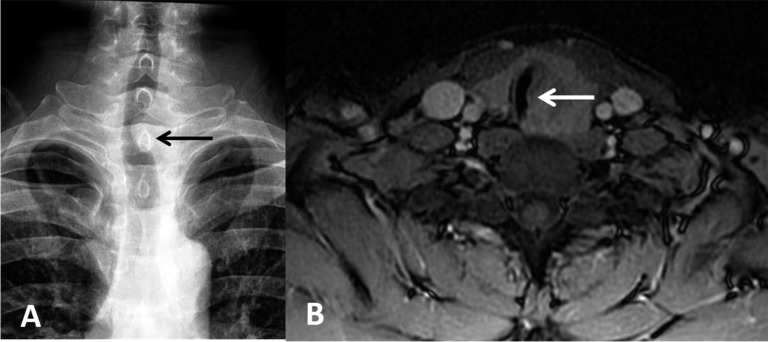
(a, b). Violation of the tracheal airway by carcinoma showing thymus-like differentiation. (a) Oesophageal barium examination showing the compression of transparent trachea (black arrow). (b) MRI performance of the visible mass surrounding the trachea wall or membrane; intraluminal extension can also be seen (white arrow).
Figure 5.
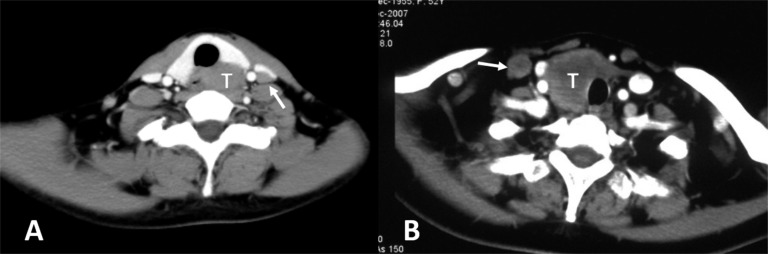
(a, b) Metastatic lymphadenopathy (white arrows) in the ipsilateral side of the tumour (white Ts). Lymph node enlargement can be found at the region of Level IV according to the imaging classification of the American Joint Cancer Committee.
Pathological findings
Grossly, all of the CASTLE tumours were solid, irregular greyish-white masses except for one expansive growth, which was a recurrent mass. Microscopically, the neoplastic cell nests or sheets were composed of irregular lobules surrounded by thick fibrous septa that were massively infiltrated with lymphocytes, resulting in a lymphoepithelial-like growth pattern (Figure 6). Thyroid carcinoma foci (either follicular or papillary types) were not found. The staining of the CASTLE specimens was similar to that of the thymic carcinoma specimens. All 10 tumours stained positively for CD5 (Figure 7) and CD117, but they were uniformly negative for thyroglobulin, thyroid transcription factor-1 (TTF-1) or calcitonin.
Figure 6.
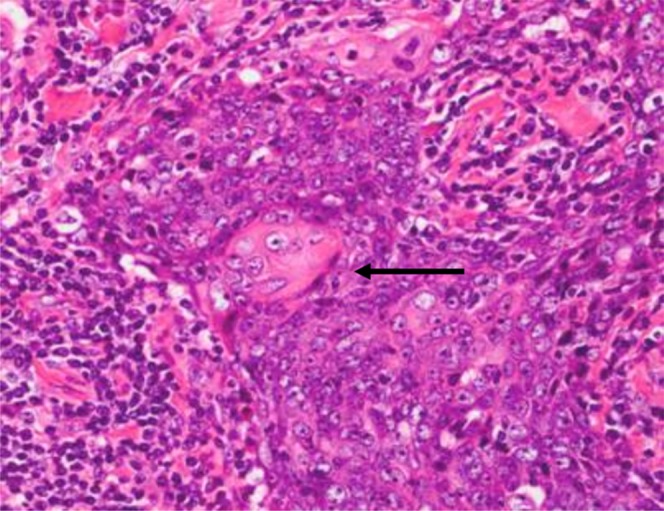
Haematoxylin–eosin stain of carcinoma showing thymus-like differentiation showing oval-to-spindle appearance of neoplastic cells separated by fibrous septa and massively infiltrated with plenty of small lymphocytes. Focal keratinization is illustrated (black arrow).
Figure 7.
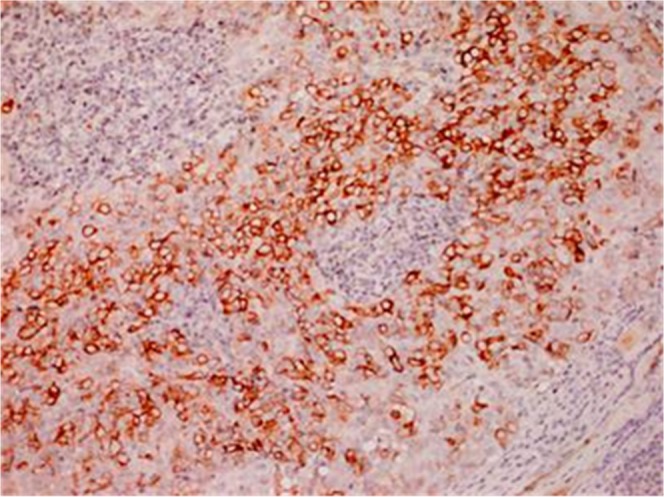
Immunostaining for CD5 showing neoplastic cells with predominant cytoplasmic membrane.
DISCUSSION
CASTLE is a rare neoplasm that arises in the thyroid gland or the soft tissue of the neck, and it presents with various degrees of morphologic resemblance to foetal, mature or involuted thymus glands or thymomas.5 CASTLE can occur in the thyroid gland, most commonly at the lower poles; rare cases may arise in the soft tissue of the neck adjacent to the thyroid.3,10 In our study, all of the tumours were located in the predilection site of the lower neck between the inferior pole of the thyroid and the upper mediastinum, which is consistent with the origin of the thymic primordium; this is in agreement with findings from previous reports.11,12 We highlight that this feature might be key point to distinguish this class of tumours from other thyroid tumours, because CASTLE is postulated to arise from either the ectopic thymic tissue or remnants of branchial pouches. For recurrent tumours, the most frequent site is the suprasternal fossa at the resection side. CASTLE also occurs most frequently in middle-aged adults, and it has a slight female predominance; the median age of our patients (46 years) is consistent with that of a previous review.5,11 Given that the number of cases in our study was relatively small, there seems to be no significant sexual predominance. CASTLE is generally considered to be an indolent low-grade malignant neoplasm with a favourable prognosis,12,13 even though invasive growths were commonly seen in our patients (90%). Most of our primary cases presented with slow-growing painless cervical masses; similarly, the longest recurrent intervals spanned up to 12 years. Physical examination may reveal rigid and fixed cervical nodules or masses upon palpation, with or without swollen lymph nodes. There is no consensus on the management of CASTLE for the rarity of the disease; generally, complete surgical excision followed by neck dissection with or without post-operative adjuvant radiation therapy depending on the nodal state is the most common therapeutic option and seems necessary to improve the long-term survival rate and reduce the locoregional recurrence rate.10
The diagnosis of CASTLE is difficult to make, not only because of its rarity but also owing to its histological and pathologic resemblance to other tumours such as lymphoepithelioma, thymic carcinoma, anaplastic thyroid carcinoma and primary or metastatic squamous-cell carcinoma of the thyroid.2 Since CASTLE exhibits favourable biologic behaviours and prognosis, it is important to distinguish it from lethal carcinomas of the thyroid.4,5,13 To our knowledge, there are only a few case reports exploring the imaging findings of CASTLE in the radiological literature. On ultrasound, CASTLE can be lobulated, solid, hypoechoic tumours without cystic components or calcification with heterogeneous internal echoes.8 On plain CT scan, uniform isodensity could be seen in most tumours without calcification; cystic change is also rare, only in one case. On contrast-enhanced CT, mild enhancement was generally noted. This was vastly different from squamous-cell carcinoma or anaplastic carcinoma of the thyroid, as the latter (whether featuring cystic necrosis, calcification or avid enhancement) was commonly seen.14 MRI is more sensitive when depicting the relationship between the tumour and thyroid tissues, and it is also more capable of determining the degree of heterogeneity inside the tumour. However, MRI does not offer an advantage for diagnosis.
CASTLE tumours may pursue a more aggressive course and they seem more likely to violate adjacent structures along the embryonic cervical descent of the thymus, mostly the nearby ipsilateral strap muscles and tracheoesophageal groove, which accounted for a relatively high proportion of our patients. The trachea can also be involved.15 Regional lymph node metastasis is not uncommon in primary CASTLE tumours according to our data. The involvement of Level IV and VI lymph nodes is also similar to other differentiated thyroid cancers. Overall, the imaging manifestations of CASTLE are not specific and are commonly seen in other aggressive head and neck tumours, particularly in thyroid cancer. In addition, the value of FNAB in the pre-operative diagnosis of CASTLE is also limited; only two cases were indicated in our study. The immunoreactivity of specific markers (CD5 and CD117) is very helpful for the diagnosis of CASTLE by revealing the thymic origin.5,16 In our study, all 10 patients with CASTLE exhibited positive staining and conversely negative to thyroid markers such as thyroglobulin, TTF-1 and calcitonin.
The main differential diagnosis for CASTLE might include other types of thyroid nodules occurring in the lower portion. CASTLE's unclear boundaries with the thyroid parenchyma are different from those of benign nodules, such as thyroid adenoma and nodular goitre. Thyroid adenoma is characterized by avid heterogeneous enhancement, while cystic changes or coarse calcification might often reflect the presence of nodular goitre. For thyroid papillary carcinomas, the distinctive acupuncture calcification pattern with the presence of psammoma bodies might be seen in 30–50% of tumours; necrosis is not uncommon. Anaplastic carcinomas and medullary carcinomas are less commonly seen. Anaplastic carcinomas typically show significant features of aggressive malignant tumours, such as rapidly enlarging mass and symptoms of obstruction,14 while medullary carcinomas show propensity for avid enhancement and to develop lymphatic infiltration.17
Our study has several limitations which have to be pointed out. The small patient population and the retrospective nature of the study do not allow us to draw any conclusion about the diagnosis of CASTLE. Furthermore, larger series with comparative study with other thyroid malignant lesions are needed in ongoing studies.
In summary, we reported 10 cases highlighting the radiological imaging features of CASTLE. The unique predilection site of the tumour might facilitate diagnosis, and differential diagnoses might include other thyroid neoplasms. Simultaneous immunohistochemical determination of CD5 and CD117 can increase the accurate diagnosis of CASTLE.
Acknowledgments
ACKNOWLEDGMENTS
English-language editing of this manuscript was provided by Journal Prep.
Contributor Information
Bin Wu, Email: wubin19780625@gmail.com.
Tuanqi Sun, Email: suntuanqifuscc@163.com.
Yajia Gu, Email: cjr.guyajia@vip.163.com.
Weijun Peng, Email: cjr.pengweijun@vip.163.com.
Zhuoying Wang, Email: wangzhuoyingfuscc@163.com.
Rui Bi, Email: br_fdcc@163.com.
Qinghai Ji, Email: jiqinghaifuscc@163.com.
REFERENCES
- 1.Miyauchi A, Kuma K, Matsuzuka F, Matsubayashi S, Kobayashi A, Tamai H, et al. Intrathyroidal epithelial thymoma: an entity distinct from squamous cell carcinoma of the thyroid. World J Surg 1985; 9: 128–35. doi: 10.1007/BF01656263 [DOI] [PubMed] [Google Scholar]
- 2.Chan JK, Rosai J. Tumors of the neck showing thymic or related branchial pouch differentiation: a unifying concept. Hum Pathol 1991; 22: 349–67. doi: 10.1016/0046-8177(91)90083-2 [DOI] [PubMed] [Google Scholar]
- 3.Cheuk W, Chan JKC, Dorfmann DM, Giordano T. Spindle cell tumor with thymus-like differentiation. In World Health Organization classification of Tumours. Pathology and Genetics of Tumours of endocrine organs. Edited by DeLellis RA, Lloyd RV, Heitz PU, Eng C. Lyon: IARC Press; 2004. pp. 96–7. [Google Scholar]
- 4.Dorfman DM, Shahsafaei A, Miyauchi A. Intrathyroidal epithelial thymoma (ITET)/carcinoma showing thymus like differentiation (CASTLE) exhibits CD5 immunoreactivity: new evidence for thymic differentiation. Histopathology 1998; 32: 104–9. doi: 10.1046/j.1365-2559.1998.00318.x [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Miyauchi A, Nakamura Y, Miya A, Kobayashi K, Kakudo K, et al. Clinicopathologic significance of intrathyroidal epithelial thymoma/carcinoma showing thymus-like differentiation: a collaborative study with Member Institutes of The Japanese Society of Thyroid Surgery. Am J Clin Pathol 2007; 127: 230–6. doi: 10.1309/VM7E52B6U9Q729DQ [DOI] [PubMed] [Google Scholar]
- 6.Ahuja AT, Chan ES, Allen PW, Lau KY, King W, Metreweli C. Carcinoma showing thymic-like differentiation (CASTLE tumor). AJNR Am J Neuroradiol 1998; 19: 1225–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Yoneda K, Matsui O, Kobayashi T, Gabata T, Minato H, Hirokawa M. CT and MRI findings of carcinoma showing thymus-like differentiation. Radiat Med 2005; 23: 451–5. [PubMed] [Google Scholar]
- 8.Yamamoto Y, Yamada K, Motoi N, Fujiwara Y, Toda K, Sugitani I, et al. Sonographic findings three cases carcinoma showing thymus-like differentiation. J Clin Ultrasound 2013; 41: 574–8. doi: 10.1002/jcu.21997 [DOI] [PubMed] [Google Scholar]
- 9.Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol 2000; 174: 837–44. doi: 10.2214/ajr.174.3.1740837 [DOI] [PubMed] [Google Scholar]
- 10.Choi KY, Kwon MJ, Ahn HK, Kim JH, Lee DJ. Extrathyroid carcinoma showing thymus-like differentiation (CASTLE): a new case report and review of the therapeutic role of neck dissection and radiotherapy. World J Surg Oncol 2014; 12: 247–53. doi: 10.1186/1477-7819-12-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roka S, Kornek G, Schüller J, Ortmann E, Feichtinger J, Armbruster C. Carcinoma showing thymic-like elements–a rare malignancy of the thyroid gland. Br J Surg 2004; 91: 142–5. doi: 10.1002/bjs.4510 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Teng XY, Sun DX, Xu WX, Sun SL. Clinical analysis of thyroid carcinoma showing thymus-like differentiation: report of 8 cases. Int Surg 2013; 98: 95–100. doi: 10.9738/INTSURG-D-12-00034.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun T, Wang Z, Wang J, Wu Y, Li D, Ying H. Outcome of radical resection and postoperative radiotherapy for thyroid carcinoma showing thymus-like differentiation. World J Surg 2011; 35: 1840–6. doi: 10.1007/s00268-011-1151-2 [DOI] [PubMed] [Google Scholar]
- 14.Takashima S, Morimoto S, Ikezoe J, Takai S, Kobayashi T, Koyama H, et al. CT evaluation of anaplastic thyroid carcinoma. AJR Am J Roentgenol 1990; 154: 1079–85. doi: 10.2214/ajr.154.5.2108546 [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui H, Hoshi M, Kubota M, Suzuki A, Nakamura N, Usuda J, et al. Management of thyroid carcinoma showing thymus-like differentiation (CASTLE) invading the trachea. Surg Today 2013; 43: 1261–8. doi: 10.1007/s00595-013-0560-2 [DOI] [PubMed] [Google Scholar]
- 16.Reimann JD, Dorfman DM, Nosé V. Carcinoma showing thymus-like differentiation of the thyroid (CASTLE): a comparative study: evidence of thymic differentiation and solid cell nest origin. Am J Surg Pathol 2006; 30: 994–1001. doi: 10.1097/00000478-200608000-00010 [DOI] [PubMed] [Google Scholar]
- 17.Ganeshan D, Paulson E, Duran C, Cabanillas ME, Busaidy NL, Charnsangavej C. Current update on medullary thyroid carcinoma. AJR Am J Roentgenol 2013; 201: W867–87. doi: 10.2214/AJR.12.10370 [DOI] [PubMed] [Google Scholar]