Abstract
Objective:
To report clinical and dosimetric outcomes of a consecutive series of patients with anal cancer treated with volumetric-modulated arc therapy (VMAT) concomitant to chemotherapy (CT).
Methods:
A cohort of 39 patients underwent VMAT employing a schedule consisting of 50.4 Gy/28 fractions to the gross tumour volume (GTV) and 42 Gy/28 fractions to the elective nodal volumes for patients with cT2N0 disease. Patients with cT3–T4/N0–N3 tumours were prescribed 54 Gy/30 fractions to the GTV and 50.4 Gy/30 fractions to the gross nodal volumes if sized ≤3 cm or 54 Gy/30 fractions if > 3 cm. Elective nodal regions were given 45 Gy/30 fractions. CT was administered concurrently following Nigro's regimen. The primary end point was acute toxicity. Secondary end points were colostomy-free survival (CFS), disease-free survival (DFS), cancer-specific survival (CSS) and overall survival (OS). Dosimetric data are also provided.
Results:
Median follow-up was 21 months. Maximum acute toxicities were: dermatologic—G3: 18%; gastrointestinal–G3: 5%; genitourinary–G3: 2%; anaemia—G2: 7%; leukopenia—G3: 28%; G4: 8%; neutropenia—G3: 13%; G4: 18%; thrombocytopenia—G3: 11%; and G4: 2%. The actuarial 2-year CFS was 77.9% [95% confidence interval (CI): 54–90.4%]. Actuarial 2-year OS and CSS were 85.2% (95% CI: 60.1–95.1%), while DFS was 75.1% (95% CI: 52.4.7–88.1%).
Conclusion:
Our clinical results support the use of VMAT as a safe and effective intensity-modulated radiotherapy (IMRT) option in the combined modality treatment of anal cancer, with consistent dosimetry, mild toxicity and promising sphincter preservation and survival rates.
Advances in knowledge:
IMRT is a standard of care for patients with anal cancer, and VMAT is a robust technical solution in this setting.
INTRODUCTION
Combined modality therapy with concomitant chemoradiation is presently considered as the standard treatment option for patients with squamous cell anal cancer, providing consistent rates of locoregional control (LC), overall survival (OS) and sphincter preservation.1 Long-term results of the Radiation Therapy Oncology Group (RTOG) 98-11 trial combining external-beam radiotherapy (EBRT) and concurrent 5-fluorouracil (5-FU)/mitomycin-C (MMC) showed 5-year disease-free survival (DFS) and OS rates of 67.8% and 78.3%, respectively, confirming valid clinical results in this setting.2 Nevertheless, this treatment regimen is frequently associated with a relevant acute toxicity profile, regarding particularly cutaneous, genitourinary (GU) and gastrointestinal (GI) districts, which may potentially be detrimental to oncological outcomes.3 The same RTOG 98-11 trial reported crude rates of acute G3/G4 toxicities of 48% for the skin and 35% for GI, mainly owing to the utilization of non-conformal techniques, namely anteroposterior/posteroanterior parallel opposed fields or four-field conformal approaches, that deliver radiotherapy to a large amount of normal tissues, exposing organs at risk (OARs) to unintended radiation.3 Intensity-modulated radiotherapy (IMRT) may provide robust conformality and modulation, abrupt dose fall-off and reliable consistency and, hence, it has been implemented in several oncological scenarios.4–6 In the context of anal cancer, IMRT may potentially reduce the dose to critical structures such as the bowel, bladder, genitalia, perineal skin and bone marrow, increasing treatment overall tolerability.7 Volumetric-modulated arc therapy (VMAT) combines a rotational approach towards the patient and beam modulation obtained with continuous modulation of multileaf collimator, dose rate variations and gantry rotational speed dynamics. Few planning comparison studies demonstrated that rotational approaches are technically feasible in the context of anal cancer and may spare OARs.8,9 We herein present the results of a consecutive series of patients with anal cancer homogeneously treated with concurrent chemoradiotherapy employing VMAT, reporting on both clinical and dosimetric outcomes.
METHODS AND MATERIALS
Eligibility criteria
All patients included in the present analysis had a histologically proven diagnosis of squamous cell anal carcinoma involving either the anal canal or margin and were treated within the Radiation Oncology Department of the University of Turin, Italy. Disease stage was defined following the 2002 American Joint Committee on Cancer classification with patients with clinical stage T1–T4, N0–N3 included. Other inclusion criteria comprehended an Eastern Cooperative Oncology Group performance status of 0–1, age 18–80 years, suitable haematological parameters (neutrophils ≥1.5 × 109 l−1 and platelets ≥100 × 109 l−1) and adequate renal and liver functions. Exclusion criteria included systemic spread at presentation, prior pelvic radiotherapy, medical contraindications to combination therapy, bowel occlusion symptoms and/or GI bleeding, malabsorption syndrome, peripheral neuropathy, psychiatric disease hampering compliance to therapy, pregnancy and breast-feeding. Written informed consent was obtained for all patients.
Pre-treatment evaluation
Patients were evaluated by the GI Tumour Board of our hospital including all related specialities (radiation and medical oncology and abdominal surgery). The medical examination was made of whole clinical history and objective assessment with digital rectal exam and complete blood check. Staging was performed with total-body computed tomography scan, pelvic MR, 18 fludeoxyglucose-positron emission tomography (PET) and/or inguinal sentinel lymph node biopsy.1
Radiotherapy
Patients had a virtual simulation procedure in supine position with both an indexed shaped knee rest and ankle support (CIVCO Medical Solutions, Kalona, IA), without custom immobilization. A computed tomography scan was performed with 3-mm slice thickness axial images acquired from the top of the L1 vertebral body to the mid-femoral bones. An isocentre was determined on computed tomography simulation software Oncentra MasterPlan® v. 3.0 (Nucletron B.V., Veendhal, Netherlands) in the pelvic region and marked on the patient's skin under laser guidance for daily setup. The gross tumour volume (GTV) comprised all primary and nodal macroscopic disease. GTV was defined based on the MRI and PET information after a non-rigid co-registration with planning computed tomography using the Velocity software (Varian Medical System Inc., Palo Alto, CA). Primary and nodal GTVs were expanded isotropically by 2 and 1 cm, respectively, to obtain subsequent clinical target volumes (CTVs) and then optimized to avoid bones and soft tissues. The prophylactic CTV included the mesorectal region and nodal areas, namely the inguinal, external and internal iliac, obturator and perirectal. For patients with cT4 and/or N2/N3 disease, presacral nodal areas were also encompassed in the CTV. Nodal areas were delineated employing a 1-cm isotropic margin around regional vessels and then modified to exclude bones and muscles. Thereafter, a 10-mm isotropic margin was added for the corresponding planning target volume (PTV) to account for organ motion and setup errors.10,11 Dose prescription for target volumes was taken from Kachnic et al12 and related to the clinical stage at presentation. Patients with cT2N0 disease were prescribed 50.4 Gy/28 fractions to the gross tumour PTV and 42 Gy/28 fractions to the elective nodal PTV. Patients staged as cT3–T4/N0–N3 were given 54 Gy/30 fractions to the macroscopic anal PTV, while clinical nodes were prescribed 50.4 Gy/30 fractions if ≤3 cm or 54 Gy/30 fractions if >3 cm; elective nodal PTV was prescribed 45 Gy/30 fractions. During the treatment-planning phase, objectives for target volumes were set so that for PTV, V95 should be at least 95%, V110 ≤10% and ≤2% should receive <95% of prescribed dose. Dose constraints for OARs were inspired by Kachnic et al12 and included V45 < 20 ml, V35 < 150 ml and V30 < 200 ml for the small and large bowel, V50 < 5%, V40 < 35% and V35 < 50% for the bladder, V40 < 5%, V30 < 35% and V20 < 50% for the external genitalia, V44 < 5% and V40 < 35% for the femoral heads and V50 < 5% and V20 < 50% for the iliac bone. To compute VMAT, Elekta Monaco® was used as the treatment-planning system (v. 3.2) (Elekta AB, Stockholm, Sweden), optimizing treatment with biological cost functions (Poisson statistics cell kill, serial and parallel complication models). The geometrical approach to treatment volumes employed a 360° single-arc or dual-arc approach after system upgrade. A simultaneous integrated boost (SIB) approach was employed for all patients. Radiotherapy delivery was performed under cone-beam computed tomography image guidance, with daily treatment couch repositioning performed after automatic matching of cone-beam computed tomography images and reference planning computed tomography. Images were matched by employing a soft-tissue window and prioritized to the primary tumour and nodal target volumes with a favourable visualization process.
Chemotherapy
All patients received concurrent chemotherapy (CT), consisting of 5-FU (1000 mg m−2 day) given as continuous infusion along 96 h (Days 1–5 and 29–33) associated with MMC (10 mg m−2) given as bolus (Days 1 and 29). A total of two concurrent cycles were planned at the baseline for each patient. Blood cell counts were performed on a routine basis prior to each CT cycle. Prophylactic antiemetic medications were given as intravenous granisetron 3 mg and dexamethasone 8 mg. CT discontinuation or drug modification were planned in case of G3–G4 toxicities.
Clinical assessment
Acute toxicity was scored according to the Common Toxicity Criteria for Adverse Events (NCI-CTCAE) scale v. 3.0, evaluating GU, GI, haematologic, dermatologic, genital and osseous events. The worst episode of toxicity for each category was considered as an acute event, if occurring within 90 days from the end of treatment. After 90 days, all toxicities were classified as late. Follow-up included digital rectal examination and anoscopy at 4, 8, 12 and 26 weeks. MRI was performed at 12 weeks and an anal canal bioptic sampling was carried out at 26. If no residual disease was found at pathology examination, a complete response was assigned. A salvage abdominoperineal resection was generally recommended for persistent disease (at pathology) or for locally progressive or recurrent disease (at imaging and pathology). Conservative salvage treatment strategies were also considered when deemed appropriate.
Statistical analysis
Definition of disease recurrence included local recurrence if relapse in the anal canal and/or anal margin and/or mesorectum was detected. Regional relapse comprised disease at draining lymph nodes (inguinal and pelvic nodes), while systemic recurrence included evidence of disease arising elsewhere. For LC, we took into account local and regional failures. For cancer-specific survival (CSS), we considered death due to disease. OS considered death of any cause. DFS included all failures and cancer-related deaths. Colostomy-free survival took into account death of any cause or definitive colostomy. Distant metastasis-free survival included failures other than those occurring in the anal region and regional nodes. Kaplan–Meier method was used to calculate survival curves and actuarial rates of relapse. Wilcoxon signed-rank test was used to perform univariate analysis. Multivariate analysis was performed using stepwise Cox proportional hazard regression models. An eventual correlation between clinical prognostic factors and OS, CSS and DFS and between dosimetric parameters and acute toxicity was tested. The threshold for statistical significance was set at p-value <0.05. As continuous variables we considered: overall treatment time (OTT) (days), time between biopsy and radiotherapy start (days), treatment breaks (days) and age. As categorical variables we included: stage, staging procedure (PET vs inguinal sentinel lymph node biopsy), tumour location (anal canal vs anal margin), inguinal lymph node involvement, tumour grading, sex, G3-G4 acute toxicity events and eventual CT dose reduction. STATA® Statistical Software, v. 13.1 (Stata Corp, College Station, TX) was employed for analysis.
RESULTS
A total of 39 patients were enrolled from April 2011 to January 2015. Detailed patient characteristics can be found in Table 1. Patients had a mean age of 66 years (range 48–80 years) and were mainly female (71%), human immunodeficiency virus negative (92%), with an anal canal primary (80%), T2 stage (72%), N0 (72%), G2 (71%) and without a preventive colostomy (100%). Patients were mainly treated with a dual-arc VMAT approach (54%). Most of the patients received 54 Gy/30 fraction on the primary tumour PTV (66.7%) and 45 Gy/30 fraction on the prophylactic nodal PTV (74%). Those with macroscopic nodal involvement mostly received 50.4 Gy/30 fractions on the corresponding PTV (23%). The mean time occurring between biopsy and start of radiotherapy was 83 days. Mean OTT was 43 days. 12 (30%) patients had a treatment break, lasting on average 2.2 days (including machine breakdown). Patients with breaks ≥3 days were 10%. For details see Table 2.
Table 1.
Patient characteristics
| Variable | N (%) |
|---|---|
| Age | |
| Mean | 66 |
| Range | 48–80 |
| Sex | |
| Female | 27 (70) |
| Male | 12 (30) |
| HIV status | |
| Positive | 3 (8) |
| Negative | 36 (92) |
| Primary tumour site | |
| Anal canal | 31 (80) |
| Anal margin | 8 (20) |
| T stage | |
| T1 | 2 (5) |
| T2 | 28 (72) |
| T3 | 9 (23) |
| T4 | 0 |
| N stage | |
| N0 | 28 (72) |
| N1 | 2 (5) |
| N2 | 8 (20) |
| N3 | 1 (3) |
| Global stage | |
| I | 2 (5) |
| II | 26 (67) |
| IIIA | 2 (5) |
| IIIB | 9 (23) |
| Grading | |
| G1 | 1 (3) |
| G2 | 27 (71) |
| G3 | 11 (27) |
| Prophylactic colostomy | |
| Yes | 0 (0) |
| No | 39 (100) |
HIV, human immunodeficiency virus.
Table 2.
Treatment characteristics
| Variable | N (%) |
|---|---|
| IMRT approach | |
| Single arc | 17 (46) |
| Dual arc | 21 (54) |
| PTV dose tumour (Gy) | |
| 54 Gy/30 fractions | 26 (66.7) |
| 50.4 Gy/28 fractions | 13 (33.3) |
| PTV dose-positive nodes (Gy) | |
| 54 Gy/30 fractions | 2 (5) |
| 50.4 Gy/30 fractions | 9 (23) |
| PTV dose-negative nodes (Gy) | |
| 45 Gy/30 fractions | 28 (74) |
| 42 Gy/28 fractions | 11 (26) |
| Chemotherapy | |
| 5-FU + MMC | 39 (100) |
| Cycles | |
| 1 | 4 (10) |
| 2 | 35 (90) |
| Chemotherapy dose reduction | |
| Yes | 2 (5) |
| No | 37 (95) |
| Biopsy–RT interval (days) | |
| Mean | 83 |
| Range | 30–148 |
| RT duration (days) | |
| Mean | 43 |
| Range | 38–54 |
| RT breaks ≥ 3 days | |
| Yes | 4 (10) |
| No | 35 (90) |
5-FU, 5-fluorouracil; IMRT, intensity-modulated radiotherapy; MMC, mitomycin C; PTV, planning target volume; RT, radiotherapy.
Toxicity profile
Table 3 shows acute toxicity for the whole cohort. Maximum detected events included: the skin (G3): 18%; GI (G3): 5%; GU (G3): 2%; anaemia (G2): 7%; leukopenia (G3): 28%, (G4):8%; neutropenia (G3): 13%; (G4): 18%; thrombocytopenia (G3): 11%; and (G4): 2%. Moist desquamation (skin), diarrhoea with >7 stools per day (GI) and cystitis interfering with activities of daily living (GU) were considered as G3 events. A total of 4 (10%) out of 39 patients underwent a single cycle of concurrent 5-FU and MMC because of major haematological toxicity, while 2 (5%) patients had a 20% dose reduction during the second CT cycle. No dosimetric factors [mean doses and dose–volume points (Vx) on the dose–volume histogram] were found to be predictors of acute toxicity. Only external genitalia V20 was found borderline significant for acute G3–G4 events regarding the genitalia on univariate analysis [odds ratio (OR): 1.04; p = 0.055; 95% confidence interval (CI): 0.997–1.006].
Table 3.
Acute toxicity
| Acute toxicity |
N (%) |
||||
|---|---|---|---|---|---|
| G0 | G1 | G2 | G3 | G4 | |
| Skin | 1 (2) | 5 (13) | 26 (67) | 7 (18) | 0 |
| Gastrointestinal | 1 (2) | 12 (31) | 24 (62) | 2 (5) | 0 |
| Genitourinary | 7 (18) | 25 (64) | 6 (16) | 1 (2) | 0 |
| Genitalia | 5 (13) | 24 (62) | 10 (25) | 0 | 0 |
| Anaemia | 24 (62) | 12 (31) | 3 (7) | 0 | 0 |
| Leukopenia | 5 (13) | 9 (23) | 11 (28) | 11 (28) | 3 (8) |
| Neutropenia | 12 (31) | 8 (20) | 7 (18) | 7 (18) | 5 (13) |
| Thrombocytopenia | 23 (59) | 8 (20) | 3 (8) | 4 (11) | 1 (2) |
Dosimetric outcomes
Dosimetric parameters regarding both treatment volumes and OARs are presented in Table 4. For the bladder and large bowel, planned dose constraints could not be met in 2 patients (5%). For the external genitalia, constraints could not be met in 23/39 (59%) patients for V20, in 15/39 (38%) patients for V30 and in 29/39 (74%) patients for V40. All others were met in the whole cohort.
Table 4.
Dosimetric results
| Volumes | Parameters | Mean | SD |
|---|---|---|---|
| PTV | |||
| PTV—tumour | D98 (Gy)—50.4 Gy | 47.8 | 0.8 |
| D2 (Gy)—50.4 Gy | 53.2 | 1.2 | |
| D98 (Gy)—54 Gy | 50.6 | 1.2 | |
| D2 (Gy)—54 Gy | 57.3 | 1.1 | |
| V95 (%) | 96.2 | 3.1 | |
| V105 (%) | 8.5 | 8.6 | |
| V107 (%) | 2.2 | 4.1 | |
| PTV—elective volumes | D98 (Gy)—42 Gy | 39.4 | 0.6 |
| D2 (Gy)—42 Gy | 52.3 | 1.5 | |
| D98 (Gy)—45 Gy | 41.5 | 1.5 | |
| D2 (Gy)—45 Gy | 55.1 | 1.3 | |
| V95 (%) | 95.6 | 2.1 | |
| V105 (%) | 36.2 | 12.5 | |
| V107 (%) | 26.3 | 11.8 | |
| OARs | |||
| Bladder | V35 (%) | 35.1 | 17.6 |
| V40 (%) | 17.7 | 13.1 | |
| V45 (%) | 5.9 | 8.4 | |
| V50 (%) | 1.3 | 2.9 | |
| D2 (Gy) | 45.5 | 4.7 | |
| Mean dose (Gy) | 30.2 | 4.9 | |
| Small intestine | V30 (cm3) | 149 | 110.6 |
| V35 (cm3) | 108 | 89.4 | |
| V45 (cm3) | 15 | 9.4 | |
| D2 (Gy) | 44.4 | 3.6 | |
| Mean dose (Gy) | 25.4 | 8.2 | |
| Large intestine | V30 (cm3) | 123 | 116.2 |
| V35 (cm3) | 78 | 65.2 | |
| V45 (cm3) | 13 | 12.4 | |
| D2 (Gy) | 42.9 | 6.9 | |
| Mean dose (Gy) | 18.9 | 4.7 | |
| External genitalia | V30 (%) | 59.5 | 19.1 |
| V35 (%) | 35.9 | 16.4 | |
| V45 (%) | 21.7 | 13.4 | |
| D2 (Gy) | 52.3 | 5.6 | |
| Mean dose (Gy) | 26.2 | 6.7 | |
| Femural heads | V30 (%) | 36.3 | 17.1 |
| V40 (%) | 7.1 | 9.3 | |
| V45 (%) | 0.6 | 1.8 | |
| D2 (Gy) | 41.4 | 3.6 | |
| Mean dose (Gy) | 26.5 | 4.7 | |
| PBM | V5 (%) | 93.6 | 5.9 |
| V10 (%) | 89.2 | 7.4 | |
| V20 (%) | 74.2 | 8.7 | |
| V30 (%) | 50.2 | 8.9 | |
| V40 (%) | 23.9 | 7.5 | |
| Mean dose (Gy) | 28.8 | 2.9 | |
OARs, organs at risk; PBM, pelvic bone marrow; PTV, planning target volume; SD, standard deviation.
Clinical outcomes
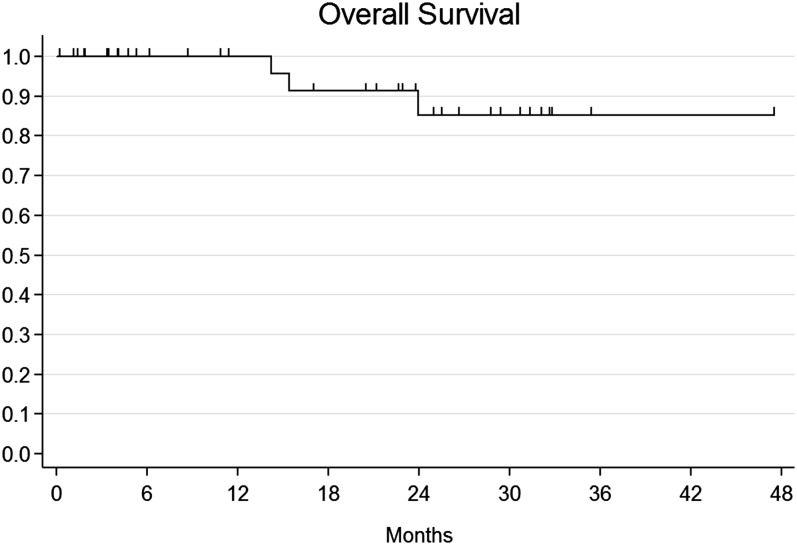
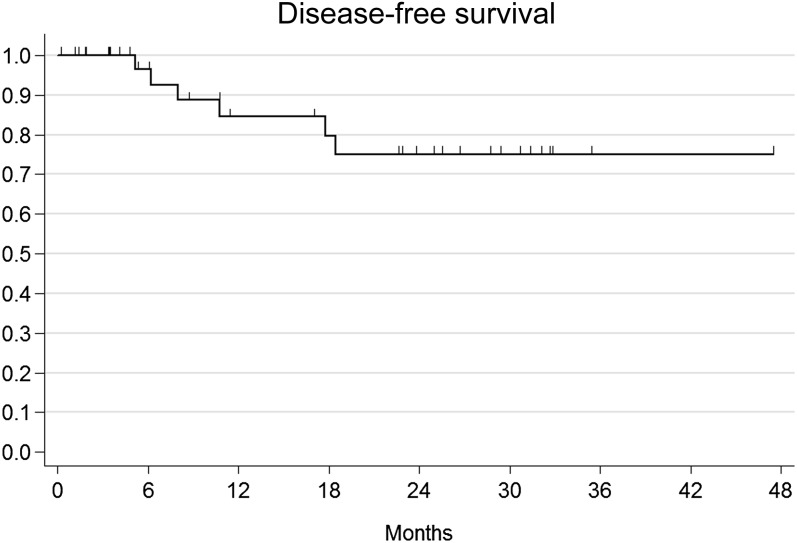
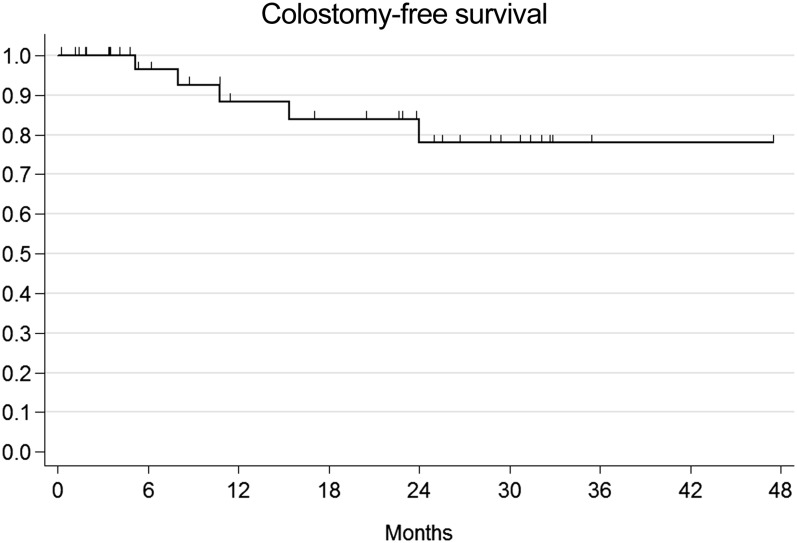
The median observation time was 21.5 months (range 6–50 months). A total of five patients presented with local relapse after combination therapy: four patients had local relapse only, while one patient experienced both local and metastatic (liver and lung) failure. Among locally relapsing patients, three patients were salvaged with abdominoperineal resection, while one patient received local excision and consolidation interstitial brachytherapy after initial failure within the anal margin. One remaining patient experienced nodal relapse within pelvic, lumbar-aortic and mediastinal lymph nodes. All metastatic patients received CT as part of their salvage treatment. Overall, four patients died and all deaths were cancer-related events. Actuarial 1-year and 2-year OS were 100% and 85.2% (95% CI: 60.1–95.1%) (Figure 1). Given that all deaths have a cancer-related cause, CSS corresponds to OS. Actuarial 1-year and 2-year DFS were 100% and 84.5% (95% CI: 63.7–93.9%) and 75.1% (95% CI: 52.4.7–88.1%) (Figure 2). The actuarial probability of being alive at 1 and 2 years without a colostomy (colostomy-free survival) was 88.4 % (95% CI: 68–96.1%) and 77.9% (95% CI: 54–90.4%) (Figure 3). On univariate and multivariate analysis, no independent variables were found to be related to clinical outcomes.
Figure 1.
Actuarial overall survival.
Figure 2.
Actuarial disease-free survival.
Figure 3.
Actuarial colostomy-free survival.
DISCUSSION
Radiotherapy for the treatment of patients with anal cancer has been historically delivered by employing two-dimensional or three-dimensional (3D) solutions.2 This leads to the consequence that a consistent part of normal tissues may be exposed to unintended irradiation, with several OARs such as the bladder, bowel, perineal region and bone marrow included within treatment fields receiving medium-to-high-dose radiation.12 Combined concurrent chemoradiotherapy in anal cancer with no IMRT use has been observed to have a high rate of GI (G3–G4: 35%, mainly diarrhoea) and haematologic (G3–G4: 61%) side effects, as demonstrated in the RTOG 98-11 trial.3 IMRT provides dosimetric advantages in terms of both target coverage and OARs avoidance compared to 3D conformal EBRT in several oncological scenarios.13–15 This is also evident in the context of anal cancer, where several reports are available in the literature. In a multi-institutional retrospective study, Salama et al reported about IMRT as part of combined modality therapy, observing a 15.1% G3 acute rate for GI, 37% for skin toxicity and 58.5% for G3–G4 haematologic side effects.16 All G4 events were haematologic (leukopenia: 30.2%; neutropenia: 34%). Up to 41.5% of patients had treatment breaks, lasting an average 4 days.7 In another interesting article, within a retrospective multi-institutional study, Kachnic et al12 employed dose-painted IMRT and reported a 7% G3 acute toxicity rate for GI, 5% rate for GU, 5% for skin and 49% for haematologic events. In the aforementioned series, G4 events included haematologic (12%), GU (2%) and dermatologic (5%) toxicities. No G4 events were seen for the GI tract. Radiotherapy breaks were observed in 40% of patients. Median time was 2 days. Patients experiencing breaks lasting more than 3 days were up to 35%. IMRT has been demonstrated to be able to decrease the acute toxicity profile in patients with anal cancer as compared with 3D conformal treatments, as shown by Bazan et al and Choung et al, with a consequent further advantage in terms of reduction in treatment breaks.16,17. This side effect decrease has been shown not to affect oncological outcomes as Dasgupta et al18 observed, within a propensity score analysis, that LC is not compromised by the use of IMRT compared with standard techniques. All the aforementioned studies investigated the role of IMRT in anal cancer within a retrospective framework. The only study that prospectively tested the hypothesis that IMRT may potentially enlarge the therapeutic window was the RTOG 05-29 phase II trial that investigated whether dose-painted IMRT may reduce by at least 15% the ≥ G2 GI and GU toxicity rates, compared with conventional EBRT concurrent to 5-FU/MMC as delivered in the RTOG 98-11 trial.19 Even if the primary end point of the trial was not reached, the study showed a significant reduction in acute G2 haematologic (73% vs 85% for RTOG 98-11), G3 GI (21% vs 36% for RTOG 98-11) and G3 dermatologic acute adverse events (23% vs 49% for RTOG 98-11) compared with standard EBRT.19 Our results compared similarly with those of the aforementioned reported series. For instance, G3 acute events were 18% for dermatologic toxicity, 5% for GI, 2% for GU, 28% for leukopenia, 18% for neutropenia and 11% for thrombocytopenia. Grade 4 events were seen only for haematological toxicity (23% overall). The acute toxicity profile of our series homogeneously treated with a VMAT approach compares favourably with similar reports employing other types of IMRT performed with different volumetric approaches. Tozzi et al reported on a consecutive series of 36 patients with anal cancer treated with the RapidArc solution, observing a 13.9% rate of G3 dermatologic toxicity, 17.9% for G3 GI events and no GU G3 toxicity (33.3% of G2). Patients were compared with a historical series of matched patients treated with 3D conformal EBRT, finding a significant decrease in toxicity.20 Yeung et al21 compared static vs volumetric IMRT delivered with helical tomotherapy, observing an advantage in terms of target coverage and lower dose to the bone marrow and external genitalia for tomotherapy and better dosimetric outcomes with respect to the bladder, femoral heads and peritoneal space for static IMRT. The rate of ≥ G2 non-haematologic toxicities were 88.2%, 53% and 5.9% for the skin, GI and GU, respectively, with the use of helical tomotherapy, compared with 85%, 67% and 18% in our series. Leukopenia, neutropenia, thrombocytopenia and anaemia ≥ G2 were 82.4%, 70.6%, 41.2% and 35.3%, respectively, for tomotherapy and 64%, 49%, 21% and 7% in our series. In our cohort, all patients were irradiated by employing an unconstrained IMRT approach towards the pelvic bone marrow, since osseous structures were not included within the optimization process for selective avoidance. It has been demonstrated, for both cervical and anal cancer treated with concomitant CT, that specific doses given to certain volumes of pelvic bones have a significant correlation with acute haematologic toxicity.22,23 On multiple regression analysis, a higher volume of pelvic and lumbosacral bone marrow receiving 5, 10, 15 and 20 Gy was significantly associated with a decreased white-blood-cell count and absolute neutrophil count nadirs.23 The inclusion of osseous structures within the optimization process may be a viable solution to decrease the rate, intensity and duration of haematologic toxicity. In our patient cohort, treatment breaks were seen in 30% of patients with a short mean duration of 2.2 days. Those having a break of ≥3 days were 10%. The RTOG 05-29 trial reported an OTT of 43 days (range 32–59 days), similar to that of our cohort (43 days; range 38–54 days) and shorter than that reported in the RTOG 98-11 trial (49 days; range 4–100 days). Up to 49% of patients experienced treatment breaks, with a higher rate than in our data set (30%), but a lower one than that in RTOG 98-11. On the contrary, a lower median interruption time was seen than in ours (0 vs 2.2 days as median time) and in RTOG 98-11 (3 days). Actuarial rates of DFS, CSS and OS were 84.5%, 85.2% and 85–2% at 2 years, respectively. Moreover, the actuarial probability of being alive at 2 years without a colostomy was 77.9%. These results are comparable with those reported in the RTOG 98-11 trial. Our findings can be considered as a further proof of principle about the feasibility of IMRT in the combination therapy of anal cancer, with similar clinical outcomes to those of the literature, suggesting that on an average, IMRT does not decrease tumour control, survival and sphincter preservation rate if compared with conventional techniques. Acute skin toxicity and compliance to treatment are improved with respect to historical data. Treatment breaks have a lower rate and duration than the series employing standard techniques. No dosimetric parameters were found to be predictive for acute toxicity in our series, except a borderline statistical significance for V20 to the external genitalia at univariate analysis. This may reflect the intrinsic difficulty with respect to dose constraints to the genitalia owing to the close proximity to high-dose target volumes. In our series, external genitalia constraints could not be met in 23/39 (59%) patients for V20, in 15/39 (38%) patients for V30 and in 29/39 (74%) patients for V40. Thus, a specific focus should be addressed to these structures during the planning process. Furthermore, we employed a SIB approach as in the RTOG 0529 trial, delivering a different daily dose to selected treatment volumes during the same treatment fraction. SIB provides dosimetric benefits in terms of target volume dose conformity and homogeneity and normal tissue sparing in the setting of anal cancer. This is noteworthy as the SIB approach is able to shorten the OTT with a consequent potential benefit on treatment outcomes In general, our finding further supports the implementation and use of IMRT on a routine basis for the treatment of cancer of the anal canal in combination with concurrent CT.
Contributor Information
Pierfrancesco Franco, Email: pierfrancesco.franco@unito.it.
Francesca Arcadipane, Email: francesca.arcadipane@gmail.com.
Riccardo Ragona, Email: riccardo.ragona@unito.it.
Massimiliano Mistrangelo, Email: mistrangelo@katamail.com.
Paola Cassoni, Email: paola.cassoni@unito.it.
Fernando Munoz, Email: fmunoz@iol.it.
Nadia Rondi, Email: nrondi@cittadellasalute.to.it.
Mario Morino, Email: mario.morino@unito.it.
Patrizia Racca, Email: pracca@cittadellasalute.to.it.
Umberto Ricardi, Email: umberto.ricardi@unito.it.
REFERENCES
- 1.Franco P, Mistrangelo M, Arcadipane F, Munoz F, Sciacero P, Spadi R, et al. Intensity-modulated radiation therapy with simultaneous integrated boost combined with concurrent chemotherapy for the treatment of anal cancer patients: 4-year results of a consecutive case series. Cancer Invest 2015; 33: 259–66. doi: 10.3109/07357907.2015.1028586 [DOI] [PubMed] [Google Scholar]
- 2.Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB, 3rd, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomicyn versus fluorouracil/cisplatin. J Clin Oncol 2012; 30: 4344–51. doi: 10.1200/JCO.2012.43.8085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008; 299: 1914–21. doi: 10.1001/jama.299.16.1914 [DOI] [PubMed] [Google Scholar]
- 4.Franco P, Zeverino M, Migliaccio F, Sciacero P, Cante D, Casanova Borca V, et al. Intensity-modulated adjuvant whole breast radiation delivered with static angle tomotherapy (TomoDirect): a prospective case series. J Cancer Res Clin Oncol 2013; 139: 1927–36. doi: 10.1007/s00432-013-1515-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlotti A, Alterio D, Vigna-Taglianti R, Muraglia A, Lastrucci L, Manzo R, et al. ; Italian Association of Radiation Oncology. Technical guidelines for head and neck IMRT on behalf of the Italian association of radiation oncology – head and neck working group. Radiat Oncol 2014; 9: 264. doi: 10.1186/s13014-014-0264-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bari B, Fiorentino A, Greto D, Ciammella P, Arcangeli S, Avuzzi B, et al. Prostate cancer as a paradigm of multidisciplinary approach? Highlights from the Italian young radiation oncologist meeting. Tumori 2013; 99: 637–49. doi: 10.1700/1390.15450 [DOI] [PubMed] [Google Scholar]
- 7.Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal cancer patients: a multicenter experience. J Clin Oncol 2007; 25: 4581–6. doi: 10.1200/JCO.2007.12.0170 [DOI] [PubMed] [Google Scholar]
- 8.Vieillot S, Azria D, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, et al. Plan comparison of volumetric modulated arc therapy (RapidArc) and conventional intensity modulated radiation therapy (IMRT) in anal canal cancer. Radiat Oncol 2010; 5: 92. doi: 10.1186/1748-717X-5-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vannetti E, Wyttenbach R, et al. Volumetric modulated arc radiotherapy for carcinomas of the anal canal: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 118–24. doi: 10.1016/j.radonc.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 10.Myerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009; 74: 824–30. doi: 10.1016/j.ijrobp.2008.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng M, Leong T, Chander S, Chu J, Kneebone A, Carroll S, et al. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys 2012; 83: 1455–62. doi: 10.1016/j.ijrobp.2011.12.058 [DOI] [PubMed] [Google Scholar]
- 12.Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 2012; 82: 153–8. doi: 10.1016/j.ijrobp.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 13.Borca VC, Franco P, Catuzzo P, Migliaccio F, Zenone F, Aimonetto S, et al. Does TomoDirect 3DCRT represent a suitable option for post-operative whole breast irradiation? A hypothesis-generating pilot study. Radiat Oncol 2012; 7: 211. doi: 10.1186/1748-717X-7-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco P, Catuzzo P, Cante D, La Porta MR, Sciacero P, Girelli G, et al. TomoDirect: an efficient means to deliver radiation at static angles with tomotherapy. Tumori 2011; 97: 498–502. doi: 10.1700/950.10404 [DOI] [PubMed] [Google Scholar]
- 15.Franco P, Zeverino M, Migliaccio F, Cante D, Sciacero P, Csanova Borca V, et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol 2014; 140: 167–77. doi: 10.1007/s00432-013-1560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazan JG, Hara W, Hsu A, Kunz PA, Ford J, Fisher GA, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011; 117: 3342–51. doi: 10.1002/cncr.25901 [DOI] [PubMed] [Google Scholar]
- 17.Chuong MD, Freilich JM, Hoffe SE, Fulp W, Weber JM, Almhanna K, et al. Intensity-modulated radiation therapy vs 3d conformal radiation therapy for squamous cell carcinoma of the anal canal. Gastrointest Cancer Res 2013; 6: 39–45. [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta T, Rothenstein D, Chou JF, Zhang Z, Wright JL, Saltz LB, et al. Intensity-modulated radiotherapy vs conventional radiotherapy in the treatment of anal squamous cell carcinoma: a propensity score analysis. Radiother Oncol 2013; 107: 189–94. doi: 10.1016/j.radonc.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013; 86: 27–33. doi: 10.1016/j.ijrobp.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tozzi A, Cozzi L, Iftode C, Ascolese A, Campisi MC, Clerici E, et al. Radiation therapy of anal canal cancer: from conformal therapy to volumetric arc therapy. BMC Cancer 2014; 14: 833. doi: 10.1186/1471-2407-14-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung R, McConnell Y, Warkentin H, Graham D, Warkentin B, Joseph K, et al. Intensity-modulated (IMRT) vs helical tomotherapy (HT) in concurrent chemoradiotherapy (CRT) for patients with anal canal carcinoma (ACC): an analysis of dose distribution and toxicities. Radiat Oncol 2015; 10: 92. doi: 10.1186/s13014-015-0398-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70: 1431–7. doi: 10.1016/j.ijrobp.2007.08.074 [DOI] [PubMed] [Google Scholar]
- 23.Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2006; 66: 1356–65. doi: 10.1016/j.ijrobp.2006.03.018 [DOI] [PubMed] [Google Scholar]