Abstract
Objective:
To evaluate prospectively the performance of diffusion-weighted imaging (DWI) for the detection of active lesions on MR enterography (MRE) in children with inflammatory bowel disease (IBD).
Methods:
MRE of 48 children (mean age 13 years) with suspected or known IBD were blindly analysed by 2 independent readers for the presence of active lesions. Two sets of imaging including DWI and gadolinium-enhanced imaging (GEI) were reviewed. A reader consensus was obtained. The gold standard was histopathological findings. In patient-level analysis and segment-level analysis, sensitivity and specificity were calculated for DWI and GEI and compared using McNemar's test or logistic random-effects models.
Results:
At least 1 active lesion was confirmed in 42 (87.5%) children. Sensitivity and specificity for the detection of at least one lesion were 88.1% (95% CI, 74.3–96.1) and 83.3% (95% CI, 35.9–99.6), respectively, for DWI and 66.7% (95% CI, 50.4–80.4) and 83.3% (95% CI, 35.9–99.6), respectively, for GEI. In segment-level analysis, sensitivity and specificity for the detection of specific segment lesions were 62.5% (95% CI, 48.1–75) and 97.1% (95% CI, 93.5–98.7), respectively, for DWI and 45.7% (95% CI, 30.8–61.3) and 98.2% (95% CI, 95.3–99.4), respectively, for GEI. The sensitivity of DWI was significantly better than that of GEI per patient (p = 0.004) and per segment (p = 0.028).
Conclusion:
DWI demonstrates better performance than GEI for the detection of active lesions in children with IBD.
Advances in knowledge:
Examination with no intravenous injection–DWI can replace T1 weighted images when paediatric patients are screened with MRE for IBD. Examination performed in free breathing is better tolerated by children.
INTRODUCTION
Inflammatory bowel disease (IBD) refers to three specific entities: Crohn's disease (CD), ulcerative colitis (UC) and undetermined colitis (UnC). In Europe, 2.2 million people are affected by IBD.1 The incidence in the paediatric population has increased in recent decades.2,3 In northern France, among a population of approximately 6 million people, the incidence of CD has increased by >70% between 1988 and 20074,5 in the 10–19-year age group. The diagnosis of IBD is based on clinical examination, imaging, biological, endoscopic and histological results.6 The gold standard for the diagnosis of IBD remains endoscopy with biopsy and histological confirmation.7 Diagnosing IBD and defining its precise type is a challenge for both clinicians and radiologists. CD and UC share the same chronic evolution, alternating bouts of recurrences and periods of remission.8
The follow-up of affected children and optimization of their treatment require frequent evaluation by imaging techniques. Ultrasound is widely used as the first-line imaging technique in children for screening patients suspected of having IBD.9 Ultrasound has several limitations such as interobserver variability and poor visualization of some segments of the digestive tract.10 Therefore, MR enterography (MRE) has gained popularity and is considered as the imaging modality of choice for IBD in children.11–13 Classical MRE protocol includes T2 and T1 weighted pre- and post-contrast sequences13–16 that are intended to differentiate between active inflammation and fibrosis. Acute bowel inflammation is characterized by an increased bowel wall thickness (>3 mm) and high mural signal intensity relative to the adjacent muscle on T2 weighted images. Recently, two studies17,18 have demonstrated that active disease is characterized by mucosal enhancement.18 However, it also necessitates the placement of an intravenous catheter, that it could be poorly tolerated by younger children below 10 years.19
More recently, Diffusion-weighted imaging (DWI) sequences have been developed for MRE. Diffusion-weighted images are produced on the basis of the random (Brownian) motion of water. DWI MRI reflects the changes in the water mobility caused by interactions with cell membranes, macromolecules and alterations of the tissue. So, in water, molecules are free and the signal intensity on DWI is low and in a tissular environment, the molecular motion is restricted and the signal intensity on DWI is high. Some studies have been shown to characterize inflammatory20–24 and neoplastic conditions.25,26 Studies in adults have shown that DWI depicts inflammatory lesions in IBD, thanks to a restriction of diffusion.20–22 Only two retrospective studies had investigated the contribution of DWI in children.27,28 DWI appears well adapted for children, thanks to faster image acquisition, the possibility of free-breathing mode, reduced motion artefacts, high tissue contrast and because it obviates the need for contrast enhancement.
The objectives of our study were to evaluate prospectively the performance of DWI for the detection of active lesions of IBD and to compare the performance of diffusion-weighted images with T1 weighted images after gadolinium injection compared with histopathological findings as the standard reference.
METHODS AND MATERIALS
The protocol of this prospective study was approved by our institutional review board.
Patients
From April 2013 to December 2014, 100 MRE were performed in 100 consecutive children, who were clinically suspected of having an IBD or known to have IBD and needed a mapping of the lesions. Exclusion criteria of the study were MRE without endoscopic or histological confirmation, MRE in the supine position, MRI with a nasogastric tube, general contraindications to MRI and age over 18 years.
MR enterography protocol
All MR examinations were performed using 1.5-T MR units (SIEMENS Magnetom® AERA XQ MRI (Siemens Healthcare, Erlangen, Germany) and General Electric Signa® MRI (GE Healthcare, Milwaukee, WI), with 18-channel phased-array body coils and 8-channel phased-array cardiac coils, respectively. The children fasted for 4 hours before MRI and were asked to drink 500–1000 ml (volume adapted to the age) of a hyperosmotic solution of water mixed with Mannitol 20% (Macopharma, Mouvaux, France) 45 min before the examination. Images were acquired with patients in the prone position.29 All patients had first T2 weighted half-Fourier single-shot turbo spin-echo and true free-induction steady-state-free precession MR sequences in the axial and coronal planes of the abdomen.
DWI was performed thereafter with a single-shot spin-echoplanar diffusion-weighted technique in the axial plane with two diffusion factors (b = 0 and 1000 s mm−2 for GE and b = 50 and 800 s mm−2 for Siemens). For the examinations performed on the GE magnet, the sequence of DWI covered the entire abdomen in one single acquisition during 166 s. For the Siemens magnet, two acquisitions of DWI were needed to cover the entire abdomen, and each sequence of DWI lasted for 86 s. Fat suppression was obtained with a frequency selective for fat saturation to reduce chemical-shift artefacts for the Siemens magnet. A selective excitation of water was used for the GE equipment.
These sequences were followed by a coronal fat-saturated 3D low-angle volumetric interpolated breath-hold T1 weighted gradient-echo MR sequence (VIBE for Siemens and LAVA for GE) before and after intravenous administration of 0.2-ml Kg gadoterate meglumine (Dotarem, Guerbet, Aulnay-sous-Bois, France) at the rate of 2 ml s−1. Images were acquired during arterial- and portal-venous phases. They were followed by an axial fat-saturated two-dimensional fast spoiled gradient-echo breath-hold T1 weighted MR sequence. No spasmolytic was administrated. The MRI characteristics used are further detailed in Table 1.
Table 1.
Imaging parameters used for MR enterography
| MRI | MR sequence | Acquisition time (s) | Flip angle (degrees) | Repetition time (ms) | Echo time (ms) | Section thickness/gap (mm) | Acceleration factor | Receiver bandwidth (KHz/pixel) | Fat suppression |
|---|---|---|---|---|---|---|---|---|---|
| SIEMENS | T2 HASTE axial | 48 | 170 | 1000 | 96 | 4.0/0.0 | 2 | 401 | No |
| T2 HASTE coronal | 44 | 180 | 1000 | 96 | 4.0/0.0 | 2 | 399 | No | |
| T2 Truefisp axial | 16 | 58 | 4.09 | 2.05 | 4.0/0.0 | 2 | 488 | No | |
| T2 Truefisp coronal | 14 | 59 | 3.95 | 1.98 | 4.0/0.0 | 3 | 488 | No | |
| DWI | 86 | 4800 | 54 | 5.0/0.2 | 2 | 1468 | Yes | ||
| Coronal Gd- enhanced 3D T1 weighted VIBE | 18 | 10 | 3.19 | 1.24 | 1.6/0.2 | 2 | 450 | Yes | |
| Axial Gd-enhanced 2D T1 weighted VIBE | 22 | 10 | 3.19 | 1.24 | 1.4/0.2 | 2 | 450 | Yes | |
| GE | T2 SSFSE axial | 279 | 90 | 4000 | 120 | 4.0/0.6 | 1 | 83.33 | No |
| T2 SSFSE coronal | 150 | 90 | 4000 | 120 | 4.0/0.0 | 1 | 83.33 | No | |
| T2 Fiesta axial | 151 | 85 | 4.80 | 2.17 | 4.0/0.5 | 1 | 62.50 | Yes | |
| T2 Fiesta coronal | 99 | 85 | 5 | 2.25 | 4.0/0.0 | 1 | 62.50 | Yes | |
| DWI | 166 | - | 6400 | 73 | 4.0/1.5 | 1 | 250 | No | |
| Coronal Gd- enhanced 3D T1 weighted LAVA | 166 | 15 | 2.92 | 1.4 | 3.6/1.8 | 1 | 125 | Yes | |
| Axial Gd-enhanced 2D T1 weighted LAVA | 21 | 12 | 3.78 | 1.77 | 3.6/1.8 | 1 | 62.50 | Yes |
2D, two-dimensional; 3D, three-dimensional; DWI, diffusion-weighted imaging; SSFSE, single shot fast spin echo.
Image analysis
MRE images were reviewed on a picture archiving and communication system viewing station (iSite 4.1.114 PACS; Philips Healthcare Informatics, Foster City, CA) by two radiologists with 5 (EAB) and 2 (CD) years’ experience in abdominal imaging using a standardized evaluation form. Both were blinded to the clinical patient information. The bowel was divided into seven segments: the jejunum, ileum, terminal ileum and ileocaecal junction, ascending colon, transverse colon, descending colon and rectosigmoid colon. We discriminate the jejunum from ileum by the number of folds (jejunum >3 folds per inch and ileum <5 folds per inch). The length of the terminal ileum used for this study was 20 cm.
After a common training session, each reader scored independently the likelihood for the presence of an active lesion using a three-point scale on DWI (1= no increased signal intensity, 2= moderate increased signal intensity and 3= marked increased signal intensity) and on gadolinium imaging (1= no contrast enhancement, 2= moderate contrast enhancement and 3= marked contrast-enhancement). On DWI, an active lesion of IBD was considered if an increased signal intensity was noted on high b-value.16 Segments graded 2 or 3 for DWI and contrast enhancement images were considered significant for an active lesion. The image quality of diffusion-weighted and gadolinium-enhanced MR images was evaluated semi-quantitatively using a three-point scale as follows: 1= poor quality (with numerous artefacts), 2= moderate quality (with a few artefacts) and 3= optimal quality (without artefact).
The readers first evaluated diffusion-weighted images as a single set. During the second reading, the readers reviewed gadolinium-enhanced MR images as a single set. In the third reading session, all MR sequences (T2+DWI and T1+gadolinium enhanced) were reviewed together, and discrepancies were resolved by consensus. To minimize recall bias, the three reading sessions were performed 4 weeks apart from each other.
Standard of reference
The standard of reference for the presence of active lesion of IBD was histopathological findings (obtained by biopsy during endoscopic examination in 44 cases and following surgery in 4 cases: 1 lesion of the jejunum and 3 lesions of the ileum). Endoscopic examination was performed within 8 weeks after MRE.
Transmucosal infiltration, focal or diffuse basal plasmacytosis, the presence of neutrophils, cryptitis, cryptitis abscesses and the absence of granuloma were histopathological findings suggesting ulcerative colitis. Epithelioid granuloma is surely a key feature of CD on histological examination but is not specific and not always present (between 15% and 85%). Other histological features suggesting CD were apthoid ulcers, fissure ulcers, transmural inflammation, fistulas and lymphangiectasia. The diagnosis of CD is ascertained when an epithelioid granuloma is present along with any other criteria. The presence of three histological features without granuloma is diagnostic as well.30
Statistical analysis
Results were expressed as the median, interquartile range and range for continuous variables and as frequencies and percentages for qualitative variables. Image quality was compared between the diffusion-weighted and gadolinium-enhanced images by using the Bhapkar's test, which generalizes the McNemar's test when more than two categories are involved.31 The level of agreement between the senior and junior radiologists in the visual assessment of segments with active lesions was evaluated using the weighted kappa (κ) coefficient for three-point scale (1= no lesion, 2= probably lesion, 3= active lesion) and simple κ coefficient for a significant active lesion (defined as Grades 2 or 3); κ values <0 indicated no agreement, 0–0.20 indicated slight agreement, 0.21–0.40 indicated fair agreement, 0.41–0.60 indicated moderate agreement, 0.61–0.80 indicated substantial agreement and 0.81–1.00 indicated almost perfect agreement.32 Using the consensus between the two radiologists for the detection of a significant active lesion, we assessed the diagnostic performance of diffusion-weighted and T1 weighted images after gadolinium injection using histopathological findings as the gold standard. We firstly performed a patient-level analysis to evaluate the diagnostic performance for the detection of IBD disease (i.e. at least one lesion at any segment) and secondly a segment-level analysis, taking into account multiple observations per patient to evaluate the diagnostic performance for the detection of specific-segment lesion.33 For patient- and segment-level analyses, we have calculated the sensitivity, specificity, positive-predictive value (PPV), negative-predictive value (NPV) and accuracy of each MRE imaging for the detection of active disease. In patient-level analysis, exactly 95% confidence intervals (CIs) for the diagnostic criteria were calculated, and sensitivity was compared between the two MRE imaging using the exact McNemar's test. In segment-level analysis, we used a logistic random-effects model to estimate the diagnostic criteria and their 95% CIs and to compare the sensitivity and specificity values between the two MRE imagings;33 this model takes into account the correlation between segment measures within patients. For segment-level analysis, we assessed the sensitivity and specificity of each MRE imaging according to the type of MRI scanner (Siemens vs GE). Statistical testing was performed at the two-tailed level of 0.05. Data were analysed using the SAS® software package, release 9.3 (SAS Institute, Cary, NC).
RESULTS
During the period of the study, 100 MRE were performed in our hospital. We excluded 52 patients: 42 patients without endoscopic results or with incomplete endoscopic results, 9 patients with MRE with complete endoscopic and histopathological results but performed in supine position and 1 patient with MRE under general anaesthesia (supine position and MRI with nasogatric tube).
48 children (25 females and 23 males) could finally be included, with a median age of 13 years (range, 5–18 years). 25 (52.1%) children had a clinical suspicion of IBD and 23 (47.9%) children had follow-up examinations for known IBD, including 15 children with CD, 5 children with UC and 3 children with UnC. Two patients had had previous bowel surgery (colectomy for Hirschsprung disease and colectomy for indeterminate colitis) and five patients had had previous perineal surgery. Patient characteristics are summarized in Table 2.
Table 2.
Patient characteristics
| Characteristics | Values |
|---|---|
| Number of patients | 48 |
| Age, median (IQR), years | 13 (12–15) |
| Males, n (%) | 23 (47.9) |
| Disease duration, median (IQR), months | 23 (12–38) |
| Prior surgery, n (%) | 7 (14.6) |
| Crohn's diseasea, n (%) | 33 (68.8) |
| Age at diagnosis: A1 | 23 (47.9) |
| A2 | 10 (20.8) |
| A3 | 0 (0.0) |
| Location: L1 | 8 (16.7) |
| L2 | 8 (16.7) |
| L3 | 12 (25.0) |
| L1L4 | 1 (2.1) |
| L2L4 | 3 (6.3) |
| L3L4 | 1 (2.1) |
| Behaviour: B1b | 19 (39.6) |
| B2 | 4 (8.3) |
| B3 | 10 (20.8) |
| Ulcerative colitisa, n (%) | 8 (16.7) |
| E1 | 1 (2.1) |
| E2 | 2 (4.2) |
| E3 | 5 (10.4) |
| Underterminate colitis, n (%) | 3 (6.3) |
IQR, interquartile range.
Montreal classification for Crohn's disease or extent for ulcerative colitis.
10 patients had concomitant perianal disease (6 patients with B1, 1 patient with B2 and 3 patients with B3).
MRE was performed successfully without side effects in all children. The quality of diffusion-weighted images was significantly better than that of T1 weighted images after gadolinium injection (Bhapkar's test: p < 0.0001). No artefact was found in 37 MRE (77.1%) with diffusion-weighted images in comparison with 21 MRE (43.8%) with T1 weighted images after gadolinium injection (Figure 1). The overall interreader agreement for graded active lesions was excellent with diffusion-weighted images [accuracy = 94.5%; k (95% CI) = 0.83 (0.77–0.89)] as well as with T1 weighted images after gadolinium injection [accuracy = 95.7%; k (95% CI) = 0.82 (0.75–0.89)] (Table 3). When considered Grades 2 and 3 as significant for active lesion, the proportion of concordance was 98.5% [k (95% CI) = 0.95 (0.91–0.99)] in diffusion-weighted images and 97.0% [k (95% CI) = 0.89 (0.82–0.96)] in T1 weighted images when considering Grades 2 or 3 as significant lesions. Among the five discordances in diffusion-weighted images, the senior radiologist did not confirm the junior radiologist’s diagnosis of active lesion in one case. For T1 weighted images, the senior radiologist did not confirm the junior radiologist’s diagnosis of active lesion in four cases.
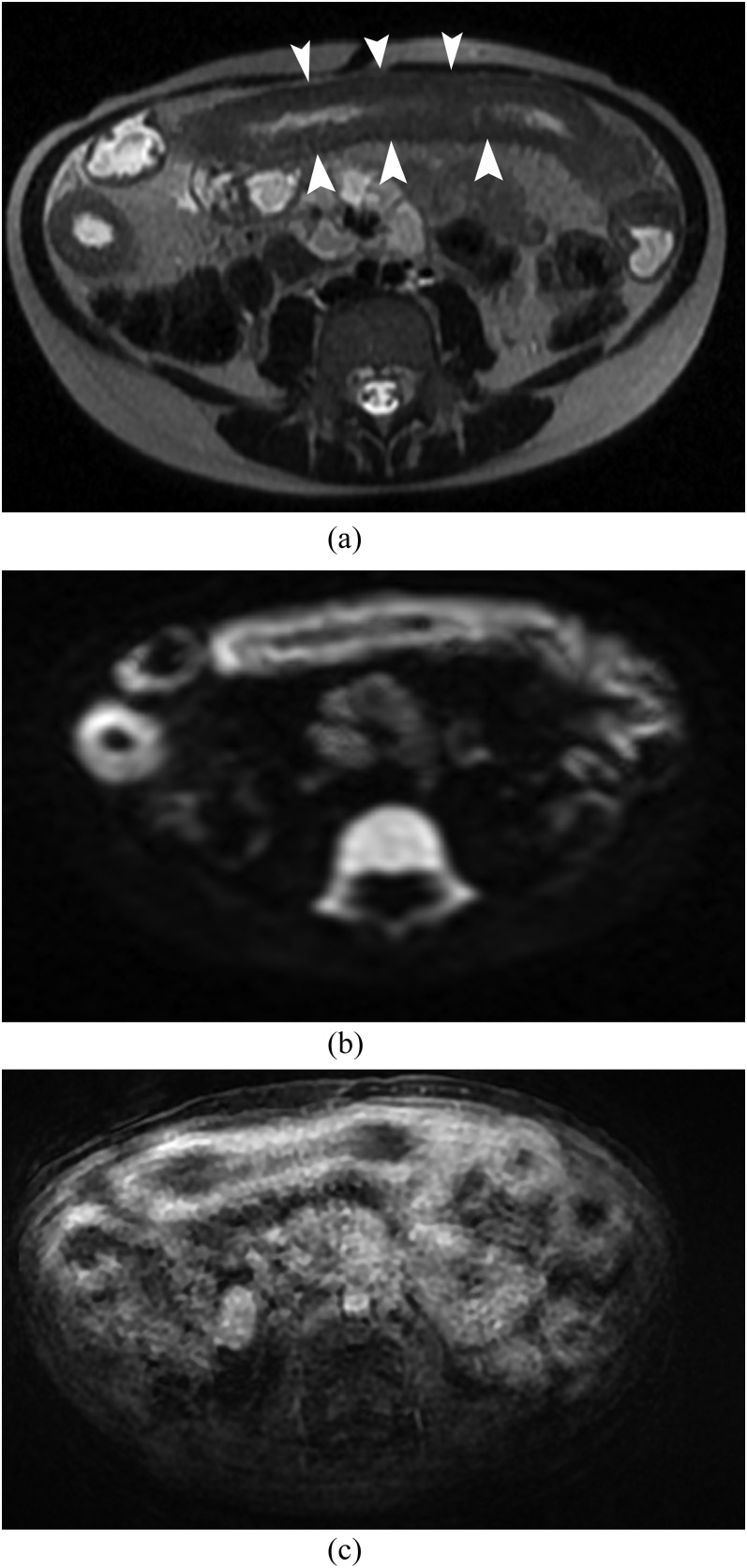
Figure 1.
MR enterography in 7-year-old female with Crohn’s disease. (a) Axial T2 single shot fast spin echo shows a long wall thickening (arrowheads) of the small bowel. (b) Axial diffusion-weighted imaging (b = 1000 s mm−2) shows a hyperintense signal of the small bowel with no artefact. (c) Axial T1 LAVA fat saturation after gadolinium enhancement shows a sequence with multiple artefacts. The enhancement is less well visualized as compared with DWI (b).
Table 3.
Observed proportion of interreader agreement for graded active lesions, overall and according to bowel segments
| Bowel segments | Diffusion-weighted images |
T1 weighted images |
||
|---|---|---|---|---|
| 3-point scale | Active diseasea | Three-point scale | Active diseasea | |
| Jejunum | 97.9 (47/48) | 97.9 (47/48) | 97.9 (47/48) | 97.9 (47/48) |
| Ileum | 93.8 (45/48) | 97.9 (47/48) | 95.8 (46/38) | 97.9 (47/48) |
| Terminal ileum and ileocaecal junction | 89.6 (43/48) | 100.0 (48/48) | 89.6 (43/48) | 100.0 (48/48) |
| Ascending colon | 89.6 (43/48) | 100.0 (48/48) | 91.7 (44/48) | 100.0 (48/48) |
| Transverse colon | 89.6 (43/48) | 97.9 (47/48) | 93.8 (45/48) | 97.9 (47/48) |
| Descending colon | 93.8 (45/48) | 100.0 (48/48) | 89.6 (43/48) | 93.8 (45/62) |
| Rectosigmoid colon | 87.5 (42/48) | 95.9 (46/48) | 91.7 (44/48) | 93.8 (45/48) |
| All segments | 94.5 (308/336) | 98.5 (331/336) | 95.7 (312/336) | 97.0 (326/336) |
Values are percentage (numbers).
Segments graded as 2 or 3 were considered to have active disease, and segments graded as 1 were considered inactive.
At least one active lesion of IBD was demonstrated in 42 (87.5%) cases using histopathological findings. Active lesion was most frequently diagnosed in the rectosigmoid colon (56.3%, n = 27), followed by in the descending colon (45.8%, n = 22), ascending colon (39.6%, n = 19), terminal ileum and ileocaecal junction (39.6%, n = 19), transverse colon (35.4%, n = 17), ileum (6.2%, n = 3) and jejunum (2.1%, n = 1) (Table 4).
Table 4.
Diagnostic performance for active IBD lesion on DWI and gadolinium using endoscopic or surgery findings as gold standard in patient-level analysis, overall and by bowel segments
| DWI and gadolinium results | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| DWI results | |||||
| All segments | 88.1 (37/42)a | 83.3 (5/6) | 97.4 (37/38) | 50.0 (5/10) | 87.5 (42/48) |
| Jejunum | 0.0 (0/1) | 100.0 (47/47) | – | 97.9 (47/48) | 97.9 (47/48) |
| Ileum | 66.7 (2/3) | 93.3 (42/45) | 40.0 (2/5) | 97.7 (42/43) | 91.7 (44/58) |
| Terminal ileum and ileo-caecal junction | 79.0 (15/19) | 93.1 (27/29) | 88.2 (15/17) | 87.1 (27/31) | 87.5 (42/48) |
| Ascending colon | 47.4 (9/19) | 96.6 (28/29) | 90.0 (9/10) | 73.7 (28/38) | 77.1 (37/48) |
| Transverse colon | 41.2 (7/17) | 96.8 (30/31) | 87.5 (7/8) | 75.0 (30/40) | 77.1 (37/48) |
| Descending colon | 59.1 (13/22) | 100.0 (26/26) | 100.0 (13/13) | 74.3 (26/35) | 81.2 (39/48) |
| Rectosigmoid colon | 63.0 (17/27) | 100.0 (21/21) | 100.0 (17/17) | 67.7 (21/31) | 79.2 (38/48) |
| Gadolinium results | |||||
| All segments | 66.7 (28/42) | 83.3 (5/6) | 96.6 (28/29) | 26.3 (5/19) | 68.7 (33/48) |
| Jejunum | 0.0 (0/1) | 100.0 (47/47) | – | 97.9 (47/48) | 97.9 (47/48) |
| Ileum | 66.7 (2/3) | 93.3 (42/45) | 40.0 (2/5) | 97.7 (42/43) | 91.7 (44/48) |
| Terminal ileum and ileocaecal junction | 63.2 (12/19) | 96.6 (28/29) | 92.3 (12/13) | 80.0 (28/35) | 83.3 (40/48) |
| Ascending colon | 42.1 (8/19) | 100.0 (29/29) | 100.0 (8/8) | 72.5 (29/40) | 77.1 (37/48) |
| Transverse colon | 35.3 (6/17) | 100.0 (31/31) | 100.0 (6/6) | 73.8 (31/42) | 77.1 (37/48) |
| Descending colon | 40.9 (9/22) | 100.0 (26/26) | 100.0 (9/9) | 66.7 (26/39) | 72.9 (35/48) |
| Rectosigmoid colon | 44.4 (12/27) | 100.0 (21/21) | 100.0 (12/12) | 58.3 (21/36) | 68.7 (33/48) |
DWI, diffusion weighted imaging; IBD, inflammatory bowel disease; NPV, negative-predictive value; PPV, positive-predictive value.
Values are percentage (numbers).
p < 0.05 for comparison with sensitivity of gadolinium by exact McNemar's test.
Diagnostic performances of diffusion-weighted images and T1 weighted images after gadolinium injection
Diagnostic performances of diffusion-weighted and gadolinium-enhanced images for the detection of active IBD lesion using histopathological findings as the gold standard are shown in Table 4 for all segments and according to each single bowel segment.
Patient-level analysis
When considering all bowel segments (i.e. a diagnosis of ≥1 IBD lesion whatever the segment), 37 true-positive and 5 true-negative results were found on DWI. One false-positive case corresponded to a normal terminal ileum at the endoscopic examination in a 17-year-old female. Five false-negative cases were found including four children with colonic lesion (one UC, one CD and two UnC) and one child with terminal ileum and colonic lesions (CD). The corresponding sensitivity, specificity, PPV, NPV and accuracy were 88.1% (95% CI, 74.3–96.1), 83.3% (95% CI, 35.9–99.6), 97.4% (95% CI, 86.2–99.9), 50.0% (95% CI, 18.7–81.3) and 87.5% (95% CI, 74.7–95.3), respectively.
Analysing the gadolinium-enhanced set, 28 true-positive and 5 true-negative results were found. One false-positive case was found and it was the same as with DWI. Nine supplementary false-negative cases were found compared with DWI including seven children with colonic lesion and two children with terminal ileum lesion. The corresponding sensitivity, specificity, PPV, NPV and accuracy were 66.7% (95% CI, 50.4–80.4), 83.3% (95% CI, 35.9–99.6), 96.6% (95% CI, 82.2–99.9), 26.3% (95% CI, 9.1–51.2) and 68.8% (95% CI, 53.7–81.3), respectively.
Sensitivity for the detection of ≥1 active IBD lesion was significantly better with DWI than with T1 weighted imaging after gadolinium injection (McNemar's test: p = 0.004), whereas the specificity was similar. Regarding sensitivity, when analysis was stratified by bowel segments, a similar difference between diffusion-weighted and T1 weighted images after gadolinium injection was observed in the most frequent sites of the IBD lesion (Table 4) (Figure 2).
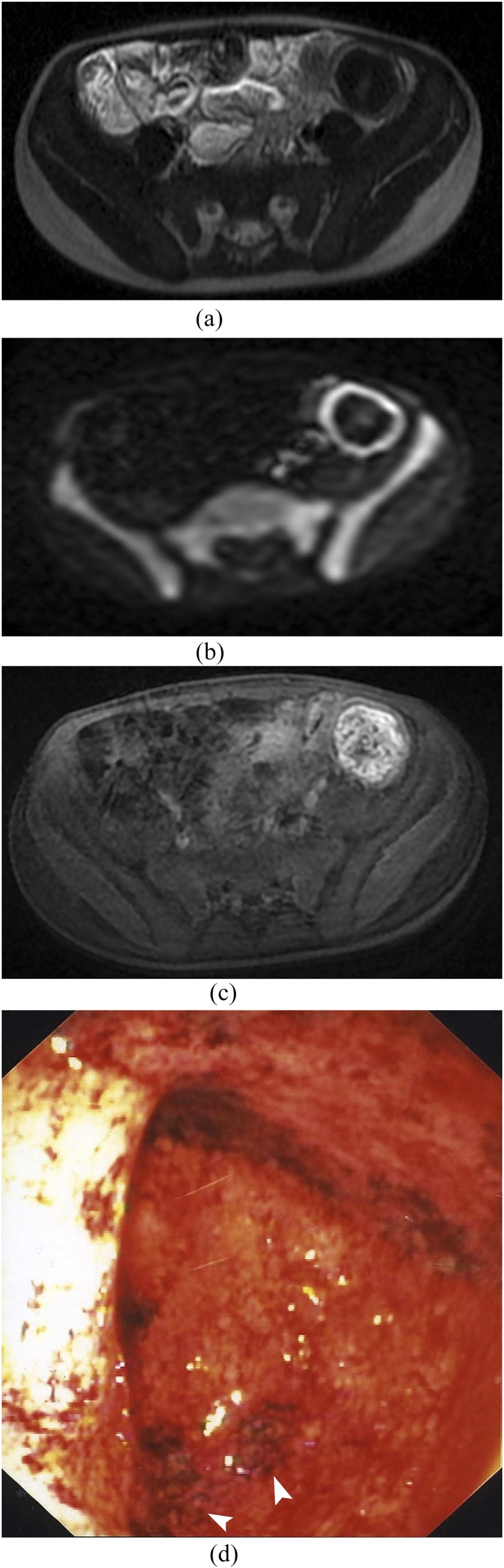
Figure 2.
MR enterography in 6-year-old female with ulcerative colitis. (a) Axial T2 single shot fast spin echo shows no colonic distension and no wall thickening. (b) Axial diffusion-weighted imaging (b = 1000 s mm−2) shows a hyperintense signal of the descendant colonic. (c) Axial T1 LAVA fat saturation after gadolinium enhancement shows mild enhancement. (d) Macroscopic photography of the endoscopy shows ulcerative lesions of the left colonic: diffuse erythema with mucosal granularity (arrowheads).
Segment-level analysis
In segment-level analysis (Table 5), we also found that the sensitivity of DWI to detect a specific-segment lesion (62.5%) was better than that of T1 weighted images after gadolinium injection (45.7%, logistic random-effect model: p = 0.028) (Figure 2). No significant difference was noted for the specificity of diffusion-weighted images and T1 weighted images after gadolinium injection (97.1% vs 98.2%, p = 0.36). When segment-level analysis was stratified accordingly between the two manufacturers, the sensitivity of diffusion-weighted images remained higher than the sensitivity of T1 weighted images after gadolinium injection, although no significant difference was noted (p = 0.19 for Siemens, and p = 0.07 for GE). The sensitivity of diffusion-weighted images was 65.9% (95% CI, 46.0–81.4) for Siemens and 54.4% (95% CI, 31.7–75.4) for T1 weighted images after gadolinium injection. The corresponding values obtained with GE were 59.4% (95% CI, 36.8–78.6) and 36.9% (95% CI, 18.6–59.9). Specificities of diffusion-weighted images and T1 weighted images after gadolinium injection were equally high in both manufacturers (>96%).
Table 5.
Summary estimates of the diagnostic criteria and their 95% confidence intervals (CIs) for active IBD lesion on DWI and gadolinium using endoscopic or surgery findings as the gold standard in segment-level analysis
| DWI | Gadolinium | p-valuea | |
|---|---|---|---|
| Sensitivity, % | 62.5 (48.1–75.0) | 45.7 (30.8–61.3) | 0.028 |
| Specificity, % | 97.1 (93.5–98.7) | 98.2 (95.3–99.4) | 0.36 |
| PPV, % | 90.3 (79.4–95.7) | 92.3 (79.3–97.4) | |
| NPV, % | 86.2 (78.9–91.3) | 81.4 (74.0–87.0) | |
| Accuracy, % | 86.9 (80.6–91.3) | 83.1 (76.5–88.1) |
DWI, diffusion-weighted imaging; IBD, inflammatory bowel disease; NPV, negative-predictive value; PPV, positive-predictive value.
Sensitivity and specificity were compared between DWI and gadolinium using a using a logistic random-effects model.
DISCUSSION
MRE combining T2 and T1 weighted images after gadolinium injection sequences is considered as an optimal imaging technique for the detection of active IBD in the adult and paediatric population. In a retrospective study, using histology as a gold standard, Dillman et al34 have shown that classical sequences (T2 weighted imaging and T1 weighted imaging after gadolinium injection) have a sensitivity of 94% for the detection of small-bowel and colonic lesions in case of CD. Gee et al35 reported similar results in a prospective study including 21 patients. They achieved a sensitivity of 90% and a specificity of 82.6% for MRE with histopathological findings as gold standard. Recently, Seo et al36 have shown in 44 adults that DWI MRE is not inferior to contrast material-enhanced MRE for the evaluation of inflammation in Crohn’s disease, except for the diagnosis of penetration. However, the “classical” technique using T2 and contrast-enhanced T1 sequences has disadvantages in children: the length of the examination, the need for contrast enhancement and catheter placement. Therefore, it would be interesting if the examination could be simplified and shortened. Our study shows that the use of DWI could achieve this.
A retrospective study by Oto et al in 200920 has shown in 11 adult patients that the inflammation of the bowel wall induces a restriction of diffusion with SE = 84% and Sp=91% for the detection of active lesions of CD with a apparent diffusion coefficient (ADC) threshold of 2. A second study22 including 18 patients and using histology and endoscopy as the gold standard showed a better sensitivity of ADC values for the detection of inflammatory lesions as compared with patterns of enhancement on T1 weighted images. In another study, Kyriu et al21 has shown that DWI had SE = 86% and an accuracy of 82.4% in the detection of active disease compared with conventional barium study or surgery (17 patients). These studies were interesting but included only small samples of adults or did not use a well-defined gold standard. Buisson et al,37 in a prospective study including 31 adult patients, showed excellent sensitivity (SE = 100%) of DWI as compared with conventional MRE. The NPV was 100%. In the paediatric population, only retrospective studies investigating the role of DWI in IBD are available. In 2012, Neubauer et al27 performed a retrospective study in a paediatric population of 33 children. They reported that the diffusion-weighted sequence combined with T2 sequences had diagnostic performances equal or superior to T1 weighted contrast-enhanced sequences for the detection of active inflammatory lesions and extraluminal complications of CD. In another retrospective study, Ream et al28 have shown that restriction of diffusion can be found in patients when active small-bowel inflammation lesions are present. Sohn et al38 showed in a retrospective study of 15 children that all MRE sequences (T2, motility imaging, DWI and dynamic enhancement imaging) have a high lesion detectability with SE = 90.2% on DWI compared with SE = 92.7–95.1% using contrast-enhanced MR. Recently, Shenoy-Bhangle et al39 have shown that the combination of DWI and MRE increased the imaging accuracy for the detection of disease activity compared with either technique alone.
To our knowledge, our study is the first that has evaluated prospectively the performance of DWI with gadolinium-enhanced sequences in the detection of active lesions of IBD in children and used anatomopathological findings as the reference standard. Our excellent results (SE = 88.1%, Sp = 83.3%, PPV = 97.4%, NPV = 50.0% and accuracy = 87.5%) are similar to those from retrospective studies.20,21 Our study demonstrates that MR images obtained with DWI could replace gadolinium chelate-enhanced MRI to detect active lesions of IBD in the protocol of MRE in a paediatric population.
Recently, Kim et al40 evaluated the performance of DWI per segment, in an adult population, and showed that DWI had a sensitivity of 83% in the terminal ileum and 60% in the colon rectum.
To our knowledge, our study was the first in children to evaluate per segment the performance of DWI compared with histopathological results. However, by comparison with patient-level analysis, the sensitivity decreased at the segment level, since every IBD lesions need to be identified separately in order to count as true positive, and thus the opportunity to miss a lesion in an individual segment increases.33 However, our results per segment (SE = 79% for the terminal ileum, SE comprised between 41.2% and 63% for the colon) are similar to those from a previous study.40 From these data, it can be concluded that DWI is an excellent screening tool, but probably lacks precision for the exact topographic diagnosis of the lesions. Furthermore, diagnostic performances are better for the evaluation of the small bowel than for the colon. Still, DWI had better diagnostic performances than gadolinium injection sequences. DWI could be less dependent on a good distension of the digestive tract.
There are other advantages in using DWI instead of T1 weighted imaging after gadolinium injection: a shorter duration of examination (if gadolinium MR imaging is not performed) and no need for catheter placement. In our study, images obtained by DWI displayed better image quality (p < 0.0001). This difference of quality may be due to a longer time of examination with T1 weighted imaging and artefacts of movements, especially at the end of MRE. Furthermore, gadolinium MR imaging is performed in apnoea, while DWI MR imaging can be performed in free breathing, which is better tolerated by paediatric patients. There are other technical explanations to the overall good performance of MRE in our study. Indeed, unlike other studies,27,28 we did not use spasmolytic medications in order to simplify the examinations in children. Also, we placed the patients in a prone position and obtained a good distension of the bowel without many artefacts from the small bowel.29 Still, there are some disadvantages of using DWI. DWI has a poorer spatial resolution that requires a joint analysis with T2 sequences; for this reason, the MR protocol requires the performance of T2 weighted imaging together with DWI. This lower spatial resolution also probably explains the lower topographic diagnostic value of DWI.
Our study has several limitations; first, the relatively small size of included patients. However, our number is larger than that in previous studies.20,21 We excluded 42 patients for whom we had no endoscopic or surgical diagnosis and 9 patients because MRE was performed in the supine position and 1 patient with MRE performed under general anaesthesia. Therefore, we could not exclude a selection bias. Another limitation of our study was the small number of true-negative patients. For ethical reasons, it was difficult to justify performing invasive examinations such as endoscopy in children with no abnormalities on MRE and moderate or low suspicion of IBD. However, in segment analysis, true-negative bowel segments were important, which allows us to accurately estimate the specificity. Another limitation of the study was that DWI evaluation was based on qualitative visual inspection rather than quantitative DWI analysis. Future studies with quantitative evaluation could be valuable.
CONCLUSION
Our study shows that DWI had better diagnostic accuracy than gadolinium MR images and might replace gadolinium MR images for screening IBD.
This would avoid the injection of contrast, reduce the examination time and is better tolerated by young paediatric patients. However, gadolinium is still necessary to detect abscess or fistulae. The respective role of T2 weighted images, DWI and T1 weighted images after gadolinium injection for the diagnosis between fibrosis and active lesions in children should be evaluated in future studies.
Contributor Information
Céline Dubron, Email: cdubron@gmail.com.
Freddy Avni, Email: favni@skynet.be.
Nathalie Boutry, Email: nathalie.boutry@chru-lille.fr.
Dominique Turck, Email: dominique.turck@chru-lille.fr.
Alain Duhamel, Email: alain.duhamel@chru-lille.fr.
Elisa Amzallag-bellenger, Email: elisa.amzallag@gmail.com.
REFERENCES
- 1.Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004; 126: 1504–17. doi: 10.1053/j.gastro.2004.01.063 [DOI] [PubMed] [Google Scholar]
- 2.Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen S, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr 2005; 146: 35–40. doi: 10.1016/j.jpeds.2004.08.043 [DOI] [PubMed] [Google Scholar]
- 3.Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr 2010; 50: 27–31. doi: 10.1097/MPG.0b013e3181b99baa [DOI] [PubMed] [Google Scholar]
- 4.Gasparetto M, Guariso G. Highlights in IBD epidemiology and its natural history in the paediatric age. Gastroenterol Res Pract 2013; 2013: 829040. doi: 10.1155/2013/829040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chouraki V, Savoye G, Dauchet L, Vernier-Massouille G, Dupas JL, Merle V, et al. The changing pattern of Crohn’s disease incidence in northern France: a continuing increase in the 10- to 19-year-old age bracket (1988–2007). Aliment Pharmacol Ther 2011; 33: 1133–42. doi: 10.1111/j.1365-2036.2011.04628.x [DOI] [PubMed] [Google Scholar]
- 6.IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis-the Porto criteria. J Pediatr Gastroenterol Nutr 2005; 41: 1–7. [DOI] [PubMed] [Google Scholar]
- 7.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. ; European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27. doi: 10.1016/j.crohns.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 8.North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America; Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007; 44: 653–74. [DOI] [PubMed] [Google Scholar]
- 9.Alison M, Kheniche A, Azoulay R, Roche S, Sebag G, Belarbi N. Ultrasonography of Crohn disease in children. Pediatr Radiol 2007; 37: 1071–82. doi: 10.1007/s00247-007-0559-1 [DOI] [PubMed] [Google Scholar]
- 10.Schreyer AG, Menzel C, Friedrich C, Poschenrieder F, Egger L, Dornia C, et al. Comparison of high-resolution ultrasound and MR-enterography in patients with inflammatory bowel disease. World J Gastroenterol 2011; 17: 1018–25. doi: 10.3748/wjg.v17.i8.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duigenan S, Gee MS. Imaging of pediatric patients with inflammatory bowel disease. AJR Am J Roentgenol 2012; 199: 907–15. doi: 10.2214/AJR.11.7966 [DOI] [PubMed] [Google Scholar]
- 12.Paolantonio P, Ferrari R, Vecchietti F, Cucchiara S, Laghi A. Current status of MR imaging in the evaluation of IBD in a pediatric population of patients. Eur J Radiol 2009; 69: 418–24. doi: 10.1016/j.ejrad.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 13.Mollard BJ, Smith EA, Dillman JR. Pediatric MR enterography: technique and approach to interpretation-how we do it. Radiology 2015; 274: 29–43. doi: 10.1148/radiol.14122449 [DOI] [PubMed] [Google Scholar]
- 14.Darge K, Anupindi SA, Jaramillo D. MR imaging of the abdomen and pelvis in infants, children, and adolescents. Radiology 2011; 261: 12–29. doi: 10.1148/radiol.11101922 [DOI] [PubMed] [Google Scholar]
- 15.Griffin N, Grant LA, Anderson S, Irving P, Sanderson J. Small bowel MR enterography: problem solving in Crohn's disease. Insights Imaging 2012; 3: 251–63. doi: 10.1007/s13244-012-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalian M, Ozturk A, Oliva-Hemker M, Pryde S, Huisman TA. MR enterography findings of inflammatory bowel disease in pediatric patients. AJR Am J Roentgenol 2011; 196: W810–16. doi: 10.2214/AJR.10.5474 [DOI] [PubMed] [Google Scholar]
- 17.Rimola J, Planell N, Rodriguez S, Delgado S, Ordas I, Ramirez-Morros A, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 432–40. doi: 10.1038/ajg.2014.424 [DOI] [PubMed] [Google Scholar]
- 18.Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 2014; 24: 619–29. doi: 10.1007/s00330-013-3015-7 [DOI] [PubMed] [Google Scholar]
- 19.Kennedy RM, Luhmann J, Zempsky WT. Clinical implications of unmanaged needle-insertion pain and distress in children. Pediatrics 2008; 122 (Suppl. 3): S130–3. doi: 10.1542/peds.2008-1055e [DOI] [PubMed] [Google Scholar]
- 20.Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn's disease. Acad Radiol 2009; 16: 597–603. doi: 10.1016/j.acra.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiryu S, Dodanuki K, Takao H, Watanabe M, Inoue Y, Takazoe M, et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn's disease. J Magn Reson Imaging 2009; 29: 880–6. doi: 10.1002/jmri.21725 [DOI] [PubMed] [Google Scholar]
- 22.Oto A, Kayhan A, Williams JT, Fan X, Yun L, Arkani S, et al. Active Crohn's disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 2011; 33: 615–24. doi: 10.1002/jmri.22435 [DOI] [PubMed] [Google Scholar]
- 23.Chavhan GB, Alsabba Z, Babyn PS. Diffusion-weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics 2014; 34: E73–88. doi: 10.1148/rg.343135047 [DOI] [PubMed] [Google Scholar]
- 24.Masselli G, Gualdi G. MR imaging of the small bowel. Radiology 2012; 264: 333–48. doi: 10.1148/radiol.12111658 [DOI] [PubMed] [Google Scholar]
- 25.Amzallag-Bellenger E, Soyer P, Barbe C, Nguyen TL, Amara N, Hoeffel C. Diffusion-weighted imaging for the detection of mesenteric small-bowel tumours with Magnetic Resonance-enterography. Eur Radiol 2014; 24: 2916–26. doi: 10.1007/s00330-014-3303-x [DOI] [PubMed] [Google Scholar]
- 26.Humphries PD, Sebire NJ, Siegel MJ, Olsen ØE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 2007; 245: 848–54. doi: 10.1148/radiol.2452061535 [DOI] [PubMed] [Google Scholar]
- 27.Neubauer H, Pabst T, Dick A, Machann W, Evangelista L, Wirth C, et al. Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol 2013; 43: 103–14. doi: 10.1007/s00247-012-2492-1 [DOI] [PubMed] [Google Scholar]
- 28.Ream JM, Dillman JR, Adler J, Khalatbari S, McHugh JB, Strouse PJ, et al. MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol 2013; 43: 1077–85. doi: 10.1007/s00247-013-2712-3 [DOI] [PubMed] [Google Scholar]
- 29.Cronin CG, Lohan DG, Mhuircheartaigh JN, McKenna D, Alhajeri N, Roche C, et al. MRI small-bowel follow-through: prone versus supine patient positioning for best small-bowel distention and lesion detection. AJR Am J Roentgenol 2008; 191: 502–6. doi: 10.2214/AJR.07.2338 [DOI] [PubMed] [Google Scholar]
- 30.Geboes K. Histopathology of Crohn’s disease and ulcerative colitis. Inflammatory Bowel Disease. Edinburgh, London, Melbourne: Churchill Livingstone; 2003. [Google Scholar]
- 31.Agresti A. Categorical data analysis , New York: John Wiley & Sons; 1990. [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 33.Genders TS, Spronk S, Stijnen T, Steyerberg EW, Lesaffre E, Hunink MG. Methods for calculating sensitivity and specificity of clustered data: a tutorial. Radiology 2012; 265: 910–16. doi: 10.1148/radiol.12120509 [DOI] [PubMed] [Google Scholar]
- 34.Dillman JR, Ladino-Torres MF, Adler J, DeMatos-Malljard V, McHugh JB, Khalatbari S, et al. Comparison of MR enterography and histopathology in the evaluation of pediatric Crohn disease. Pediatr Radiol 2011; 41: 1552–8. doi: 10.1007/s00247-011-2186-0 [DOI] [PubMed] [Google Scholar]
- 35.Gee MS, Nimkin K, Hsu M, Israel EJ, Biller JA, Katz AJ, et al. Prospective evaluation of MR enterography as the primary imaging modality for pediatric Crohn disease assessment. AJR Am J Roentgenol 2011; 197: 224–31. doi: 10.2214/AJR.10.5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo N, Park SH, Kim KJ, Kang BK, Lee Y, Yang SK, et al. MR enterography for the evaluation of small-bowel inflammation in crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 2015; [Epub Ahead of Print]. 10.1148/radiol.2015150809 [DOI] [PubMed] [Google Scholar]
- 37.Buisson A, Joubert A, Montoriol PF, Da Ines D, Hordonneau C, Pereira B, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn's disease. Aliment Pharmacol Ther 2013; 37: 537–45. doi: 10.1111/apt.12201 [DOI] [PubMed] [Google Scholar]
- 38.Sohn B, Kim MJ, Koh H, Han KH, Lee MJ. Intestinal lesions in pediatric Crohn disease: comparative detectability among pulse sequences at MR enterography. Pediatr Radiol 2014; 44: 821–30. doi: 10.1007/s00247-014-2902-7 [DOI] [PubMed] [Google Scholar]
- 39.Shenoy-Bhangle AS, Nimkin K, Aranson T, Gee MS. Value of diffusion-weighted imaging when added to MRE evaluation of Crohn disease in children. Pediatr Radiol 2016; 46: 34–42. doi: 10.1007/s00247-015-3438-1 [DOI] [PubMed] [Google Scholar]
- 40.Kim KJ, Lee Y, Park SH, Kang BK, Seo N, Yang SK, et al. Diffusion-weighted MR enterography for evaluating Crohn’s disease: how does it add diagnostically to conventional MR enterography? Inflamm Bowel Dis 2015; 21: 101–9. doi: 10.1097/MIB.0000000000000222 [DOI] [PubMed] [Google Scholar]