Abstract
Objective:
To evaluate the ability of conventional MRI, diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI to differentiate malignant and benign parotid lesions.
Methods:
A retrospective review of MRI findings was performed in patients with pathologically confirmed parotid lesions between January 2010 and December 2014. Morphological MRI characteristics and functional characteristics such as apparent diffusion coefficient (ADC) and pattern of time–signal intensity curve (TIC) were recorded and compared. For each lesion, summed scores were respectively calculated for conventional MRI alone, conventional MRI with DWI and/or with DCE-MRI. Statistical analyses were performed to assess the association of these characteristics and summed scores with malignancy.
Results:
A total of 207 patients (111 males and 96 females; age: 48.4 ± 17.0 years) were included, consisting of 156 benign and 51 malignant tumours. After adjusting for age, sex, smoking status, alcohol use and tumour size, the lesions with ill-defined margin, adjacent tissue infiltration, cervical lymphadenopathy, ADC ≤1.01 × 10−3 mm2 s−1 and plateau TIC pattern are more likely to be malignant than those without these findings. Significant difference in receiver operator characteristic was detected after adding DWI to conventional MRI (p = 0.003), generating a sensitivity and specificity of 54.05% and 91.30%, respectively. Compared with lesions score <3, lesions with score ≥5 in the combination of conventional MRI and DWI were approximately 90 times more likely to be malignant parotid tumour. Additional DCE-MRI did not improve differential ability of conventional MRI.
Conclusion:
Morphological and functional MRI characteristics are associated with malignancy in parotid gland. The diagnostic value of MRI would increase when DWI is applied in combination with conventional MRI.
Advances in knowledge:
The parotid lesions with ill-defined margin, adjacent tissue infiltration, cervical lymphadenopathy, ADC ≤1.01 × 10−3 mm2 s−1 and plateau TIC pattern are more likely to be malignant. The diagnostic value of conventional MRI would be increased when DWI is applied in combination, whereas additional DCE-MRI did not improve differential ability of conventional MRI.
INTRODUCTION
Modern imaging plays a key role in discriminating benign parotid lesion (BPL) and malignant parotid tumour (MPT), selecting appropriate surgical strategy and making radiation therapy plan.1 High-resolution ultrasonography, CT and conventional MRI are primary imaging modalities. MRI is superior in demonstrating the interface of lesion and surrounding tissue without radiation exposure. Typically, conventional MRI findings of MPT include ill-defined border and infiltration into adjacent structure.2 However, it is reported that margin, internal texture and signal intensity are not optimal factors to discriminate BPL and MPT.3
Over the past decades, the roles of functional MRI, such as diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI, have been evaluated in head and neck lesions. DWI quantifies the diffusional mobility of water protons with apparent diffusion coefficient (ADC). DCE-MRI provides information related to the physiology of the microcirculation and vascularity. Functional MRI characteristics of MPT include a lower ADC value on DWI and lower washout on DCE-MRI than those of BPL.4 However, additional ability of functional MRI in differential diagnosis of parotid lesions is still controversial because overlap is detected between MPT and BPL. Larger studies combining morphological and functional findings of MRI are warranted. Therefore, we retrospectively reviewed conventional MRI, DWI and DCE-MRI images of parotid lesions. The purpose was to determine whether MPTs differ from BPLs in conventional and functional MRI characteristics and to identify the characteristics most useful for making a differential diagnosis in patients with parotid lesions.
METHODS AND MATERIALS
Patients
We retrospectively reviewed the MRI findings of patients with histopathologically confirmed primary parotid lesions between 1 January 2010 and 31 December 2014. We recorded the demographic and clinical characteristics, including sex, age, smoking status and alcohol use. “Ever drinkers” were defined as patients who had drunk at least one alcoholic beverage per week for at least 1 year. Patients who had smoked at least 100 cigarettes in their lifetime were defined as “ever smokers”.5 Patients were excluded in any of the following conditions: (1) a head and neck cancer was previously diagnosed; (2) surgical management or radiotherapy treatment in head and neck region was performed; or (3) the parotid lesion received biopsy, surgery, chemotherapy or radiotherapy. This study was approved by the institutional review board.
MRI acquisition
MRI examinations were performed on a 1.5-T imager (Signa® Excite; GE Medical Systems, Milwaukee, WI). The conventional MRI sequences included axial T1 weighted imaging (T1WI) [repetition time (TR)/echo time (TE), 400–600/10 ms], axial and coronal T2 weighted imaging (T2WI) (TR/TE, 3200/100 ms). Other parameters included matrix, 256 × 192 mm; field of view (FOV), 240 × 240 mm and thickness/gap, 5/1 mm. DWI was performed using a single-shot spin echo echoplanar imaging sequence (TR/TE, 2775/70 ms; matrix, 128 × 128 mm; FOV, 240 × 240 mm; thickness/gap, 5.0/0.5 mm; b, 0 and 800 s mm−2; x-, y-, and z-gradient directions). DCE-MRI was obtained after intravenous injection of gadopentetate dimeglumine (Magnevist®; Schering, Berlin, Germany) at a dosage of 0.1 mmol kg−1 of body weight (TR/TE, 4.8/2.2 ms; FOV, 240 × 240 mm; matrix, 256 × 192 mm; thickness/gap, 5.0/0 mm). Contrast-enhanced transverse and coronal T1 weighted images were also obtained. The main parameters were the same as those used pre-injection.
MRI interpretation
All MRI images were evaluated by consensus by two radiologists (YY and WT) with over 8 years' experience in interpretation of head and neck MRI. They were blinded to results from other imaging modalities and histopathological reports. When lesions were multiple, the largest lesion was selected for the study. Tumour size was defined as maximal dimension on transverse plane. On conventional MRI, each lesion was evaluated according to enhancement (enhanced or not enhanced), inner texture (homogeneous or heterogeneous based on pre-contrast T1WI and T2WI and post-contrast T1WI), margin [well-defined (more than two-thirds of the margin was sharply demarcated from the surrounding tissue) or ill-defined (less than one-third of the margin was sharply defined)6] and adjacent structure infiltration [extension into adjacent tissue (muscle, fat, bone or neurovascular structures) or no involvement of adjacent tissue]. Lymphadenopathy was defined as a cervical lymph node with minimal axial diameter >10 mm or with visualized necrosis.7
The DWI and DCE-MRI data were post-processed using the FuncTool software (GE Healthcare). Two radiologists (YY and WT) selected representative regions of interest (ROIs) by consensus. For ADC measurements, freehand ROI was drawn, taking care to exclude obvious necrotic or non-perfused areas by visual correlation with pre- and post-contrast T1 weighted images and to avoid the most peripheral portions because of partial volume effects of adjacent tissue. Three ROIs were manually placed within each lesion, and the averaged ADC value was obtained. For post-processing of DCE-MRI, we placed ROIs (approximately 25–50 mm2) over the lesion and time–intensity curve (TIC) for each ROI was generated. The TIC with the most prominent enhancement was selected as the representation for each lesion. The detail method was described in our studies.8,9 The TICs (axis co-ordinate, time; vertical, signal intensity) were categorized into three patterns: (1) Type A (persistent pattern): Tpeak >120 s; (2) Type B (plateau pattern): Tpeak ≤120 s, washout ratio (WR) ≤30%; (3) Type C (washout pattern): Tpeak ≤120 s, WR >30%; (4) Type D (flat pattern): flat, without prominent enhancing.10,11
Statistical analysis
Statistical analysis was carried out using STATA® v. 12.0 (StataCorp, College Station, TX). p < 0.05 was considered as statistically significant. Clinical and MRI characteristics were compared between MPT and BPL using the χ2 testing (the Fisher exact testing where appropriate) for categorical variables and t test for non-categorical data. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using logistic regression models with adjustment for possible confounding factors to determine the association between malignancy and each MRI characteristic. To find the optimal ADC value to distinguish MPT from BPL, we conducted receiver operator characteristic analysis. The sensitivity vs (1 − specificity) was plotted across the range of ADC values to generate ROC curve and the area under the ROC curve (AUC) was assessed. The cut-off ADC value was determined as the point that maximized the sum of sensitivity and specificity.
For the evaluation of combined MRI findings, MRI characteristics were scored as 0 for negative results and 1 for positive results as follows: enhancement (with enhancement, 1; without, 0), inner texture (heterogeneous, 1; homogeneous, 0), margin (ill-defined, 1; well-defined, 0), adjacent structure infiltration (with infiltration, 1; without, 0), lymphadenopathy (with cervical lymphadenopathy, 1; without, 0), ADC value (ADC ≤ cut-off value, 1; ADC > cut-off value, 0). The TIC pattern was scored as Type A or Type D, 0; Type B, 1; and Type C, 2. For each lesion, summed scores were respectively calculated for conventional MRI alone, conventional MRI with DWI and/or DCE-MRI. We trisected the patients into three groups in each combined set. The ability of summed scores in differentiating MPT from BPLs was assessed using logistic regression model and ROC analysis. The ROCs for conventional MRI alone, conventional MRI with DWI and/or DCE-MRI were generated and compared. The cut-off scores with the maximized sum of sensitivity and specificity were calculated.
RESULTS
Patients and clinical characteristics
A total of 207 patients [111 males and 96 females; age: 48.4 ± 17.0 years (mean ± standard deviation)] with pathologically confirmed primary parotid lesions were included, consisting of 156 benign and 51 malignant lesions. Of the malignant entities, the most prevalent tumours were lymphoepithelial carcinomas (n = 12), malignant mixed tumours (n = 10) and mucoepidermoid carcinomas (n = 6). The most prevalent BPLs were pleomorphic adenomas (n = 60), Warthin tumours (n = 38) and basal cell adenomas (n = 8). The clinical characteristics of patients are summarized in Table 1. There was no statistical difference in age, sex, smoking status or alcohol use between patients with MPT and BPL (p > 0.05).
Table 1.
Clinical characteristics of patients
| Characteristics | MPT (51), n (%) | BPL (156), n (%) | p-valuea |
|---|---|---|---|
| Mean age ± SD (years) | 45.0 ± 17.4 | 49.5 ± 16.8 | 0.104 |
| Sex |
1.000 | ||
| Male | 27 (52.9) | 84 (53.8) | |
| Female | 24 (47.1) | 72 (46.2) | |
| Smoking |
0.747 | ||
| Ever | 27 (52.9) | 77 (49.4) | |
| Never | 24 (47.1) | 79 (50.6) | |
| Alcohol |
0.631 | ||
| Ever | 26 (51.0) | 73 (46.8) | |
| Never | 25 (49.0) | 83 (53.2) | |
BPL, benign parotid lesion; MPT, malignant parotid tumour; SD, standard deviation.
p-values of χ2 test (the Fisher exact test was used where appropriate) for categorical variables and unpaired t test for non-categorical data.
MRI findings
Conventional MRI was available for all patients, and 129 and 154 of them also underwent DWI and DCE-MRI, respectively. All MPTs demonstrated enhancement after intravenous injection of contrast agent. The MRI characteristics of enhancement, margin, adjacent tissue infiltration, cervical lymphadenopathy, ADC value and TIC type were all significantly different between MPTs and BPLs (p < 0.05) (Table 2). The association between MRI characteristics and malignancy are summarized in Table 2. After adjusting for age, sex, smoking status, alcohol use and tumour size in logistic regression model, the lesions with adjacent tissue infiltration were approximately 15 times more likely to be malignant than those without adjacent structure involvement (OR, 15.1, 95% CI, 3.5–65.8). The lesions with ill-defined margin and cervical lymphadenopathy were 7–8 times more likely to be MPT than those with well-defined border and no lymphadenopathy (OR, 7.3, 95% CI, 3.4–15.5 for margin; OR, 8.8, 95% CI, 3.3–23.1 for lymphadenopathy).
Table 2.
Association between MRI characteristics and malignancy
| Characteristics | MPT (51), n (%) | BPL (156), n (%) | p-valuea | Adjusted ORb (95% CI) |
|---|---|---|---|---|
| Enhancement |
0.041 | |||
| No | 0 | 12 (7.7) | ||
| Yes | 51 (100.0) | 144 (92.3) | NA | |
| Inner texture |
0.657 | |||
| Homogeneous | 9 (3.1) | 23 (14.7) | 1.0 | |
| Heterogeneous | 42 (96.9) | 133 (85.3) | 0.8 (0.3–1.9) | |
| Margin |
<0.001 | |||
| Well-defined | 23 (45.1) | 133 (85.3) | 1.0 | |
| Ill-defined | 28 (54.9) | 23 (14.7) | 7.3 (3.4–15.5) | |
| Adjacent tissue extension |
<0.001 | |||
| No | 40 (78.4) | 153 (98.1) | 1.0 | |
| Yes | 11 (21.6) | 3 (1.9) | 15.1 (3.5–65.8) | |
| Lymphadenopathy |
<0.001 | |||
| No | 34 (66.7) | 147 (94.2) | 1.0 | |
| Yes | 17 (33.3) | 9 (5.8) | 8.8 (3.3–23.1) | |
| ADC (mm2 s−1)c |
<0.001 | |||
| >1.01 × 10−3 | 5(13.5) | 55 (59.8) | 1.0 | |
| ≤1.01 × 10−3 | 32 (86.5) | 37 (40.2) | 23.6 (6.3–88.5) | |
| TICc |
<0.001 | |||
| Type A | 7 (17.9) | 46 (40.0) | 1.0 | |
| Type B | 29 (74.4) | 22 (19.1) | 15.0 (4.5–49.6) | |
| Type C | 3 (7.7) | 34 (29.6) | 1.1 (0.2–5.3) | |
| Type D | 0 | 13 (11.3) | NA | |
ADC, apparent diffusion coefficient; BPL, benign parotid lesion; CI, confidence interval; MPT, malignant parotid tumour; NA, not available; OR, odds ratio; TIC, time–signal intensity curve.
p-values of χ2 test (the Fisher exact test was used where appropriate) for categorical variables and unpaired t-test for non-categorical data.
Adjusted for age, sex, smoking status, alcohol use and tumour size in a logistic regression model.
Diffusion-weighted imaging and dynamic contrast-enhanced MRI images were not available in 78 and 53 patients, respectively.
The mean ADC values of BPL (0.89 × 10−3 mm2 s−1) were significantly higher than those of the MPT (1.19 × 10−3 mm2 s−1) (p < 0.001). ROC was generated for ADC and the AUC was 0.72 ± 0.05 (mean ± standard error). The optimal differential performance was obtained at a cut-off ADC value of 1.01 × 10−3 mm2 s−1, which suggested the malignant diagnosis with ADC ≤1.01 × 10−3 mm2 s−1 and the benign diagnosis with ADC >1.01 × 10−3 mm2 s−1, with sensitivity and specificity of 86.49% and 59.78%, respectively. Lesions with ADC ≤1.01 × 10−3 mm2 s−1 were about 24 times more likely to be malignant than those with an ADC value >1.01 × 10−3 mm2 s−1 (OR, 23.6, 95% CI, 6.3–88.5). 13 BPLs and no MPTs presented flat TIC pattern. When compared with Type A and D, TIC of Type B was more associated with malignancy (OR, 15.0, 95% CI, 4.5–49.6). TIC of Type C did not predict MPT (Table 2). Figures 1 and 2 show two representative cases with conventional MRI, DWI and DCE-MRI results.
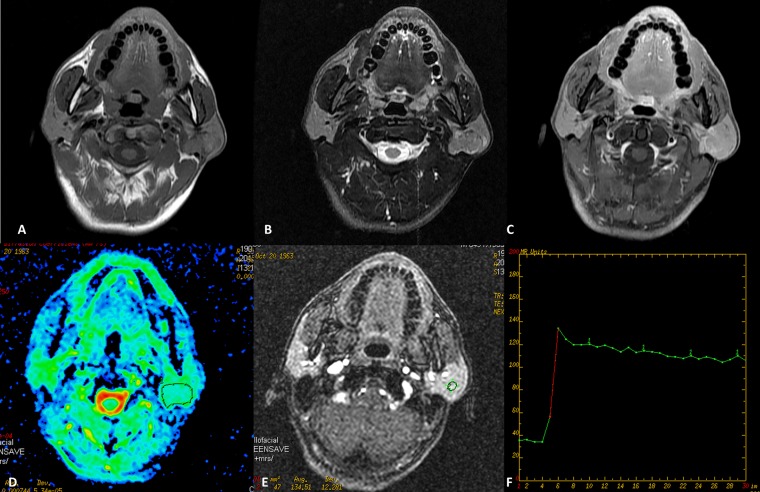
Figure 1.
Warthin tumour of the left parotid gland in a 49-year-old male. (a) Axial T1 weighted imaging (b) T2 weighted imaging and (c) contrast-enhanced T1 weighted imaging show a well-defined mass without infiltration into adjacent structure. The round cursors mark the regions of interest selected (d) for the measurement of the apparent diffusion coefficient (ADC) value in ADC map and (e) for signal intensity measurement at dynamic MRI. The ADC value is 0.752 × 10−3 mm2 s−1, which is lower than the cut-off value. (f) Time–signal intensity curve shows a plateau enhancement pattern (Type B). TE, echo time; TR, repetition time.
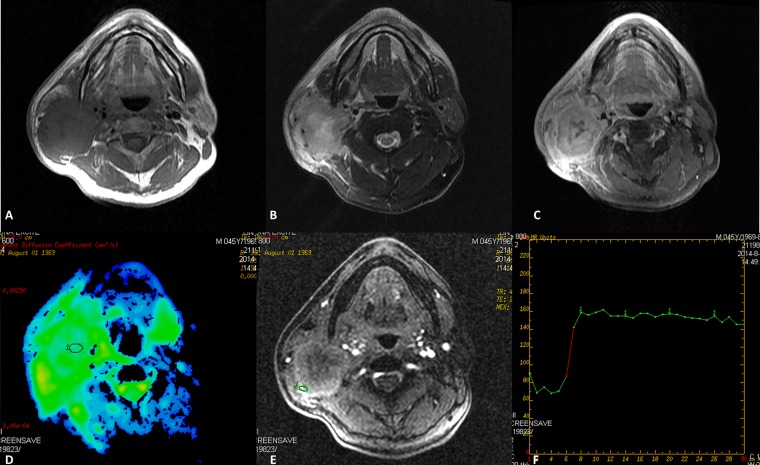
Figure 2.
Mucoepidermoid carcinomas of the right parotid gland in a 45-year-old male. (a) Axial T1 weighted imaging (b) T2 weighted imaging and (c) contrast-enhanced T1 weighted imaging show an ill-defined heterogeneously enhanced mass with infiltration into adjacent structure. The round cursors mark the regions of interest selected (d) for the measurement of the apparent diffusion coefficient (ADC) value in ADC map and (e) for signal intensity measurement at dynamic MRI. The ADC value is 0.980 × 10−3 mm2 s−1, which is lower than the cut-off value. (f) Time–signal intensity curve shows a plateau enhancement pattern (Type B). TE, echo time; TR, repetition time.
Combined MRI characteristics
Table 3 shows the association between malignancy and summed MRI scores. Patients were respectively divided into three groups according to the summed scores of conventional MRI: (1) score < 2, (2) 2 ≤ score < 4 and (3) score ≥ 4; conventional MRI and DWI/DCE-MRI: (1) score < 3, (2) 3 ≤ score < 5 and (3) score ≥ 5; conventional MRI and DWI and DCE-MRI: (1) score < 4, (2) 4 ≤ score < 6 and (3) score ≥ 6. Lesions with higher score were more likely to be malignant (p < 0.05). The most prominent association was found between malignancy and combination of conventional MRI and DWI. Compared with lesions with score <3, lesions with score ≥5 in combination of conventional MRI and DWI were almost 90 times more likely to be MPT (OR, 86.5, 95% CI, 9.1–817.3).
Table 3.
Association of combined MRI characteristics with malignancy
| Combined characteristics | MPT (51), n (%) | BPL (156), n (%) | Adjusted ORa (95% CI) |
|---|---|---|---|
| Conventional MRI | |||
| Score < 2 | 5 (9.8) | 25 (16.0) | 1.0 |
| 2 ≤ score < 4 | 27 (52.9) | 126 (80.8) | 1.0 (0.4–3.0) |
| Score ≥ 4 | 19 (37.3) | 5 (3.2) | 21.9 (4.9–97.7) |
| Conventional + DWIb | |||
| Score < 3 | 8 (21.6) | 53 (57.6) | 1.0 |
| 3 ≤ score < 5 | 16 (43.2) | 38 (41.3) | 3.4 (1.3–9.5) |
| Score ≥ 5 | 13 (35.1) | 1 (1.1) | 86.5 (9.1–817.3) |
| Conventional + DCE-MRIb | |||
| Score < 3 | 7 (17.9) | 50 (43.5) | 1.0 |
| 3 ≤ score < 5 | 19 (48.7) | 61 (53.0) | 3.2 (1.1–8.9) |
| Score ≥ 5 | 13 (33.3) | 4 (3.5) | 35.5 (7.8–161.1) |
| Conventional + DWI + DCE-MRIb | |||
| Score < 4 | 7 (20.6) | 53 (58.9) | 1.0 |
| 4 ≤ score < 6 | 16 (47.1) | 35 (38.9) | 4.9 (1.6–14.7) |
| Score ≥ 6 | 11 (32.3) | 2 (2.2) | 49.7 (8.1–304.3) |
BPL, benign parotid lesion; CI, confidence interval; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging; MPT, malignant parotid tumour; OR, odds ratio.
Adjusted for age, sex, smoking status, alcohol use and tumour size in a logistic regression model.
DWI, DCE-MRI and both were available in 37, 39 and 34 patients with MPT and 92, 115 and 90 patients with BPL, respectively. Conventional MRI was available in all included patients.
To define the diagnostic value for combined MRI characteristics to distinguish between malignant and benign lesions, ROCs were generated. The optimal differential performance was obtained at a cut-off score of 3, 4, 5 and 6 for conventional MRI only, conventional MRI and DWI, conventional MRI and DCE-MRI and conventional MRI and DWI and DCE-MRI respectively, which suggested the malignant diagnosis with score ≥cut-off score. Significant difference was detected in ROC after adding DWI to conventional MRI (p = 0.003), generating sensitivity and specificity of 54.05% and 91.30%, respectively. No statistical difference was found after adding DCE-MRI to conventional MRI or to the combination of conventional MRI and DWI (p = 0.359 and 0.856, respectively). The results of ROC analysis and corresponding differential performance are presented in Table 4.
Table 4.
Differential performance of combined MRI scores
| Combined characteristics | AUC (SE) | Pa | Pa | Sensitivityb (%) | Specificityb (%) |
|---|---|---|---|---|---|
| Conventional MRI | 0.72 (0.04) | Ref. | 56.86 | 81.41 | |
| Conventional + DWIc | 0.78 (0.05) | 0.003 | Ref. | 54.05 | 91.30 |
| Conventional + DCE-MRIc | 0.70 (0.05) | 0.359 | 33.33 | 96.52 | |
| Conventional + DWIc + DCE-MRIc | 0.76 (0.05) | 0.043 | 0.856 | 32.35 | 97.7 |
AUC, area under receiver operator characteristic curve; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging; Ref., reference; ROC, receiver operator characteristic; SE, standard error.
Comparison of AUC.
The values were generated from the ROC which maximized the sum of sensitivity and specificity.
DWI, DCE-MRI and both were available in 37, 39 and 34 patients with malignant parotid tumour and 92, 115 and 90 patients with benign parotid lesion, respectively. Conventional MRI was available in all included patients.
DISCUSSION
We analysed clinical and MRI characteristics from 207 patients with pathologically confirmed primary parotid lesions (156 benign and 51 malignant). The percentage of malignant tumours is consistent with previous reports (20–30%).12,13 No statistical difference was found in age, sex, smoking status or alcohol use between patients with MPT and those with BPL. Warthin tumour typically shows male predilection and is related to smoking and ionizing radiation.14 High percentage of males and smokers in patients with Warthin tumour was also noticed in our study. In a total of 38 patients with Warthin tumour, only 1 patient was female and 4 patients were never smokers.
MPTs typically show aggressive manner of ill-defined and infiltrative border. The surrounding soft tissue, subcutaneous tissue and skin may be infiltrated.1,3,15 In the present study, the morphological MRI characteristics of enhancement, margin, adjacent tissue infiltration and cervical lymphadenopathy were all significantly different between MPTs and BPLs. After adjusting for age, sex, smoking status, alcohol use and tumour size, the lesion with ill-defined margin, adjacent tissue infiltration and cervical lymphadenopathy was 7–15 times more likely to be malignant than those without these findings. One exception was noticed in the inner texture, which showed no significant association with malignancy. Teresi et al16 also reported that tumour homogeneity was not a useful criterion for distinguishing benign from MPTs. Although benign lesion tends to have relatively homogeneous signal intensity, haemorrhage and calcification may result in a heterogeneous appearance simulating malignancy.1 However, the visual assessment employed in the present study might obscure the results. Computer-assisted technique such as MaZda, which depicts the grey level distribution of the pixels of digital images, has been applied in evaluating texture of parotid lesions and proved different texture between benign and malignant lesions, as well as between pleomorphic adenomas and Warthin tumours.17
Although lesions with score ≥4 in conventional MRI were approximately 22 times more likely to be MPT than those with score <2, the sensitivity and specificity (56.86% and 81.41%, respectively) generated from ROC of conventional MRI were far from promising. DWI and DCE-MRI are functional MRI techniques independent of morphological information and are used to characterize lesions that are indefinite on conventional MRI. In malignant tumours, water molecule diffusion is restricted resulting in low ADC values compared with benign lesions.4,11,18,19 However, controversy exists in clinical application of DWI in differential diagnosis of parotid lesions. Eida et al4 performed DWI in 31 patients with salivary gland tumours resulting in 97% accuracy, 100% positive-predictive value and 96% negative-predictive value. Celebi et al20 revealed that an ADC value of 1.165 optimizing the sensitivity and specificity (63.3% and 71.9%, respectively) of DWI for distinguishing the parotid malignancy. Fruehwald-Pallamar et al17 reported an AUC of only 0.584 for ADC to discriminate malignant and benign tumours. Matsushima et al and Habermann et al19,21 concluded that ADC value alone is not sufficient to differentiate benign from MPTs. Yerli et al22 consecutively included 25 patients with parotid masses and found that diagnostic accuracy did not increase by adding DWI to conventional MRI. In the present study, we found significantly lower ADC values for MPTs than those for BPLs. The optimal differential performance was obtained at a cut-off ADC value of 1.01 × 10−3 mm2 s−1, with sensitivity of 86.49% and specificity of 59.78%. Statistically, better discriminative performance was achieved after adding DWI to conventional MRI; however, the sensitivity and specificity (54.05% and 91.30%, respectively) were still not satisfactory.
DCE-MRI monitors the entrance of diffusible contrast agents into the tumour tissue over time. On dynamic MRI, malignant tumours are supposed to typically show rapid enhancement and rapid or slow washout.23,24 A persistent or flat TIC pattern on DCE-MRI indicates benign disease. Yabuuchi et al10 examined 22 benign and 11 malignant salivary gland tumours and concluded that gadolinium-enhanced dynamic MRI is useful for differentiating benign from malignant salivary gland tumours. High sensitivity (91%) and specificity (91%) in the differentiation of benign and malignant tumours was achieved at a threshold Tpeak of 120 s and a WR of 30%. Another study was conducted by Lam et al25 for 98 salivary gland tumours (74 benign and 24 malignant). Tpeak <150 s with WR <30% comprised the optimal diagnostic criterion and provided a sensitivity of 79% and specificity of 95%. In the present study, however, no significant additional value was detected by adding DCE-MRI to conventional MRI or to the combination of conventional MRI and DWI. The AUC of DCE-MRI alone was only 0.57 for discriminating MPT from BPL.
Previous studies were conducted to evaluate the functional MRI finding of specific pathological entities as well. Significant differences were found in the mean ADC values between Warthin tumours and pleomorphic adenomas, as well as between Warthin tumours and benign lesions.17 Motoori et al26 reported higher ADC values in pleomorphic adenomas than malignant tumours. An ADC cut-off value of 1.315 × 10−3 mm2 s−1 had sensitivity and specificity of 82.1% and 81.2%, respectively, for distinguishing between pleomorphic adenomas and malignant tumours.20 Owing to the lymphoid accumulation of Warthin tumour resembling lymphoma, the ADC of Warthin tumour seems to be even smaller than some of the carcinomas, showing an overlap with examined malignant lesions.19 Most pleomorphic adenoma shows persistent TIC pattern, while Warthin tumour shows rapid uptake and washout pattern.23,27 Most Warthin tumours in the present study exhibited a washout TIC pattern (26/35, 74.2%), and the mean ADC was 0.91 × 10−3 mm2 s−1, significantly lower than other BPLs. Thus, for parotid lesions with washout TIC pattern and low ADC, Warthin tumour should be differentiated. In addition, 3 cases of oncocytoma (3/7, 42.9%) and 3 cases of basal cell adenoma (3/3, 100%) showed washout TIC pattern. Larger study is needed to validate the finding.
Our study had some limitations. First is related to the reproducibility of ADC and TIC. The cut-off ADC value derived may not be transferable to other institutions owing to interobserver variation and the equipment and methodology used. Selection of b-value and localization of ROIs would affect the consistent acquisition of ADC values and TIC patterns, although in order to ensure the representative and reproductive of results, we have selected the mean ADC value of three ROIs (taking care to exclude obvious necrotic or non-perfused areas and to avoid the most peripheral portions) and the TIC with the most prominent enhancement. Second, normal gland measurements were not included in our study since we aimed to compare malignant with benign lesions, while parotid fat content in healthy adults is related with age and sex,28 which would influence parotid ADC measurements. To rule out possible influence, we adjusted confounding factors including age and sex in logistic regression model. Further studies on comparing normal-side gland, benign and malignant lesions can be performed. Third, we evaluated the most clinical available MRI techniques, while other functional imaging methods such as arterial spin labelling and MR elastography have also be adopted to assess parotid masses. Kato et al29 included 10 pleomorphic adenomas, 12 Warthin tumours and 9 malignant tumours of the parotid glands and reported the sensitivity and specificity of 91.7% and 94.7%, respectively, for diagnosis of Warthin tumours with arterial spin labelling. Yeung et al30 implemented a new MR elastographic driver for the mechanical assessment of tissues in the head and neck. Furthermore, we focused on the application of conventional and functional MRI in parotid lesions, without comparison of ultrasound or CT results, which are commonly employed for initial assessment and biopsy. Finally, although we included a relatively larger sample of patients, not every examined tumour entity was available in a satisfying number of patients. Data from larger studies are required to define the applications of DWI, DCE-MRI and other functional techniques in specific pathological entities.
CONCLUSION
The results of our study show that conventional MRI, DWI and DCE-MRI are associated with malignancy of parotid gland. The diagnostic value of MRI would increase when DWI is applied in combination with morphological analyses. However, owing to an overlap between benign and malignant lesions, optimal diagnoses could not be addressed on combination of conventional MRI, DWI and DCE-MRI, and probably other MRI techniques seem to be warranted. Data from larger studies are required to define the applications of functional MRI in specific pathological entities.
FUNDING
This work was supported by grants from National Natural Science Foundation of China (81402461, 81471709); Subject Chief Scientist of Shanghai, Science and Technology Commission of Shanghai Municipality (13XD1402400).
Contributor Information
Ying Yuan, Email: yuany83@163.com.
Weiqing Tang, Email: manu9radiology1@163.com.
REFERENCES
- 1.Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol 2008; 66: 419–36. doi: 10.1016/j.ejrad.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 2.Freling NJ, Molenaar WM, Vermey A, Mooyaart EL, Panders AK, Annyas AA, et al. Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiology 1992; 185: 691–6. doi: 10.1148/radiology.185.3.1438746 [DOI] [PubMed] [Google Scholar]
- 3.Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H. MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 2011; 32: 1202–7. doi: 10.3174/ajnr.A2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eida S, Sumi M, Sakihama N, Takahashi H, Nakamura T. Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJNR Am J Neuroradiol 2007; 28: 116–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Sturgis EM, Zheng H, Song X, Wei P, Jin L, et al. Genetic variants in TNF-α promoter are predictors of recurrence in patients with squamous cell carcinoma of oropharynx after definitive radiotherapy. Int J Cancer 2014; 134: 1907–15. doi: 10.1002/ijc.28512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ban X, Wu J, Mo Y, Yang Q, Liu X, Xie C, et al. Lymphoepithelial carcinoma of the salivary gland: morphologic patterns and imaging features on CT and MRI. AJNR Am J Neuroradiol 2014; 35: 1813–19. doi: 10.3174/ajnr.A3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990; 177: 379–84. doi: 10.1148/radiology.177.2.2217772 [DOI] [PubMed] [Google Scholar]
- 8.Yuan Y, Yue XH, Tao XF. The diagnostic value of dynamic contrast-enhanced MRI for thyroid tumors. Eur J Radiol 2012; 81: 3313–18. doi: 10.1016/j.ejrad.2012.04.029 [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Kuai XP, Chen XS, Tao XF. Assessment of dynamic contrast-enhanced magnetic resonance imaging in the differentiation of malignant from benign orbital masses. Eur J Radiol 2013; 82: 1506–11. doi: 10.1016/j.ejrad.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 2003; 226: 345–54. doi: 10.1148/radiol.2262011486 [DOI] [PubMed] [Google Scholar]
- 11.Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, et al. Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology. 2008; 249: 909–16. doi: 10.1148/radiol.2493072045 [DOI] [PubMed] [Google Scholar]
- 12.Auclair PL, Ellis GL, Gnepp DR, Wening BM, Janney CG. Salivary gland neoplasms: general considerations. In: Eliis GL, Audair PL, Gnepp DR, eds. Surgical pathology of the salivary glands. Philadelphia, PA: WB Saunders Co.; 1991. pp. 135–64. [Google Scholar]
- 13.Rankow RM, Polayes IM. Surgical treatment of salivary gland tumours. In: Rankow RM, Polayes IM, eds. Disease of the salivary glands. Philadelphia, PA: WB Saunders Co.; 1976. pp. 239–83. [Google Scholar]
- 14.Som PM, Brandwein MS. Salivary glands: anatomy and pathology. In: Som PM, Curtin DC, eds. Head and neck imaging. 4th edn. St. Louis, MO: Mosby; 2003. pp. 2005–133. [Google Scholar]
- 15.Som PM, Biller HF. High-grade malignancies of the parotid gland: identification with MR imaging. Radiology 1989; 173: 823–6. doi: 10.1148/radiology.173.3.2813793 [DOI] [PubMed] [Google Scholar]
- 16.Teresi LM, Lufkin RB, Wortham DG, Abemayor E, Hanafee WN. Parotid masses: MR imaging. Radiology 1987; 163: 405–9. doi: 10.1148/radiology.163.2.3562818 [DOI] [PubMed] [Google Scholar]
- 17.Fruehwald-Pallamar J, Czerny C, Holzer-Fruehwald L, Nemec SF, Mueller-Mang C, Weber M, et al. Texture-based and diffusion-weighted discrimination of parotid gland lesions on MR images at 3.0 Tesla. NMR Biomed 2013; 26: 1372–9. doi: 10.1002/nbm.2962 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology 2001; 220: 621–30. doi: 10.1148/radiol.2202010063 [DOI] [PubMed] [Google Scholar]
- 19.Habermann CR, Arndt C, Graessner J, Diestel L, Petersen KU, Reitmeier F, et al. Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol 2009; 30: 591–6. doi: 10.3174/ajnr.A1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celebi I, Mahmutoglu AS, Ucgul A, Ulusay SM, Basak T, Basak M. Quantitative diffusion-weighted magnetic resonance imaging in the evaluation of parotid gland masses: a study with histopathological correlation. Clin Imaging 2013; 37: 232–8. doi: 10.1016/j.clinimag.2012.04.025 [DOI] [PubMed] [Google Scholar]
- 21.Matsushima N, Maeda M, Takamura M, Takeda K. Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol 2007; 34: 183–9. doi: 10.1016/j.neurad.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 22.Yerli H, Aydin E, Haberal N, Harman A, Kaskati T, Alibek S. Diagnosing common parotid tumours with magnetic resonance imaging including diffusion-weighted imaging vs fine-needle aspiration cytology: a comparative study. DentomaxillofacRadiol 2010; 39: 349–55. doi: 10.1259/dmfr/15047967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takashima S, Noguchi Y, Okumura T, Aruga H, Kobayashi T. Dynamic MR imaging in the head and neck. Radiology 1993; 189: 813–21. doi: 10.1148/radiology.189.3.8234709 [DOI] [PubMed] [Google Scholar]
- 24.Eida S, Sumi M, Nakamura T. Multiparametric magnetic resonance imaging for the differentiation between benign and malignant salivary gland tumors. J Magn Reson Imaging 2010; 31: 673–9. doi: 10.1002/jmri.22091 [DOI] [PubMed] [Google Scholar]
- 25.Motoori K, Yamamoto S, Ueda T, Nakano K, Muto T, Nagai Y, et al. Inter- and intratumoral variability in magnetic resonance imaging of pleomorphic adenoma: an attempt to interpret the variable magnetic resonance findings. J Comput Assist Tomogr 2004; 28: 233–46. doi: 10.1097/00004728-200403000-00014 [DOI] [PubMed] [Google Scholar]
- 26.Lam PD, Kuribayashi A, Imaizumi A, Sakamoto J, Sumi Y, Yoshino N, et al. Differentiating benign and malignant salivary gland tumours: diagnostic criteria and the accuracy of dynamic contrast-enhanced MRI with high temporal resolution. Br J Radiol 2015; 88: 20140685. doi: 10.1259/bjr.20140685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechner Goyault J, Riehm S, Neuville A, Gentine A, Veillon F. Interest of diffusion-weighted and gadolinium-enhanced dynamic MR sequences for the diagnosis of parotid gland tumors. J Neuroradiol 2011; 38: 77–89. doi: 10.1016/j.neurad.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 28.Chang HC, Juan CJ, Chiu HC, Cheng CC, Chiu SC, Liu YJ, et al. Effects of gender, age, and body mass index on fat contents and apparent diffusion coefficients in healthy parotid glands: an MRI evaluation. Eur Radiol 2014; 24: 2069–76. doi: 10.1007/s00330-014-3265-z [DOI] [PubMed] [Google Scholar]
- 29.Kato H, Kanematsu M, Watanabe H, Kajita K, Mizuta K, Aoki M, et al. Perfusion imaging of parotid gland tumours: usefulness of arterial spin labeling for differentiating Warthin's tumours. Eur Radiol 2015; 25: 3247–54. doi: 10.1007/s00330-015-3755-7 [DOI] [PubMed] [Google Scholar]
- 30.Yeung DK, Bhatia KS, Lee YY, King AD, Garteiser P, Sinkus R, et al. MR elastography of the head and neck: driver design and initial results. Magn Reson Imaging 2013; 31: 624–9. doi: 10.1016/j.mri.2012.09.008 [DOI] [PubMed] [Google Scholar]