Abstract
Objective:
Non-invasive biomarkers to monitor cerebral function in treated human immunodeficiency virus (HIV) disease are required. Cerebral metabolite ratios (CMRs) measured by proton-MR spectroscopy (1H-MRS) are a potential biomarker. Here, we compare two post-processing software packages to quantify CMRs.
Methods:
Cerebral 1H-MRS data from 11 HIV-positive subjects before and after antiretroviral therapy intensification with maraviroc were quantified using a java-based version of the MR user interface package (jMRUI) and the totally automatic robust quantitation in nuclear MR (TARQUIN). 1H-MRS data included N-acetylaspartate (NAA), creatine (Cr), choline (Cho) and myo-inositol (mI) from three cerebral locations. Differences in quantification and clinical associations of CMRs measured by the two packages were evaluated.
Results:
Mean CMRs were generally lower when measured by TARQUIN than by jMRUI (NAA/Cr, Cho/Cr, mI/Cr ratios of 1.78, 0.83, 0.81 for jMRUI, and 1.27, 0.25, 0.81 for TARQUIN). Longitudinal changes were observed in CMRs in the basal ganglia voxel although these changes were not statistically significant [+7.1% (p = 0.18), +0.0% (p = 0.91) and −6.6% (p = 0.61) and +14.8% (p = 0.18), +17.9% (p = 0.07) and +34.8% (p = 0.17) for NAA/Cr, Cho/Cr and mI/Cr ratios measured by TARQUIN and jMRUI, respectively]. Plasma maraviroc concentration was associated with a decrease in mI/Cr ratio measured via TARQUIN (p = 0.049).
Conclusion:
Although CMRs differed when quantified by jMRUI vs TARQUIN, these differences were consistently observed across three cerebral locations, and clinical associations were evident by both methods.
Advances in knowledge:
TARQUIN and jMRUI are viable options to use in the post-processing of cerebral MRS data acquired in HIV disease.
INTRODUCTION
30 years since its emergence, human immunodeficiency virus-1 (HIV-1) infection remains a global healthcare issue affecting 35 million individuals worldwide, with 2.3 million new infections reported in 2012.1 Before the advent of combination antiretroviral therapy (cART), HIV-associated dementia affected between 5% and 20% of patients, with an annual incidence of new diagnosis of 7% among individuals with acquired immune deficiency syndrome.2 The widespread use of cART has seen significant reductions in HIV-associated morbidity and mortality, with patients now benefiting from longer and healthier lives.3 Although incidence rates for HIV-associated dementia have declined, the prevalence of mild to moderate forms of cognitive dysfunction remains high,4 with neuropsychological deficits reported in 15–50% of patients despite long-term cART and suppression of viraemia.5
Rapid, sensitive and reliable screening tool for the presence of HIV-associated cognitive disorders are needed. Specific bedside tools such as the HIV dementia scale and the International HIV Dementia Scale have been shown to lack diagnostic effectiveness owing to inconsistencies in language comprehension and confounding clinical parameters.6 Furthermore, potential plasma biomarkers have shown little specificity in detecting HIV-associated cognitive disorders.7 Although cerebrospinal fluid (CSF) markers are considered a more valuable clinical tool,8 the requirement for CSF examination is challenging, and less invasive methods are preferable. Measurement of cerebral metabolites as biomarkers, via proton-MR spectroscopy (1H-MRS), is one attractive approach for the assessment of cerebral function in HIV disease.
1H-MRS is a non-invasive method of analysing metabolite concentrations in targeted anatomical locations, and abnormalities have been correlated with clinical disease status in large case series which have adequate power to detect such associations. N-acetylaspartate (NAA), myo-inositol (mI) and choline (Cho)-containing compounds are measured to reflect neuronal damage and inflammation that are reported to be altered in HIV infection.9 A reduction in the neuronal marker NAA has been correlated with the presence of dementia in HIV-infected individuals and with the clinical severity of this dementia.10 Elevated levels of both mI and Cho, representing neuroinflammation, have been reported in neurologically symptomatic patients with HIV infection.11
The scope of 1H-MRS in clinical medicine is expanding,12 but the use of this method requires accurate post-acquisition quantification of the 1H-MRS spectra.13 Prior work has been undertaken to compare the sensitivity of quantification methods over a range of diseased states,14,15 but to our knowledge, no comparisons have been made of quantification models in the analysis of cerebral metabolites in HIV disease. Although such comparisons should not differ in HIV disease compared with other disease states, data on the utility of these software packages within the specific disease area provides clinical researchers with the confidence to use such modalities within a clinical research setting. The aim of this study is to directly compare two 1H-MRS analysis models and to identify any differences present in cerebral metabolite ratios (CMRs) in HIV-positive individuals.
METHODS AND MATERIALS
Subject selection
Eligible individuals were neurologically asymptomatic adults with chronic HIV-infection, receiving tenofovir/emtricitabine/lopinavir/ritonavir as antiretroviral therapy with plasma HIV RNA levels of <50 copies/ml (Bayer Quantiplex Assay™; Bayer Diagnostics, Walpole, MA) for at least 3 months prior to study entry. Cerebral 1H-MRS imaging was undertaken prior to antiretroviral intensification with maraviroc (baseline scan) and after 14 days of antiretroviral intensification with maraviroc (follow-up scan). Results from this study have previously been published.16 Here, we report differences in CMRs within this antiretroviral intensification study measured by two methods. Local human ethical approval was obtained (Imperial College Healthcare National Health Service Trust local ethics approval), and all subjects provided written informed consent prior to undertaking any study procedures.
Cerebral proton-MR spectroscopy
1H-MRS imaging was performed on a Phillips Achieva™ 1.5-T scanner (Phillips NV, Best, Netherlands) at Hammersmith Hospital, London, UK. To ensure accurate positioning of the voxels, T1 weighted MR images of the brain were performed in the sagittal, coronal and axial planes, and T2 weighted images were obtained to exclude any visible cerebral pathology. An MR spectroscopy (MRS) operations manual was used to ensure all MRS examinations were undertaken using identical operational settings. An experienced radiographer selected voxel locations using a standardized procedure ensuring consistent voxel positioning across subjects. Voxels were placed on areas of the brain which appeared anatomically normal on T1 and T2 images. 1H-MRS data were acquired by single-voxel examination in three locations: mid-frontal grey matter, right frontal white matter (FWM) and the right basal ganglia (RBG). Voxel size was approximately 5.5 cm3 (1.75 × 1.75 × 1.75 cm). Spectra were obtained using a double spin-echo point-resolved spectroscopy sequence with the following settings: echo time, 36 ms; repetition time, 3000 ms; 2048 data points; spectral width, 2500 Hz; and 128 data acquisitions. Cerebral metabolites NAA, mI and Cho were measured and expressed as ratios to creatine (Cr). All spectra were inspected visually by an experienced physicist to ensure quality of acquisition, and in cases with low signal-to-noise ratio, acquisition was repeated.
Cerebrospinal fluid analysis
Lumbar puncture examination was performed using aseptic technique. Maraviroc concentrations were measured in CSF samples at the University of Liverpool, UK, using high-performance liquid chromatography–tandem mass spectrometry.17
Analysis models
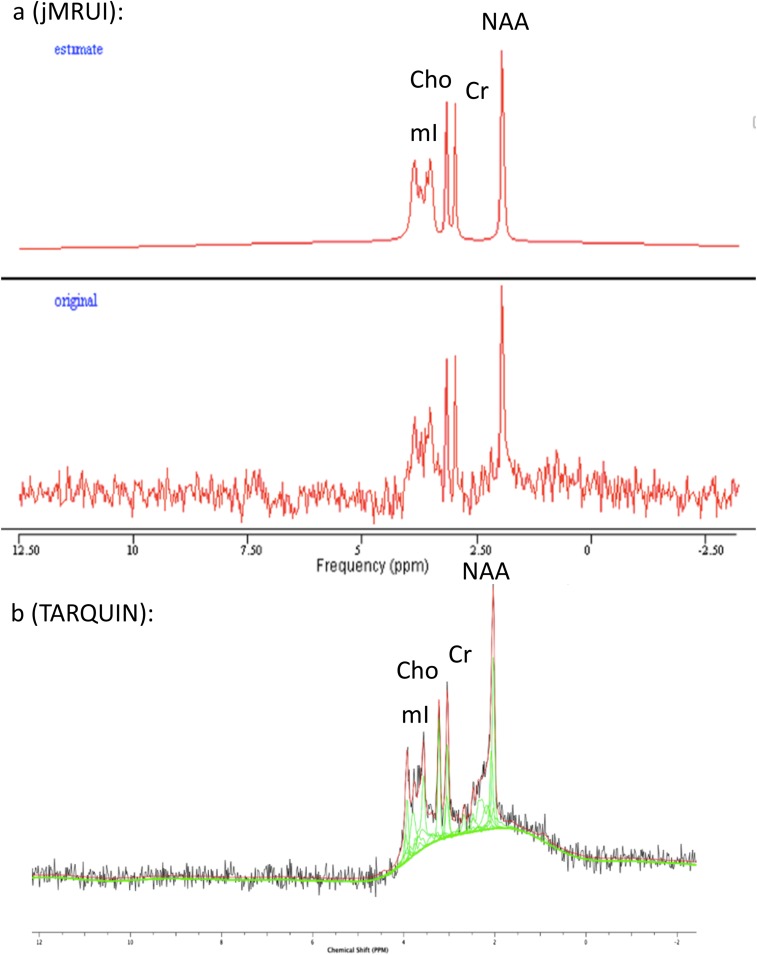
MR spectra were analysed and quantified using two programs: a java-based version of the MR user interface package (jMRUI v. 5.0)18 and the totally automatic robust quantitation in nuclear MR (TARQUIN) algorithm.19 These were chosen because jMRUI has been used previously by our group and others to quantify CMRs in HIV disease. TARQUIN is a new algorithm, that to our knowledge has not been used to quantify CMRs in HIV disease. It offers several potential advantages over jMRUI and LCmodel, another widely used software package, namely it is free to use and modify under the General Public License, is available across computer platforms and is designed to give rapid and automated quantitation of CMRs. jMRUI enables time domain analysis of in vivo MRS data in two stages. Pre-processing requires user interaction largely to suppress residual water molecules using the HLSVD/HLSVDPro filters20 and the use of the Cadzow function to filter the signal.18 This manual pre-processing step may influence the results of model fitting and thus affect the accuracy of signal quantification.13 Quantitation of MR spectra utilizes the Advanced Method for Accurate, Robust and Efficient Spectral Fitting (AMARES) algorithm,20 requiring the input of prior knowledge to estimate peak frequency and decay constants.21 The same prior knowledge of the estimated peaks was inputted for all analyses in this study with peaks set at the following positions; 2.0 parts per million (ppm) and 3.9 line width [LW (Hz)] for NAA, 3.01 ppm and 4.9 LW for Cr, 3.2 ppm and 4.9 LW for Cho and 3.54 ppm and 4.9 LW for mI. An example spectra being read by jMRUI is provided in Figure 1a.
Figure 1.
Examples of MRS spectra. (a) Original signal (bottom) and jMRUI model (top). (b) Original signal (black), TARQUIN model (red) with individual peaks (green). Cho, choline; Cr, creatine, jMRUI, java-based version of the MR user interface; NAA, N-acetylaspartate; mI, myo-inositol; ppm, parts per million; TARQUIN, totally automatic robust quantitation in nuclear MR. For colour image see online.
TARQUIN (v. 4.3.2) is a novel time domain–fitting algorithm used to quantify 1H-MRS data.19 A least squares approach similar to the AQSES algorithm22 is used to estimate signal amplitudes, yet TARQUIN imposes a new method of soft constraints on the projection to reduce errors associated with overfitting.19 Baseline interference is reduced by point truncation and HSVD water removal23 in a fully automated process, and thus user variability is eliminated. Point truncation eliminates the very early points of the free induction decay that contains very broad signals that are difficult to model and the last few that contain only noise. An example spectra being read by TARQUIN can be seen in Figure 1b. This algorithm has been found to compare favourably with LCmodel using both clinical and simulated data.19 Cerebral metabolites NAA, mI and Cho were quantified by both methods and expressed as ratios to cerebral Cr.
Statistical analysis
All statistical analyses were performed using SPSS® v. 21.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). CMRs were evaluated using paired sample t-tests to compare jMRUI with TARQUIN. Absolute changes in CMRs between baseline and Day 14 were evaluated using paired sample t-tests. Linear regression analyses were used to assess the correlation between changes in CMRs over the study period with pharmacokinetic parameters measured in this study (maraviroc plasma and CSF concentration) with no correction for multiple comparisons as CMRs are correlated to some degree. p-values <0.05 were considered statistically significant.
RESULTS
1H-MRS quantification comparing jMRUI and TARQUIN analysis models
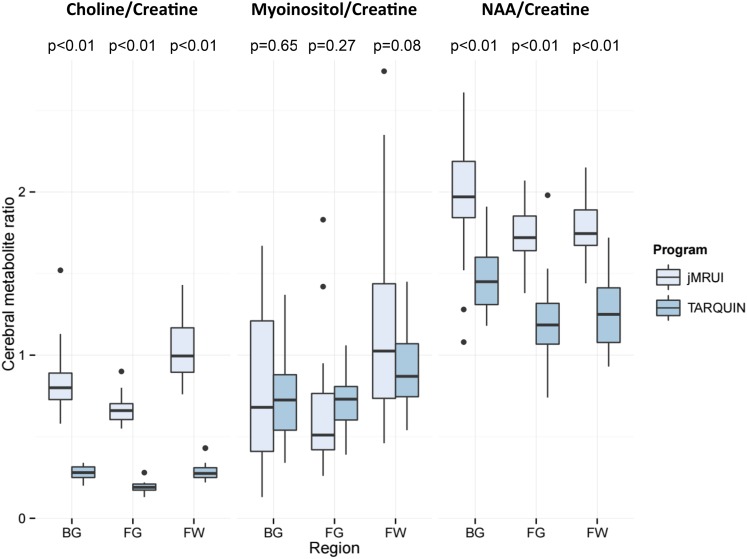
A comparison of all CMRs from both study time points by anatomical region, quantified by the two analysis models is shown in Figure 2. CMRs were significantly higher for NAA/Cr and Cho/Cr when quantified via jMRUI across all three voxels. Coefficients of variation (CoVs) and 95% confidence intervals for each CMR by voxel and algorithm are shown in Table 1. In general, these were higher for NAA/Cr but lower for Cho/Cr and mI/Cr quantified by TARQUIN. Furthermore, CoVs were generally consistent across voxels for NAA/Cr and Cho/Cr quantified by both algorithms. However, mI/Cr showed considerably more variation than the other two CMRs, particularly when quantified using jMRUI. Pearson's correlation coefficients for CMR values are shown in Table 2.
Figure 2.
Box plots of cerebral metabolite ratios quantified using java-based version of the MR user interface (jMRUI) and totally automatic robust quantitation in nuclear MR (TARQUIN) by anatomical region (includes Day 0 and Day 14 data). Box plots show median values (solid horizontal lines), interquartile range (IQR, box), 1.5 × IQR (vertical lines) and outlier values (solid circles). p-values computed using the paired t-test. BG, basal ganglia; FG, frontal grey; FW, frontal white; NAA, N-acetylaspartate.
Table 1.
Coefficients of variation (95% confidence intervals) for each cerebral metabolite ratio by voxel and algorithm
| Voxel location | Algorithm | Cerebral metabolite ratio |
||
|---|---|---|---|---|
| NAA/Cr | Cho/Cr | mI/Cr | ||
| Frontal grey matter | TARQUIN | 25.5 (17.5–47.2) | 16.2 (11.3–29.1) | 24.1 (16.6–44.4) |
| jMRUI | 10.8 (7.5–20.0) | 14.9 (10.2–27.8) | 52.4 (33.8–121) | |
| Frontal white matter | TARQUIN | 21.2 (14.7–38.7) | 13.5 (9.4–24.1) | 30.7 (20.9–58.1) |
| jMRUI | 11.8 (8.2–20.9) | 20.9 (14.4–37.9) | 59.7 (38.6–138) | |
| Right basal ganglia | TARQUIN | 15.2 (10.6–27.3) | 13.3 (9.2–23.6) | 39.0 (26.2–77.1) |
| jMRUI | 21.2 (14.6–38.7) | 14.8 (10.3–26.5) | 72.9 (45.6–192) | |
Cho, choline; Cr, creatine; jMRUI, java-based version of the MR user interface; mI, myo-inositol; NAA, N-acetylaspartate; TARQUIN, totally automatic robust quantitation in nuclear MR.
Table 2.
Pearson's correlations of cerebral metabolite ratios by region (includes Day 0 and Day 14 data)
| CMR | Frontal white matter (n = 22) |
Frontal grey matter (n = 20) |
Right basal ganglia (n = 22) |
|||
|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | |
| NAA/Cr | 0.74 | <0.01 | 0.15 | 0.53 | 0.38 | 0.08 |
| Cho/Cr | 0.61 | <0.01 | 0.37 | 0.11 | 0.25 | 0.26 |
| mI/Cr | 0.22 | 0.34 | 0.41 | 0.07 | −0.10 | 0.66 |
Cho, choline; CMR, cerebral metabolite ratio; Cr, creatine; mI, myo-inositol; NAA, N-acetylaspartate.
Longitudinal changes in cerebral metabolite ratios
Table 3 shows the results of the baseline (study entry) and Day 14 (post-antiretroviral intensification) CMRs. In the three voxels analysed, no significant changes were observed in any of the CMRs over the study period using either quantification method.
Table 3.
Longitudinal changes in cerebral metabolite ratio parameters
| Parameter mean (SD) | Day 0 (n = 11) | Day 14 (n = 11) | Change over study period | p-value for change over study period |
|---|---|---|---|---|
| TARQUIN | ||||
| Frontal grey matter | ||||
| NAA/Cr | 1.13 (0.21) | 1.16 (0.20) | 0.03 (0.15) | 0.88 |
| Cho/Cr | 0.19 (0.033) | 0.20 (0.025) | 0.01 (0.025) | 0.42 |
| mI/Cr | 0.75 (0.17) | 0.68 (0.19) | −0.07 (0.14) | 0.17 |
| Frontal white matter | ||||
| NAA/Cr | 1.25 (0.26) | 1.25 (0.24) | 0.00 (0.27) | 0.83 |
| Cho/Cr | 0.28 (0.045) | 0.30 (0.068) | 0.02 (0.076) | 0.22 |
| mI/Cr | 0.91 (0.28) | 0.97 (0.23) | 0.06 (0.27) | 0.23 |
| Right basal ganglia | ||||
| NAA/Cr | 1.41 (0.24) | 1.51 (0.21) | 0.10 (0.14) | 0.18 |
| Cho/Cr | 0.28 (0.033) | 0.28 (0.034) | 0.00 (0.060) | 0.91 |
| mI/Cr | 0.76 (0.27) | 0.71 (0.28) | −0.05 (0.35) | 0.61 |
| jMRUI16 | ||||
| Frontal grey matter | ||||
| NAA/Cr | 1.75 (0.19) | 1.72 (0.20) | −0.02 (0.21) | 0.74 |
| Cho/Cr | 0.66 (0.10) | 0.67 (0.47) | 0.02 (0.14) | 0.73 |
| mI/Cr | 0.64 (0.33) | 0.65 (0.01) | 0.01 (0.63) | 0.96 |
| Frontal white matter | ||||
| NAA/Cr | 1.76 (0.20) | 1.77 (0.19) | 0.01 (0.19) | 0.99 |
| Cho/Cr | 1.05 (0.22) | 1.05 (0.18) | 0.00 (0.23) | 0.98 |
| mI/Cr | 1.09 (0.65) | 1.25 (0.55) | 0.17 (0.65) | 0.41 |
| Right basal ganglia | ||||
| NAA/Cr | 1.82 (0.39) | 2.09 (0.31) | 0.27 (0.61) | 0.18 |
| Cho/Cr | 0.78 (0.12) | 0.92 (0.24) | 0.14 (0.23) | 0.07 |
| mI/Cr | 0.69 (0.50) | 0.93 (0.48) | 0.24 (0.60) | 0.17 |
Cho, choline; Cr, creatine; jMRUI, java-based version of the MR user interface; mI, myo-inositol; NAA, N-acetylaspartate; SD, standard deviation; TARQUIN, totally automatic robust quantitation in nuclear MR.
Associations between pharmacokinetic results and cerebral metabolite ratios
Utilizing TARQUIN, the mI/Cr ratio in the RBG on Day 14 was negatively correlated with trough maraviroc plasma concentration (Cmin; r = −0.60, p = 0.049) but not with maraviroc CSF concentration (r = 0.47, p = 0.15). No significant associations between maraviroc Cmin or CSF concentration with other CMR changes were observed (p > 0.08 all values, data not shown) or associations between Day 14 metabolite results and either plasma or CSF maraviroc concentration. In a previous publication utilizing jMRUI,16 the only significant association was a positive correlation of NAA/Cr ratio in the RBG with plasma maraviroc Cmin (r = 0.61, p = 0.047).
DISCUSSION
In this report, 1H-MRS data from HIV-infected patients on a stable antiretroviral regimen were analysed using two quantification models: jMRUI and TARQUIN. This is the first report to compare these particular software packages with regard to CMRs in HIV-positive subjects. We observed differences between CMRs, determined by jMRUI and TARQUIN, in all brain areas, which were most marked for Cho/Cr.
In a previous report describing CMRs quantified using the LCmodel in HIV disease at a higher magnetic field strength than our study,24 mean CMRs in the FWM were 1.28, 0.32 and 0.56 for NAA/Cr, Cho/Cr and mI/Cr, respectively. These values are similar to our findings in the FWM utilizing the TARQUIN package (1.25, 0.28 and 0.91 for NAA/Cr, Cho/Cr and mI/Cr, respectively) and differed to our findings utilizing the jMRUI package (1.76, 1.05 and 1.05 for NAA/Cr, Cho/Cr and mI/Cr, respectively).16 In view of this, we hypothesise the Cho/Cr and mI/Cr ratios reported in our study, quantified by TARQUIN, may be a more accurate reflection of the true concentrations of these metabolites. However, in other studies where the LCmodel model for spectra quantification had been used, different spectral acquisition parameters were utilized. This limits our ability to determine actual values for these cerebral metabolites.
Our data determined by the TARQUIN package show less variability than those with jMRUI for measurements of mI/Cr. A possible explanation for this is that TARQUIN is fully automated and therefore analysed spectra are not dependent on user interaction. Manual removal of water peaks and the reduction of residual MRS noise in jMRUI are user dependent and could affect quantification of spectra and reproducibility of results.
We also assessed longitudinal 1H-MRS data. No statistically significant longitudinal changes in CMR were evident when analysed using TARQUIN or jMRUI. We did observe a negative correlation between mI/Cr ratio measured by TARQUIN with higher maraviroc plasma Cmin. We hypothesize that this effect may be secondary to an anti-inflammatory property of maraviroc mediated by antagonism by CCR5 or by suppression of residual HIV replication.25 However, such observations need to be interpreted with caution given the multiple comparisons and that our study is a descriptive analysis rather than one with a sample size powered to determine such observations. This association was not observed using jMRUI. Although this may be due to chance and the lack of power we have to make such observations, another possible explanation is due to the higher CoV of mI/Cr when measured by jMRUI.
In summary, we have observed that CMRs in HIV-positive subjects differ when quantified utilizing jMRUI and TARQUIN. However, we did not observe significant differences in the clinical associations with CMRs using the two different software packages giving some confidence to future researchers if they wished to adopt the newer TARQUIN package. Future work to assess the accuracy, reproducibility and clinical associations of CMRs measured via 1H-MRS in HIV-positive subjects is warranted.
Acknowledgments
ACKNOWLEDGMENTS
All authors are grateful to the National Institute for Health Research Biomedical Facility at Imperial College London for infrastructure support.
Contributor Information
Joseph Scott, Email: joseph.scott10@imperial.ac.uk.
Jonathan Underwood, Email: jonathan.underwood@imperial.ac.uk.
Lucy J Garvey, Email: lucy.garvey@imperial.nhs.uk.
Borja Mora-Peris, Email: b.mora-peris@imperial.ac.uk.
Alan Winston, Email: a.winston@imperial.ac.uk.
FUNDING
The maraviroc intensification study was funded by an investigator-initiated grant to Imperial College London (AW) from ViiV Healthcare.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS report on the global AIDS epidemic 2013. Switzerland: UNAIDS; 2013. [Google Scholar]
- 2.McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993; 43: 2245–52. doi: 10.1212/WNL.43.11.2245 [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338: 853–60. doi: 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol 2002; 8(Suppl. 2): 115–21. [DOI] [PubMed] [Google Scholar]
- 5.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24: 1243–50. [DOI] [PubMed] [Google Scholar]
- 6.Skinner S, Adewale AJ, DeBlock L, Gill MJ, Power C. Neurocognitive screening tools in HIV/AIDS: comparative performance among patients exposed to antiretroviral therapy. HIV Med 2009; 10: 246–52. doi: 10.1111/j.1468-1293.2008.00679.x [DOI] [PubMed] [Google Scholar]
- 7.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 2004; 63: 2084–90. doi: 10.1212/01.WNL.0000145763.68284.15 [DOI] [PubMed] [Google Scholar]
- 8.Price RW, Peterson J, Fuchs D, Angel TE, Zetterberg H, Hagberg L, et al. Approach to cerebrospinal fluid (CSF) biomarker discovery and evaluation in HIV infection. J Neuroimmune Pharmacol 2013; 8: 1147–58. doi: 10.1007/s11481-013-9491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. NeuroImage 2002; 17: 1638–48. doi: 10.1006/nimg.2002.1254 [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999; 52: 100–8. doi: 10.1212/WNL.52.1.100 [DOI] [PubMed] [Google Scholar]
- 11.Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol 2011; 17: 220–9. doi: 10.1007/s13365-011-0030-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiology 2009; 64: 12–21. doi: 10.1016/j.crad.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 13. Magnetic Resonance Spectroscopy, InTech. Available from: http://www.intechopen.com/books/magnetic-resonance-spectroscopy/quantification-improvements-of-1h-mrssignals. [Google Scholar]
- 14.Pels P, Ozturk-Isik E, Swanson MG, Vanhamme L, Kurhanewicz J, Nelson SJ, et al. Quantification of prostate MRSI data by model-based time domain fitting and frequency domain analysis. NMR Biomed 2006; 19: 188–97. doi: 10.1002/nbm.1008 [DOI] [PubMed] [Google Scholar]
- 15.Mullins PG, Rowland L, Bustillo J, Bedrick EJ, Lauriello J, Brooks WM. Reproducibility of 1H-MRS measurements in schizophrenic patients. Magn Reson Med 2003; 50: 704–7. doi: 10.1002/mrm.10598 [DOI] [PubMed] [Google Scholar]
- 16.Garvey L, Nelson M, Latch N, Erlwein OW, Allsop JM, Mitchell A, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother 2012; 67: 206–12. doi: 10.1093/jac/dkr427 [DOI] [PubMed] [Google Scholar]
- 17.Else L, Watson V, Tjia J, Hughes A, Siccardi M, Khoo S, et al. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 1455–65. doi: 10.1016/j.jchromb.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 18.Naressi A, Couturier C, Devos J, Janssen M, Mangeat C, De Beer R, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001; 12: 141–52. [DOI] [PubMed] [Google Scholar]
- 19.Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC. A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn Reson Med 2011; 65: 1–12. doi: 10.1002/mrm.22579 [DOI] [PubMed] [Google Scholar]
- 20.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997; 129: 35–43. doi: 10.1006/jmre.1997.1244 [DOI] [PubMed] [Google Scholar]
- 21.van den Boogaart A. A users guide to the magnetic resonance user interface software package MRUI manual V96.3. Delft, Netherlands. 1997. [Google Scholar]
- 22.Poullet JB, Sima DM, Simonetti AW, De Neuter B, Vanhamme L, Lemmerling P, et al. An automated quantitation of short echo time MRS spectra in an open source software environment: AQSES. NMR Biomed 2007; 20: 493–504. doi: 10.1002/nbm.1112 [DOI] [PubMed] [Google Scholar]
- 23.Vanhamme L, Fierro RD, Van Huffel S, de Beer R. Fast removal of residual water in proton spectra. J Magn Reson 1998; 132: 197–203. doi: 10.1006/jmre.1998.1425 [DOI] [PubMed] [Google Scholar]
- 24.Mohamed MA, Barker PB, Skolasky RL, Selnes OA, Moxley RT, Pomper MG, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging 2010; 28: 1251–7. doi: 10.1016/j.mri.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 2005; 49: 4721–32. doi: 10.1128/AAC.49.11.4721-4732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]